Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines
Abstract
1. Introduction
2. Vaccines
3. Liposomes as Vaccine Adjuvants
3.1. Effect of Liposome Composition on Their Performance
3.2. Mechanistic Understanding of Lipid-Based Vaccines
4. Liposome Application in Severe Acute Respiratory Syndrome (SARS)
5. Published Data for Liposomal Vaccines for SARS-CoV-2
6. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Application of Liposomes
7. Liposomal Vaccines for Respiratory Syncytial Virus (RSV)
8. Application of Liposomes in Influenza Vaccines
8.1. Mucosal Influenza Liposomal Vaccines
8.2. Oral Liposomal Influenza Vaccines
9. Conclusions
10. Current Problems and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, L.; Waterer, G. Control Measures for Human Respiratory Viral Infection. Semin. Respir. Crit. Care Med. 2016, 37, 631–639. [Google Scholar]
- Mäkelä, M.J.; Puhakka, T.; Ruuskanen, O.; Leinonen, M.; Saikku, P.; Kimpimäki, M.; Blomqvist, S.; Hyypiä, T.; Arstila, P. Viruses and Bacteria in the Etiology of the Common Cold. J. Clin. Microbiol. 1998, 36, 539–542. [Google Scholar] [CrossRef]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission Routes of Respiratory Viruses among Humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Lipscomb, M.F.; Hutt, J.; Lovchik, J.; Wu, T.; Lyons, C.R. The Pathogenesis of Acute Pulmonary Viral and Bacterial Infections: Investigations in Animal Models. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 223–252. [Google Scholar] [CrossRef]
- Ferkol, T.; Schraufnagel, D. The Global Burden of Respiratory Disease. Ann. ATS 2014, 11, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Severe Acute Respiratory Syndrome (SARS): Report by the Secretariat; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization. Rolling Updates on Coronavirus Disease (COVID-19)—Events as They Happen. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 10 June 2020).
- World Health Organization. Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: http://www.who.int/emergencies/mers-cov/en/ (accessed on 10 June 2020).
- Memish, Z.A.; Perlman, S.; Kerkhove, M.D.V.; Zumla, A. Middle East Respiratory Syndrome. Lancet 2020, 395, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, H.; Gu, J.; Li, H.; Zheng, L.; Zou, Q. Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines 2020, 8, 153. [Google Scholar] [CrossRef]
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the Liver: Little Cause for Concern. Lancet Gastroenterol. Hepatol. 2020, 5, 529–530. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the Cardiovascular System. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Narasimhan, B.; Mallapragada, S.K. Respiratory Nanoparticle-Based Vaccines and Challenges Associated with Animal Models and Translation. J. Control. Release 2015, 219, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Zepp, F. Principles of Vaccine Design-Lessons from Nature. Vaccine 2010, 28 (Suppl. 3), C14–C24. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.; Romeu, B.; Cabrera, O.; Gonzalez, E.; Batista-Duharte, A.; Labrada, A.; Pérez, R.; Reyes, L.; Ramírez, W.; Sifontes, S.; et al. Adjuvants Are Key Factors for the Development of Future Vaccines: Lessons from the Finlay Adjuvant Platform. Front. Immunol. 2013, 4, 407. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Wu, C.-Y. Carbohydrate-Based Vaccines: Challenges and Opportunities. Expert Rev. Vaccines 2010, 9, 1257–1274. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as Vaccine Delivery Systems: A Review of the Recent Advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Leroux-Roels, G. Unmet Needs in Modern Vaccinology. Vaccine 2010, 28, C25–C36. [Google Scholar] [CrossRef]
- Alving, C.R.; Peachman, K.K.; Rao, M.; Reed, S.G. Adjuvants for Human Vaccines. Curr. Opin. Immunol. 2012, 24, 310–315. [Google Scholar] [CrossRef]
- Sibbald, B. Death but One Unintended Consequence of Gene-Therapy Trial. CMAJ 2001, 164, 1612. [Google Scholar]
- Park, Y.-M.; Lee, S.J.; Kim, Y.S.; Lee, M.H.; Cha, G.S.; Jung, I.D.; Kang, T.H.; Han, H.D. Nanoparticle-Based Vaccine Delivery for Cancer Immunotherapy. Immune Netw. 2013, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.-W.; Chattopadhyay, S.; Lin, J.-C.; Hu, C.-M.J. Advances and Opportunities in Nanoparticle- and Nanomaterial-Based Vaccines against Bacterial Infections. Adv. Healthc. Mater. 2018, 7, 1701395. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Banchereau, J. Taking Dendritic Cells into Medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev.™ Ther. Drug Carr. Syst. 2010, 46, 523–580. [Google Scholar] [CrossRef]
- Andersson, L.C.; Häyry, P.; Bach, M.A.; Bach, J.F. Differences in the Effects of Adult Thymectomy on T-Cell Mediated Responses in Vitro. Nature 1974, 252, 252–254. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes Used as a Vaccine Adjuvant-Delivery System: From Basics to Clinical Immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Rao, M.; Peachman, K.K.; Alving, C.R. Liposome Formulations as Adjuvants for Vaccines; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- De Serrano, L.O.; Burkhart, D.J. Liposomal Vaccine Formulations as Prophylactic Agents: Design Considerations for Modern Vaccines. J. Nanobiotechnol. 2017, 15, 83. [Google Scholar] [CrossRef]
- Marasini, N.; Ghaffar, K.A.; Skawczynski, M.; Toth, I. Liposomes as a Vaccine Delivery System in Micro and Nanotechnology in Vaccine Development; Skawczynski, M., Toth, I., Eds.; Elsevier: Oxford, UK, 2017; pp. 221–239. [Google Scholar]
- Essa, E.A.; Bonner, M.C.; Barry, B.W. Electrically Assisted Skin Delivery of Liposomal Estradiol; Phospholipid as Damage Retardant. J. Control. Release 2004, 95, 535–546. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Khemka, V.; See, D.; See, J.; Chang, J.; Chou, S.; Tilles, J. The Capacity of a Combined Liposomal Hepatitis B and C Vaccine to Stimulate Humoral and Cellular Responses in Mice. Viral Immunol. 1998, 11, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Comparison of the Depot Effect and Immunogenicity of Liposomes Based on Dimethyldioctadecylammonium (DDA), 3β-[N-(N′,N′-Dimethylaminoethane)Carbomyl] Cholesterol (DC-Chol), and 1,2-Dioleoyl-3-Trimethylammonium Propane (DOTAP): Prolonged Liposome Retention Mediates Stronger Th1 Responses. Mol. Pharm. 2011, 8, 153–161. [Google Scholar]
- Adler-Moore, J.; Munoz, M.; Kim, H.; Romero, J.; Tumpey, T.; Zeng, H.; Petro, C.; Ernst, W.; Kosina, S.; Jimenez, G.; et al. Characterization of the Murine Th2 Response to Immunization with Liposomal M2e Influenza Vaccine. Vaccine 2011, 29, 4460–4468. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Bramwell, V.W.; Christensen, D.; Agger, E.-M.; Andersen, P.; Perrie, Y. Liposomes Based on Dimethyldioctadecylammonium Promote a Depot Effect and Enhance Immunogenicity of Soluble Antigen. J. Control. Release 2010, 142, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic Liposomes as Vaccine Adjuvants. Expert. Rev. Vaccines 2011, 10, 513–521. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of Nanotechnology for Immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Latif, N.; Bachhawat, B.K. The Effect of Surface Charges of Liposomes in Immunopotentiation. Biosci. Rep. 1984, 4, 99–107. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kunisawa, J.; Hayashi, A.; Tsutsumi, Y.; Kubo, K.; Nakagawa, S.; Fujiwara, H.; Hamaoka, T.; Mayumi, T. Positively Charged Liposome Functions as an Efficient Immunoadjuvant in Inducing Immune Responses to Soluble Proteins. Biochem. Biophys. Res. Commun. 1997, 240, 793–797. [Google Scholar] [CrossRef]
- Christensen, D.; Korsholm, K.S.; Rosenkrands, I.; Lindenstrøm, T.; Andersen, P.; Agger, E.M. Cationic Liposomes as Vaccine Adjuvants. Expert. Rev. Vaccines 2007, 6, 785–796. [Google Scholar] [CrossRef]
- Foged, C.; Arigita, C.; Sundblad, A.; Jiskoot, W.; Storm, G.; Frokjaer, S. Interaction of Dendritic Cells with Antigen-Containing Liposomes: Effect of Bilayer Composition. Vaccine 2004, 22, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhuang, Y.; Xie, X.; Wang, C.; Wang, F.; Zhou, D.; Zeng, J.; Cai, L. The Role of Surface Charge Density in Cationic Liposome-Promoted Dendritic Cell Maturation and Vaccine-Induced Immune Responses. Nanoscale 2011, 3, 2307–2314. [Google Scholar] [CrossRef]
- Yanasarn, N.; Sloat, B.R.; Cui, Z. Negatively Charged Liposomes Show Potent Adjuvant Activity When Simply Admixed with Protein Antigens. Mol. Pharm. 2011, 8, 1174–1185. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Senior, J. The Phospholipid Component of Small Unilamellar Liposomes Controls the Rate of Clearance of Entrapped Solutes from the Circulation. FEBS Lett. 1980, 119, 43–46. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified MRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The Dawn of MRNA Vaccines: The COVID-19 Case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.-R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.M.; et al. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; Zhang, L.; Welte, T.; Zabel, P.; Chung, K.F. Severe Acute Respiratory Syndrome: Global Initiatives for Disease Diagnosis. QJM Int. J. Med. 2003, 96, 845–852. [Google Scholar] [CrossRef][Green Version]
- Seto, W.; Tsang, D.; Yung, R.; Ching, T.; Ng, T.; Ho, M.; Ho, L.; Peiris, J. Effectiveness of Precautions against Droplets and Contact in Prevention of Nosocomial Transmission of Severe Acute Respiratory Syndrome (SARS). Lancet 2003, 361, 1519–1520. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Ohno, S.; Kohyama, S.; Taneichi, M.; Moriya, O.; Hayashi, H.; Oda, H.; Mori, M.; Kobayashi, A.; Akatsuka, T.; Uchida, T.; et al. Synthetic Peptides Coupled to the Surface of Liposomes Effectively Induce SARS Coronavirus-Specific Cytotoxic T Lymphocytes and Viral Clearance in HLA-A*0201 Transgenic Mice. Vaccine 2009, 27, 3912–3920. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Update on COVID-19—27 July 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-july-2021 (accessed on 5 August 2021).
- Milane, L.; Amiji, M. Clinical Approval of Nanotechnology-Based SARS-CoV-2 MRNA Vaccines: Impact on Translational Nanomedicine. Drug Deliv. Transl. Res. 2021, 11, 1309–1315. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA Vaccines: Ready for Prime Time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The New Revolution in Nucleic Acid Vaccines. Semin. Immunol. 2013, 25, 152–159. [Google Scholar] [CrossRef]
- Redding, L.; Weiner, D.B. DNA Vaccines in Veterinary Use. Expert Rev. Vaccines 2009, 8, 1251–1276. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-Viral COVID-19 Vaccine Delivery Systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.R.; Boczkowski, D.; Nair, S.K.; Snyder, D.; Gilboa, E. Vaccination with MRNAs Encoding Tumor-Associated Antigens and Granulocyte-Macrophage Colony-Stimulating Factor Efficiently Primes CTL Responses, but Is Insufficient to Overcome Tolerance to a Model Tumor/Self Antigen. Cancer Immunol. Immunother. 2006, 55, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A Comprehensive Status Report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid Nanoparticles for Nucleic Acid Delivery: Current Perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Carstens, M.G.; Camps, M.G.M.; Henriksen-Lacey, M.; Franken, K.; Ottenhoff, T.H.M.; Perrie, Y.; Bouwstra, J.A.; Ossendorp, F.; Jiskoot, W. Effect of Vesicle Size on Tissue Localization and Immunogenicity of Liposomal DNA Vaccines. Vaccine 2011, 29, 4761–4770. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Klimek, L.; Hamelmann, E.; Pfaar, O.; Taube, C.; Wagenmann, M.; Werfel, T.; Worm, M. Severe Allergic Reactions to the COVID-19 Vaccine—Statement and Practical Consequences. Allergol. Sel. 2021, 5, 26–28. [Google Scholar] [CrossRef]
- Prüβ, B.M. Current State of the First COVID-19 Vaccines. Vaccines 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Chen, H.; Liu, H.; Gao, Q.; Cong, F.; Gao, G.; Chen, Y. Subunit Nanovaccine with Potent Cellular and Mucosal Immunity for COVID-19. ACS Appl. Bio Mater. 2020, 3, 5633–5638. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.; Yang, S.; Xiao, W.; Zheng, Q.; Song, X. The Investigation of MRNA Vaccines Formulated in Liposomes Administrated in Multiple Routes against SARS-CoV-2. J Control Release 2021, 335, 449–456. [Google Scholar] [CrossRef]
- Lui, P.-Y.; Wong, L.-Y.R.; Fung, C.-L.; Siu, K.-L.; Yeung, M.-L.; Yuen, K.-S.; Chan, C.-P.; Woo, P.C.-Y.; Yuen, K.-Y.; Jin, D.-Y. Middle East Respiratory Syndrome Coronavirus M Protein Suppresses Type I Interferon Expression through the Inhibition of TBK1-Dependent Phosphorylation of IRF3. Emerg. Microbes Infect. 2016, 5, e39. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Lee, S.I.; Bae, J.-Y.; Park, M.-S.; Lee, Y.; Kwon, H.-J. Production of a Monoclonal Antibody Targeting the M Protein of MERS-CoV for Detection of MERS-CoV Using a Synthetic Peptide Epitope Formulated with a CpG–DNA–Liposome Complex. Int. J. Pept. Res. Ther. 2019, 25, 819–826. [Google Scholar] [CrossRef]
- Numata, M.; Chu, H.W.; Dakhama, A.; Voelker, D.R. Pulmonary Surfactant Phosphatidylglycerol Inhibits Respiratory Syncytial Virus-Induced Inflammation and Infection. Proc. Natl. Acad. Sci. USA 2010, 107, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, G.L.; Velazquez, L.; Pham, S.; Qaisar, N.; Delaney, J.C.; Viswanathan, K.; Albers, L.; Comolli, J.C.; Shriver, Z.; Knipe, D.M.; et al. Heparin Octasaccharide Decoy Liposomes Inhibit Replication of Multiple Viruses. Antivir. Res. 2015, 116, 34–44. [Google Scholar] [CrossRef]
- Park, B.K.; Choi, S.H.; Kim, Y.-E.; Park, S.; Lee, Y.; Lee, K.-W.; Kwon, H.-J. Monoclonal Antibodies Against the Human Respiratory Syncytial Virus Obtained by Immunization with Epitope Peptides and CpG-DNA-Liposome Complex. Monoclon. Antibodies Immunodiagn. Immunother. 2015, 34, 101–109. [Google Scholar] [CrossRef]
- Joshi, S.; Chaudhari, A.; Dennis, V.; Kirby, D.; Perrie, Y.; Singh, S. Anti-RSV Peptide-Loaded Liposomes for the Inhibition of Respiratory Syncytial Virus. Bioengineering 2018, 5, 37. [Google Scholar] [CrossRef]
- Babai, I.; Samira, S.; Barenholz, Y.; Zakay-Rones, Z.; Kedar, E. A Novel Influenza Subunit Vaccine Composed of Liposome-Encapsulated Haemagglutinin/Neuraminidase and IL-2 or GM-CSF. I. Vaccine Characterization and Efficacy Studies in Mice. Vaccine 1999, 17, 1223–1238. [Google Scholar] [CrossRef]
- Babai, I.; Barenholz, Y.; Zakay-Rones, Z.; Greenbaum, E.; Samira, S.; Hayon, I.; Rochman, M.; Kedar, E. A Novel Liposomal Influenza Vaccine (INFLUSOME-VAC) Containing Hemagglutinin–Neuraminidase and IL-2 or GM-CSF Induces Protective Anti-Neuraminidase Antibodies Cross-Reacting with a Wide Spectrum of Influenza A Viral Strains. Vaccine 2001, 20, 505–515. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Liang, J.-X.; Dai, S.-Y.; Qiu, X.-L.; Yi, Y.-R.; Pan, Y. Immunological Effect of Subunit Influenza Vaccine Entrapped by Liposomes. Biomed. Environ. Sci. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Khantasup, K.; Kopermsub, P.; Chaichoun, K.; Dharakul, T. Targeted Small Interfering RNA-Immunoliposomes as a Promising Therapeutic Agent against Highly Pathogenic Avian Influenza A (H5N1) Virus Infection. Antimicrob. Agents Chemother. 2014, 58, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- de Haan, A.; Van Scharrenburg, G.J.M.; Masihi, K.N.; Wilschut, J. Evaluation of a Liposome-Supplemented Intranasal Influenza Subunit Vaccine in a Murine Model System: Induction of Systemic and Local Mucosal Immunity. J. Liposome Res. 2000, 10, 159–177. [Google Scholar] [CrossRef]
- Joseph, A.; Itskovitz-Cooper, N.; Samira, S.; Flasterstein, O.; Eliyahu, H.; Simberg, D.; Goldwaser, I.; Barenholz, Y.; Kedar, E. A New Intranasal Influenza Vaccine Based on a Novel Polycationic Lipid—Ceramide Carbamoyl-Spermine (CCS). Vaccine 2006, 24, 3990–4006. [Google Scholar] [CrossRef]
- Chiou, C.-J.; Tseng, L.-P.; Deng, M.-C.; Jiang, P.-R.; Tasi, S.-L.; Chung, T.-W.; Huang, Y.-Y.; Liu, D.-Z. Mucoadhesive Liposomes for Intranasal Immunization with an Avian Influenza Virus Vaccine in Chickens. Biomaterials 2009, 30, 5862–5868. [Google Scholar] [CrossRef]
- Dhakal, S.; Cheng, X.; Salcido, J.; Renu, S.; Bondra, K.; Lakshmanappa, Y.S.; Misch, C.; Ghimire, S.; Feliciano-Ruiz, N.; Hogshead, B.; et al. Liposomal Nanoparticle-Based Conserved Peptide Influenza Vaccine and Monosodium Urate Crystal Adjuvant Elicit Protective Immune Response in Pigs. IJN 2018, 13, 6699–6715. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Yorgensen, Y.M.; Morasse, A.; Evans, J.T.; Burkhart, D.J. PEG Modified Liposomes Containing CRX-601 Adjuvant in Combination with Methylglycol Chitosan Enhance the Murine Sublingual Immune Response to Influenza Vaccination. J. Control. Release 2016, 223, 64–74. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary Surfactant–Biomimetic Nanoparticles Potentiate Heterosubtypic Influenza Immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Wang, B.; Zeng, S.; Qi, F.; Lu, C.; Kimura, Y.; Liu, B. Oral Vaccination with a Liposome-Encapsulated Influenza DNA Vaccine Protects Mice against Respiratory Challenge Infection: Oral Vaccination and Influenza Virus Infection. J. Med. Virol. 2014, 86, 886–894. [Google Scholar] [CrossRef]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of Coronavirus Cell Entry Mediated by the Viral Spike Protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The Spike Glycoprotein of the New Coronavirus 2019-NCoV Contains a Furin-like Cleavage Site Absent in CoV of the Same Clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E. MERS Coronavirus: Diagnostics, Epidemiology and Transmission. Virol. J. 2015, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronaviruses—Drug Discovery and Therapeutic Options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhou, Y.; Lu, L.; Li, F.; Jiang, S. MERS-CoV Spike Protein: A Key Target for Antivirals. Expert Opin. Ther. Targets 2017, 21, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.; Batool, M.; Shah, M.; Choi, S. Middle East Respiratory Syndrome Coronavirus: Transmission, Virology and Therapeutic Targeting to Aid in Outbreak Control. Exp. Mol. Med. 2015, 47, e181. [Google Scholar] [CrossRef]
- Ki, M. 2015 MERS Outbreak in Korea: Hospital-to-Hospital Transmission. Epidemiol. Health 2015, 37, e2015033. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Epidemic and Pandemic-Prone Diseases MERS Situation Update. Available online: http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html (accessed on 11 June 2020).
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 4 June 2020).
- Tregoning, J.S.; Schwarze, J. Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology, and Immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 11, 1749–1759. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory Syncytial Virus—A Comprehensive Review. Clin. Rev. Allerg. Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Welliver, R.C. Review of Epidemiology and Clinical Risk Factors for Severe Respiratory Syncytial Virus (RSV) Infection. J. Pediatrics 2003, 143, 112–117. [Google Scholar] [CrossRef]
- Kamphuis, T.; Meijerhof, T.; Stegmann, T.; Lederhofer, J.; Wilschut, J.; de Haan, A. Immunogenicity and Protective Capacity of a Virosomal Respiratory Syncytial Virus Vaccine Adjuvanted with Monophosphoryl Lipid A in Mice. PLoS ONE 2012, 7, e36812. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Kang, S.-M.; Ko, E.-J.; Hwang, H.S.; Lee, J.S.; Kim, K.-H.; Kwon, Y.-M. Respiratory Syncytial Virus-like Nanoparticle Vaccination Induces Long-Term Protection without Pulmonary Disease by Modulating Cytokines and T-Cells Partially through Alveolar Macrophages. IJN 2015, 10, 4491. [Google Scholar] [PubMed]
- Piedimonte, G.; Perez, M.K. Respiratory Syncytial Virus Infection and Bronchiolitis. Pediatrics Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Bawage, S.S.; Tiwari, P.M.; Pillai, S.; Dennis, V.; Singh, S.R. Recent Advances in Diagnosis, Prevention, and Treatment of Human Respiratory Syncytial Virus. Adv. Virol. 2013, 2013, 595768. [Google Scholar] [CrossRef]
- Joshi, S.; Bawage, S.; Tiwari, P.; Kirby, D.; Perrie, Y.; Dennis, V.; Singh, S.R. Liposomes: A Promising Carrier for Respiratory Syncytial Virus Therapeutics. Expert Opin. Drug Deliv. 2019, 16, 969–980. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Launches New Global Influenza Strategy. Available online: https://www.who.int/news-room/detail/11-03-2019-who-launches-new-global-influenza-strategy (accessed on 14 June 2020).
- World Health Organization. Seasonal Influenza. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/seasonal-influenza (accessed on 12 June 2020).
- Jazayeri, S.D.; Poh, C.L. Development of Universal Influenza Vaccines Targeting Conserved Viral Proteins. Vaccines 2019, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A.; Krug, R.M. Orthomyxoviridae: The Viruses and Their Replication. Fields Virol. 1996, 1353–1395. [Google Scholar]
- Murphy, B.; Webster, R. Orthomyxoviruses. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Chanock, R.M., Melnick, J.L., Monath, T.P., Roizman, B., Straus, S.E., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1996. [Google Scholar]
- Kilbourne, E.D.; Arden, N.H. Inactivated Influenza Vaccine. Dalam: Plotkin SA, Orenstein WA, Penyunting. Vaccines. Edisi Ke-3. Philadelphia, London, Toronto, Montreal, 3rd ed.; WB Saunders Company: Sydney, Australia; Tokyo, Japan, 1999. [Google Scholar]
- Bernasconi, V.; Norling, K.; Bally, M.; Höök, F.; Lycke, N.Y. Mucosal Vaccine Development Based on Liposome Technology. J. Immunol. Res. 2016, 2016, 5482087. [Google Scholar] [CrossRef] [PubMed]
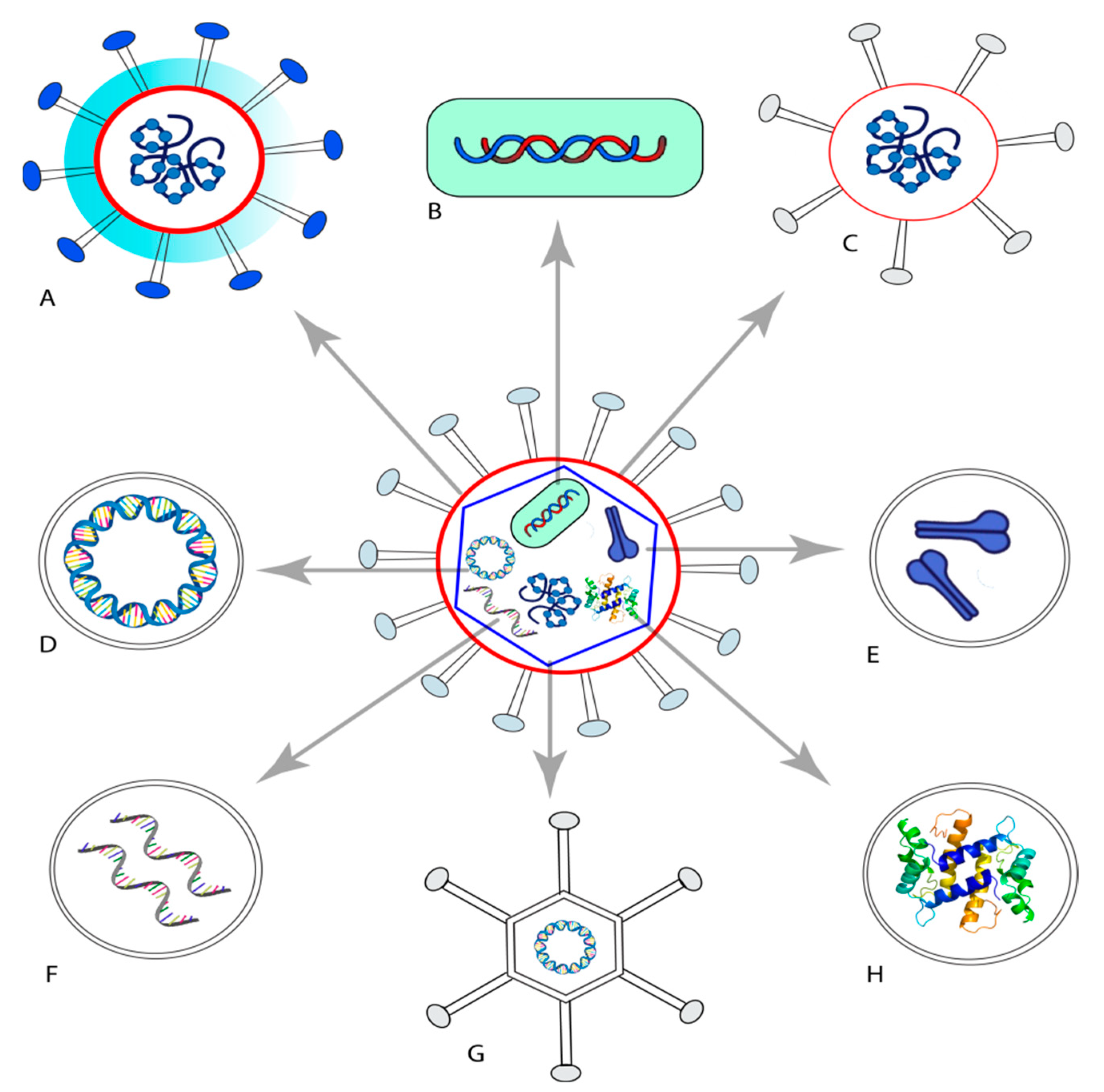


| Developers | Type of the Candidate Vaccine | Vaccine Platform Description | Phase |
|---|---|---|---|
| Sinovac Research and Development Co., Ltd. | CoronaVac; SARS-CoV-2 vaccine | Inactivated, produced in Vero cells | Phase IV |
| AstraZeneca + University of Oxford | ChAdOx1-S (AZD1222) (Covishield) | Recombinant ChAdOx1 adenoviral vector encoding the spike protein antigen of SARS-CoV-2 | Phase IV |
| CanSino Biological Inc./Beijing Institute of Biotechnology | Recombinant novel coronavirus vaccine (adenovirus type 5 vector) | Viral vector (nonreplicating) | Phase III |
| Gamaleya Research Institute; Health Ministry of the Russian Federation | Gam-COVID-Vac adeno-based (rAd26-S+rAd5-S) | Viral vector (nonreplicating) | Phase III |
| Janssen Pharmaceutical | Ad26.COV2.S | Recombinant, replication-incompetent adenovirus type 26(Ad26) vector vaccine encoding the SARS-CoV-2 spike (S) protein | Phase III |
| Novavax United Kingdom | SARS-CoV-2 rS/Matrix M1 adjuvant (full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvated with Matrix M) | Protein subunit | Phase III |
| Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | mRNA-1273mRNA-1283 | RNA-based vaccine | Phase IV |
| Pfizer/BioNTech + Fosun Pharma | BNT162b2 (three LNP-mRNAs), also known as Comirnaty | RNA-based vaccine | Phase IV |
| Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Recombinant SARS-CoV-2 vaccine (CHO cell) | Protein subunit | Phase III |
| Institute of Medical Biology + Chinese Academy of Medical Sciences | SARS-CoV-2 vaccine (Vero cells) | Inactivated virus | Phase III |
| Research Institute for Biological Safety Problems, Republic of Kazakhstan | QazCovid-in®; COVID-19 inactivated vaccine | Inactivated virus | Phase III |
| Zydus Cadila | nCov vaccine | DNA-based vaccine | Phase III |
| Bharat Biotech International Limited | Whole-virion inactivated SARS-CoV-2 vaccine (BBV152) | Inactivated virus | Phase III |
| Sanofi Pasteur + GSK | VAT00002: SARS-CoV-2 S protein with an adjuvant | Protein subunit | Phase III |
| Instituto Finlay de Vacunas | FINLAY-FR-2 anti-SARS-CoV-2 vaccine (RBD chemically conjugated to tetanus toxoid plus adjuvant) | Protein subunit | Phase III |
| Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” | EpiVacCorona (EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19) | Protein subunit | Phase III |
| Target Disease | Therapeutic Agent | Liposome Composition | Route of Administration | Liposomal Size | Experimental Stage | Preparation Method | References |
|---|---|---|---|---|---|---|---|
| SARS-CoV | Cytotoxic T lymphocytes coupled to the surface of liposomes | Dioleoyl phosphatidyl ethanolamine, dioleoyl phosphatidyl choline, dioleoyl phosphatidyl glycerol acid, and cholesterol at the 3:4:2:7 molar ratio | IV | – | In vivo (mice) | – | [63] |
| SARS-CoV by Pfizer/BioNTech | Liposome loaded with synthetic mRNA | (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 0.05 mg; ALC-0159 = 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 0.09 mg; 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 0.2 mg; cholesterol (46.3:9.4:42.7:1.6) | IM | – | Human | – | [78] |
| SARS-CoV by Moderna | Liposome loaded with synthetic mRNA | (hydroxyethyl(6-oxo-6-(undecyloxy) hexyl)amino)octanoate; PEG2000-DMG = 1-monomethoxypolyethylene glycol-2,3-dimyristylglycerol with polyethylene glycol of the average molecular weight of 2000; 1,2-distearoyl-sn-glycero-3 phosphocholine (DSPC); cholesterol (50:10:38.5:1.5) | IM | – | Human | – | [78] |
| SARS-CoV | PEG-coated liposomal pDNA | Cationic liposomes, e.g., PC, DOPE, and DOTAP | SC | 140 nm | In vivo (mice) | Dehydration–rehydration method | [75] |
| SARS-CoV | Liposomes as the carrier to anchor the anionic S1 subunit of SARS-CoV-2 | Cationic 1,2-dioleoyl-3-trimethylammonium-propane,1,2-dioleoyl-sn-glycero-3-phosphoethanolamine and cholesterol | SC | 135 nm | In vivo (mice) | Thin film hydration | [79] |
| SARS-CoV | Receptor-binding domain encoding mRNA of the S protein | Cationic liposomes composed of DOTAP chloride lipids with cholesterol | IM | 50–150 nm | In vivo (mice)/in vitro | Thin film hydration | [80] |
| MERS-CoV | Liposome-loaded epitope peptide and CpG-DNA | Phosphatidyl-β-oleoyl-γ-palmitoylethanolamine (DOPE):cholesterol hemisuccinate | IP | – | In vivo (mice) | – | [81,82] |
| RSV | – | Palmitoyl-oleoyl-phosphatidylglycerol | Inoculated intranasally | – | In vivo (mice) | – | [83] |
| RSV | Liposomal heparan sulfate octasaccharide | Negatively charged HS-octa-DOPE 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine | – | 44–62.4 nm | In vitro | – | [84] |
| RSV | RSV F protein epitope-specific monoclonal antibody | phosphatidyl-β-oleoyl-γ-palmitoylethanolamine and cholesterol hemisuccinate | IP | – | In vivo (mice)/in vitro | Thin film hydration | [85] |
| RSV | Coloaded with RF-482 (an anti-RSV peptide for RSV inhibition) | 1,2-disteroylphosphatidylcholine and cholesterol (5:2 w/w) | – | 96.91 nm | In vitro | Thin film hydration | [86] |
| Influenza | Liposome-encapsulated hemagglutinin/neuraminidase and IL-2 or GMCSF | Dimyristoyl phosphatidylcholine | IP, SC | 50 nm | In vivo (mice) | Dehydration/re-hydration | [87] |
| Influenza | Liposomes containing viral surface glycoproteins HA and NA | Dimyristoyl phosphatidylcholine (DMPC):dimyristoyl phosphatidylglycerol (DMPG) at the DMPC:DMPG molar ratio of 9:1 | IP | – | In vivo (mice) | Dehydration/re-hydration | [88] |
| Influenza | Trivalent subunit influenza | Phosphatidylcholine, cholesterol, and octadecylamine | – | 4.5–5.5 μm | In vivo (mice) | Thin film evaporation combined with freeze-drying | [89] |
| H5N1 avian influenza A | Cationic liposome coupled with a humanized single-chain Fv antibody | DC-cholesterol, DOPE, maleimide-derived PEG 2000–DSPE (Mal-PEG–DSPE), and distearoyl-sn-glycero-3-phosphoethanolamine[methoxypolyethylene glycol] (2000) (mPEG–DSPE) at the molar ratio of 48.25:48.25:1:2.5 | – | – | In vitro | Thin film hydration followed by lyophilisation | [90] |
| Influenza | Negatively charged liposomes with an influenza subunit | PC and cholesterol (molar ratio, 1:1) and DCP, PA, PS, PG, or SA at either 10 or 30 mol% | Intranasal | - | In vivo (mice) | Thin film hydration | [91] |
| Influenza | Cationic liposomes | Polycationic sphingolipidceramide carbamoyl-spermine | Intranasal | 0.05–10 m | In vivo (mice) | Thin film hydration | [92] |
| Influenza virus H5N3 | Mucoadhesive liposome loaded with inactivated H5N3 virus as a model antigen against avian influenza virus | Liposomes with the lipid molar ratio of 95% of phosphatidylcholine:cholesterol of 4:1 xanthan gum as the bioadhesive polysaccharide | Intranasal | 1953.6 nm | In vivo/in vitro | Thin film hydration | [93] |
| Influenza virus H1N1 | Liposome coloaded with conserved B and T cell epitope peptides with monosodium urate crystals as an adjuvant | Soya lecithin, cholesterol, and alpha tocopherol | Intranasal | 134 nm | In Vivo (pigs) | Thin film hydration | [94] |
| Influenza | Influenza antigens codelivered sublingually with engineered liposomes carrying the synthetic toll-like receptor 4 agonist | Pluronics or DSPE–polyethylene glycol conjugates in the presence of methylglycol chitosan as a mucoadhesive agent | Sublingual | 96–173 nm | In vivo (mice) | Thin film hydration | [95] |
| Influenza | Negatively charged liposomes encapsulating 2,3-cyclic guanosine monophosphate–adenosine monophosphate | DPPC/DPPG/DPPE-PEG/Chol at 10:1:1:1 | Intranasal | 200 nm | In vivo (mice) | Reverse-phase evaporation | [96] |
| Influenza | Cationic liposomal DNA vaccine containing the M1-encoding plasmid of influenza A | – | Oral | – | In vivo (mice) | – | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, M.A.; Essa, E.A.; Elebyary, T.T.; Faheem, A.M.; Elkordy, A.A. Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines. Pharmaceuticals 2021, 14, 1173. https://doi.org/10.3390/ph14111173
Attia MA, Essa EA, Elebyary TT, Faheem AM, Elkordy AA. Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines. Pharmaceuticals. 2021; 14(11):1173. https://doi.org/10.3390/ph14111173
Chicago/Turabian StyleAttia, Mohamed Ahmed, Ebtessam Ahmed Essa, Toka Tarek Elebyary, Ahmed Mostafa Faheem, and Amal Ali Elkordy. 2021. "Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines" Pharmaceuticals 14, no. 11: 1173. https://doi.org/10.3390/ph14111173
APA StyleAttia, M. A., Essa, E. A., Elebyary, T. T., Faheem, A. M., & Elkordy, A. A. (2021). Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines. Pharmaceuticals, 14(11), 1173. https://doi.org/10.3390/ph14111173








