Effect of Polymers and Storage Relative Humidity on Amorphous Rebamipide and Its Solid Dispersion Transformation: Multiple Spectra Chemometrics of Powder X-Ray Diffraction and Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
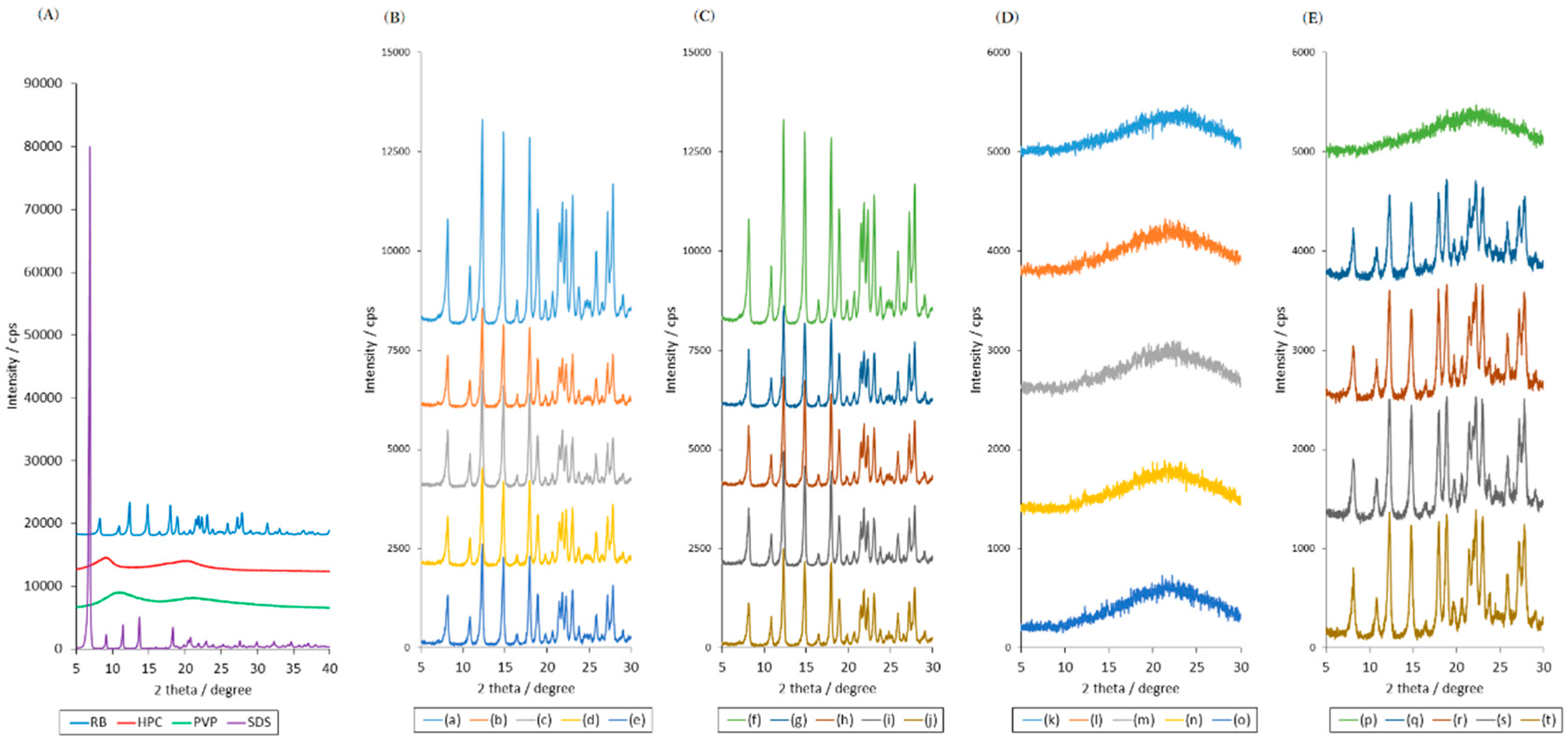
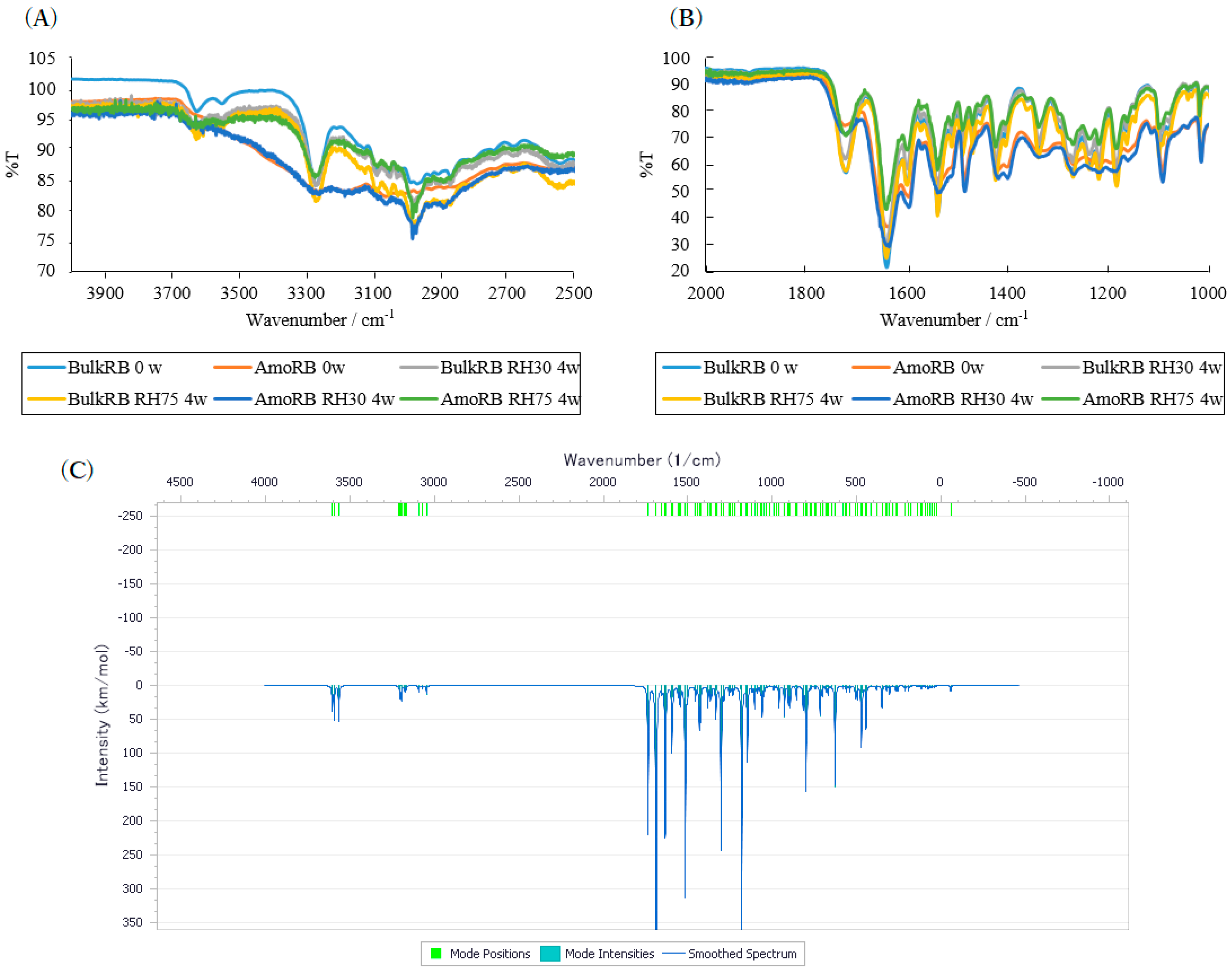
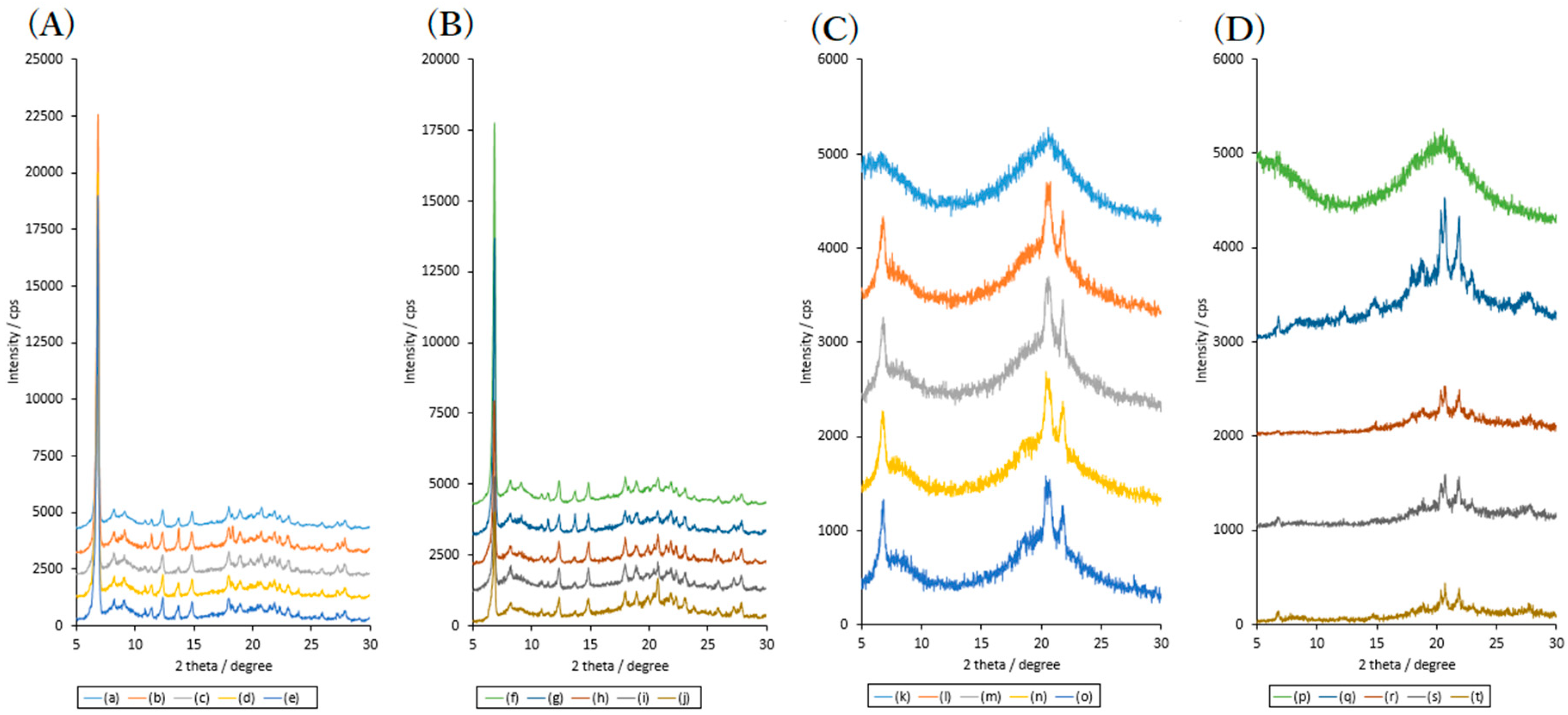
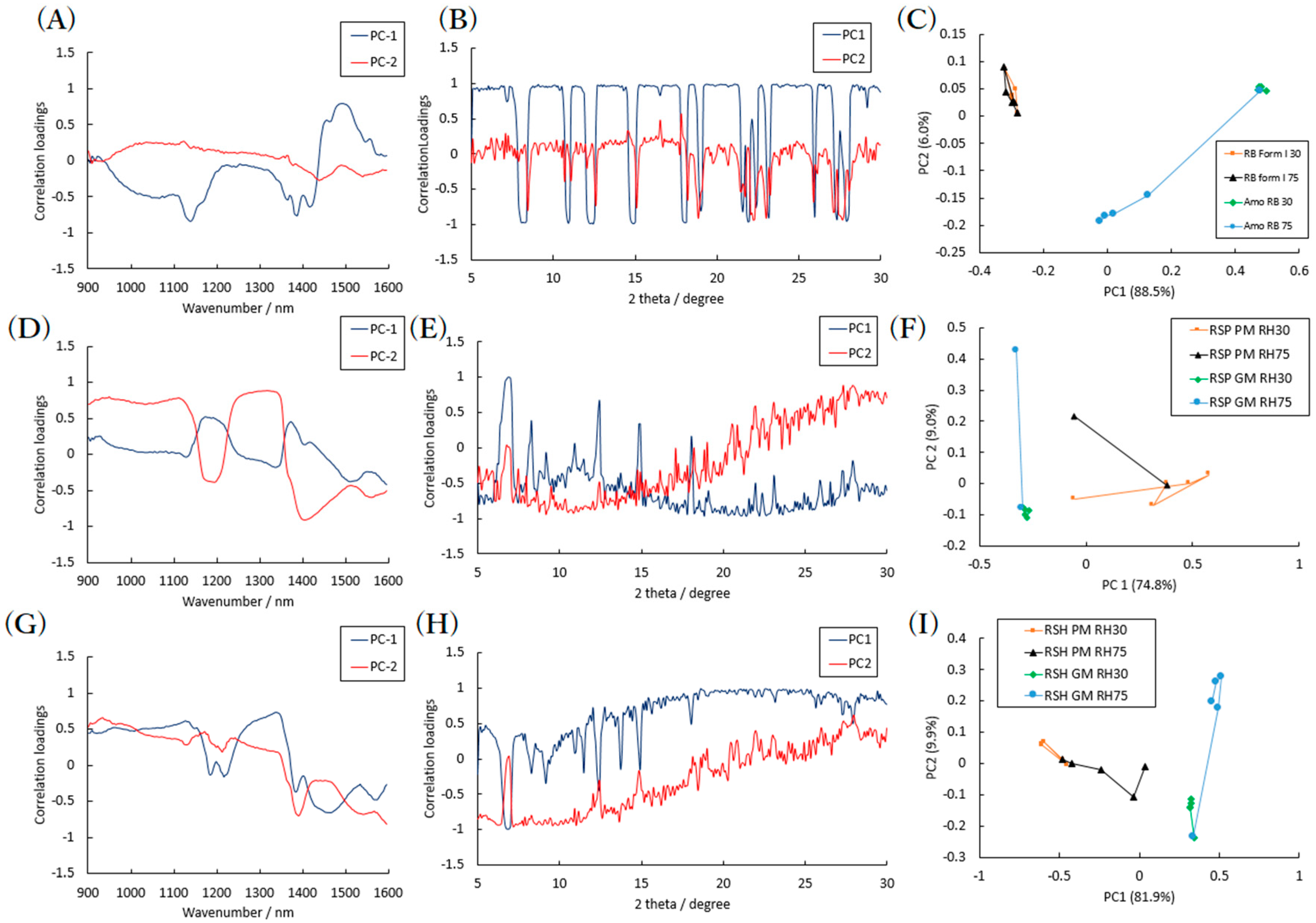
2.1. Effects of High Humidity Storage on Amorphous RB and Bulk RB Samples
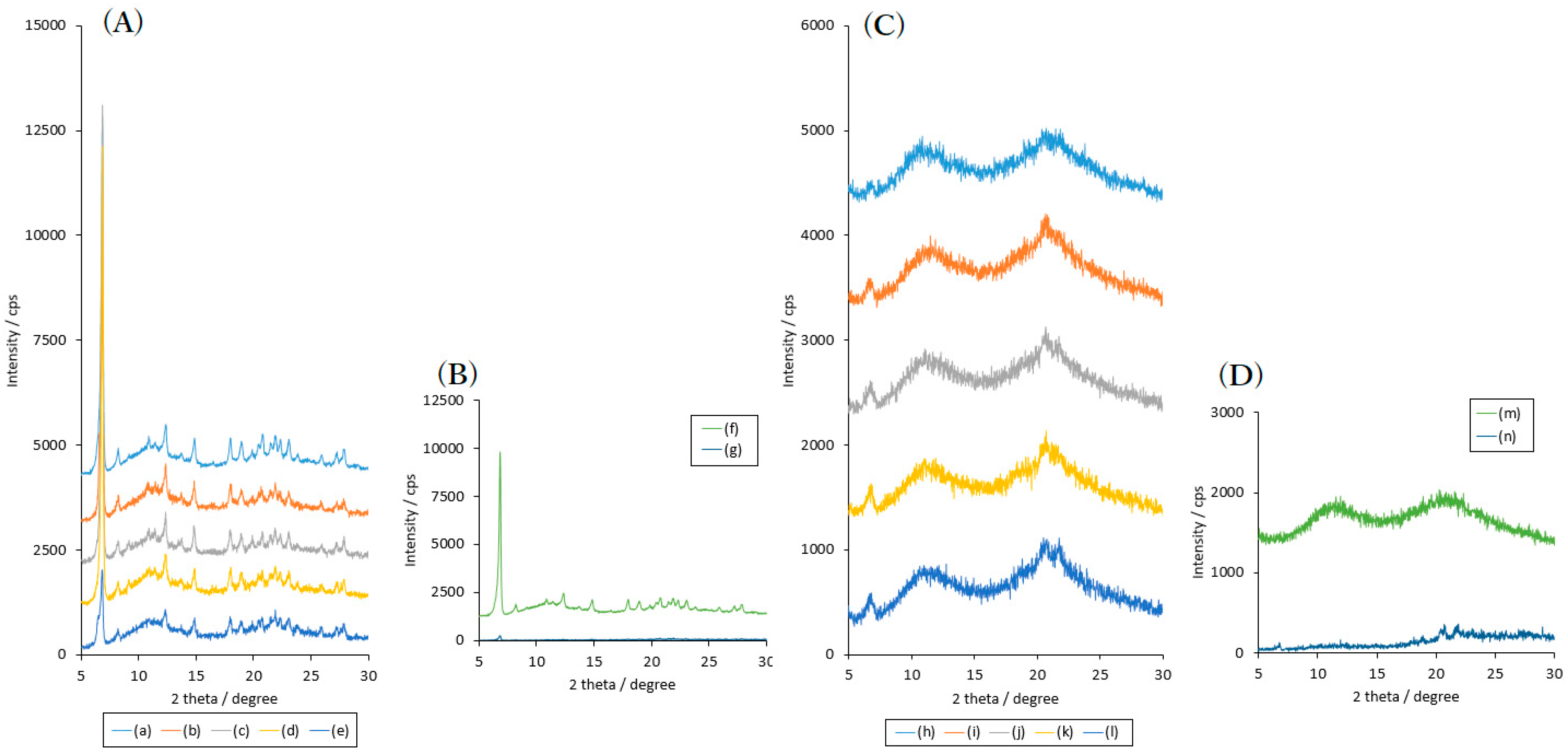
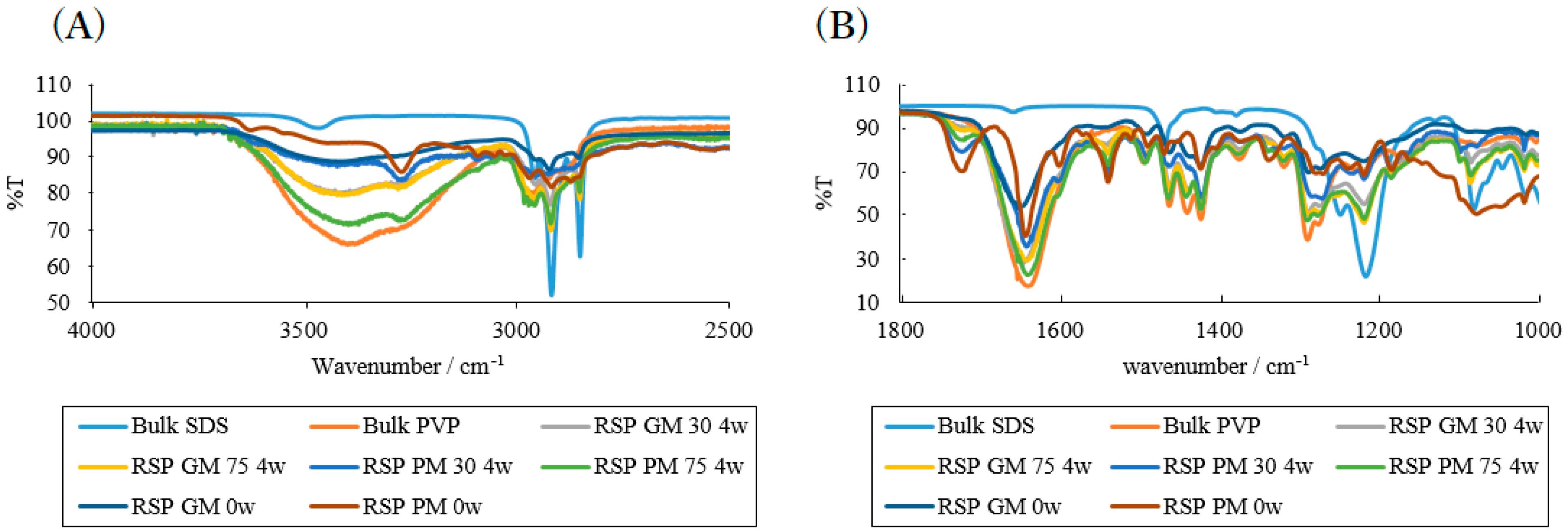
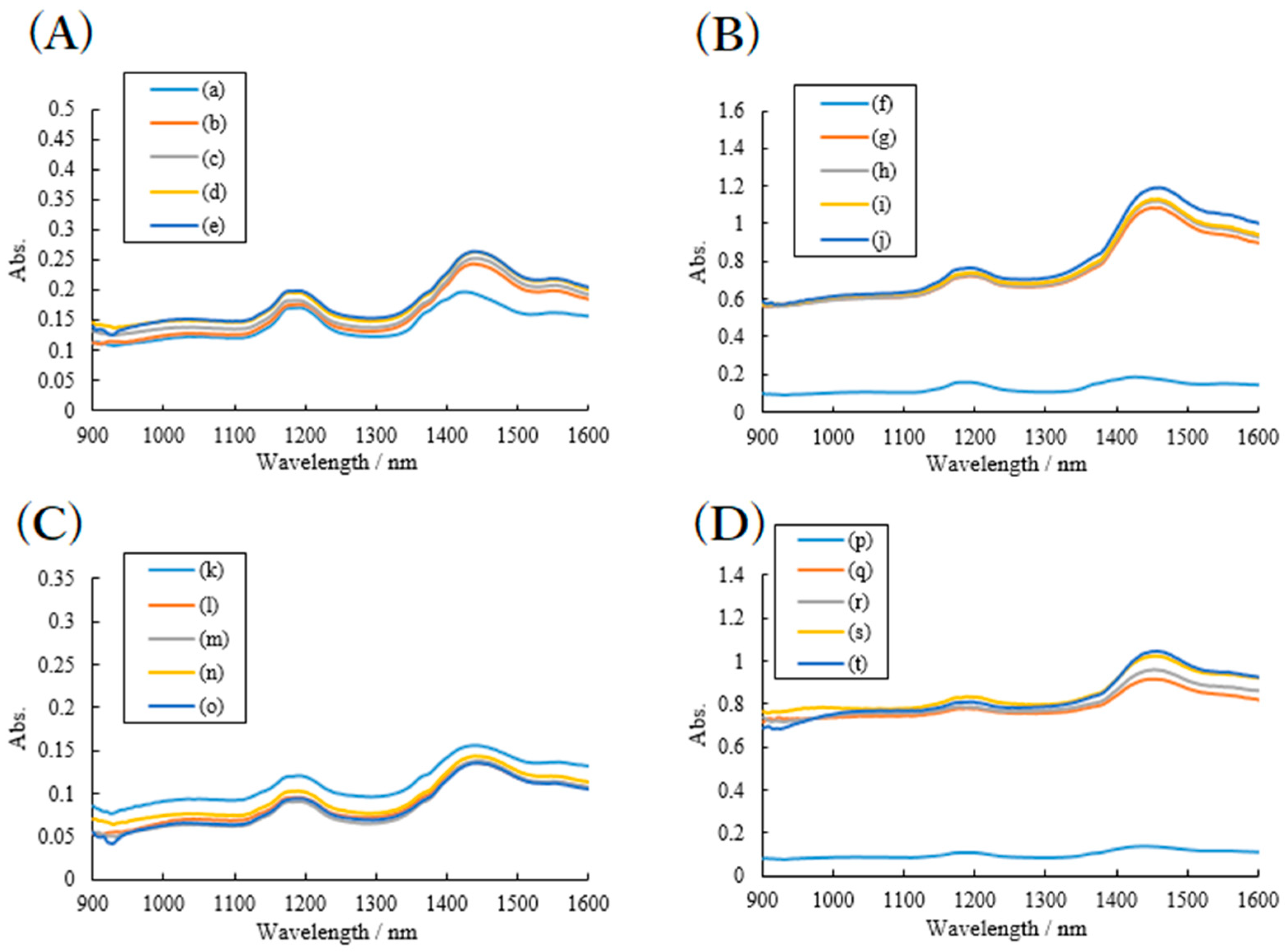
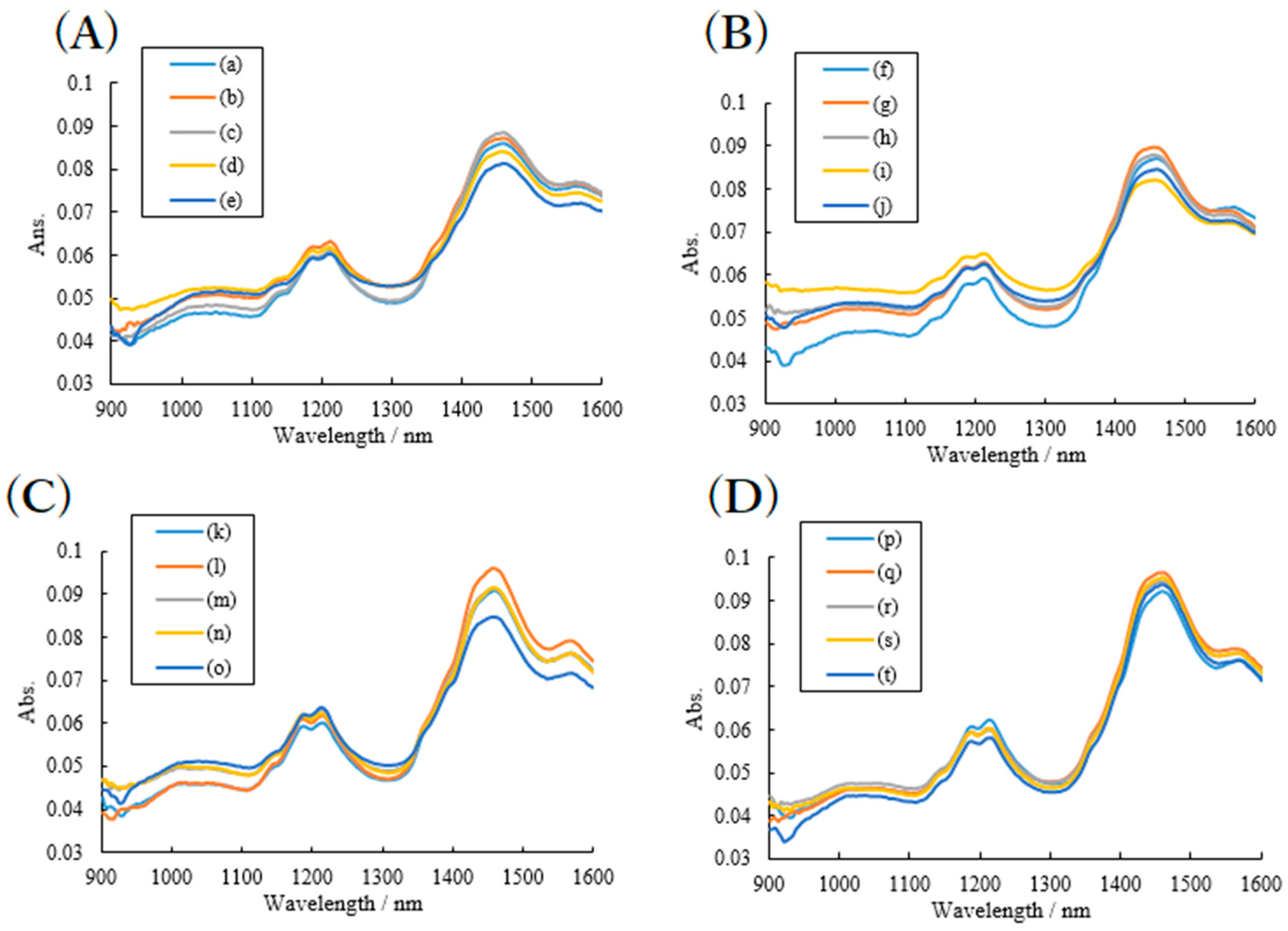
2.2. Effects of High Humidity Storage on the Mixture of RB Sample, SDS and PVP
2.3. Effects of High Humidity Storage on RB Mixture Sample with SDS and HPC
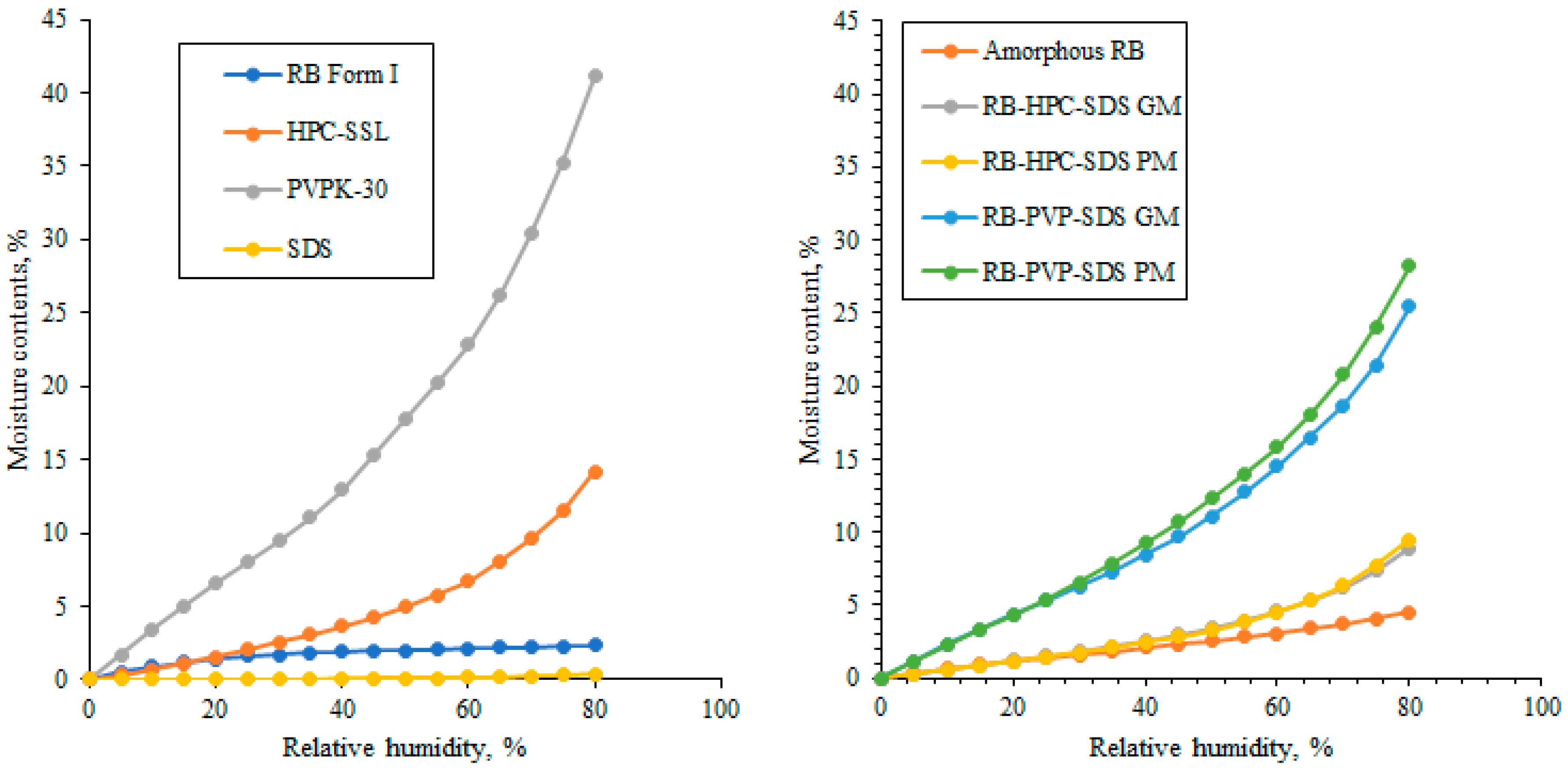
2.4. Dynamic Vapor Sorption (DVS) of Solid Dispersion Samples
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Sample Preparations
3.2.2. PXRD
3.2.3. NIR Spectroscopy
3.2.4. ATR-MIR Spectroscopy
3.2.5. Simulated Vibrational Frequencies
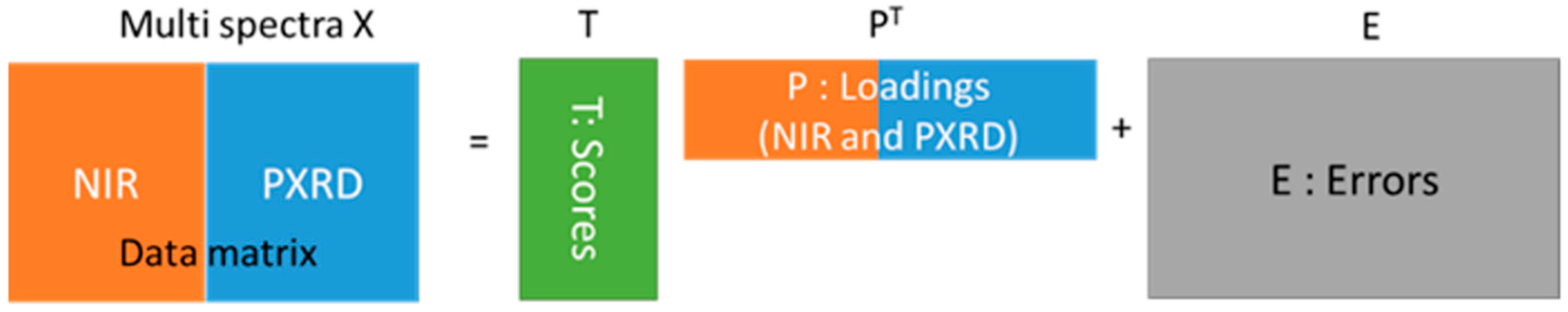
3.2.6. PCA for Multiple Datasets
3.2.7. Software
3.2.8. DVS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamasaki, K.; Kanbe, T.; Chijiwa, T.; Ishiyama, H.; Morita, S. Gastric mucosal protection by OPC-12759, a novel anti-ulcer compound, in the rat. Eur. J. Pharmacol. 1987, 142, 23–29. [Google Scholar] [CrossRef]
- Kleine, A.; Kluge, S.; Peskar, B.M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Digest. Dis. Sci. 1993, 38, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, H.; Ishihara, S.; Moriyama, N.; Kazumori, H.; Adachi, K.; Fukuda, R.; Watanabe, M.; Kinoshita, Y. Effect of rebamipide on prostaglandin EP4 receptor gene expression in rat gastric mucosa. J. Lab. Clin. Med. 2000, 136, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Sasabe, H.; Koga, T.; Konishi, T. Mechanism of hydroxyl radical scavenging by rebamipide: Identification of mono-hydroxylated rebamipide as a major reaction product. Free Radic. Res. 2004, 38, 487–494. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Tanigawa, T.; Sakurai, K.; Yamasaki, K.; Uchida, M.; Kondo, M. Hydroxyl radical scavenging by rebamipide and related compounds: Electron paramagnetic resonance study. Free Radic. Biol. Med. 1995, 18, 117–123. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Tanigawa, T.; Kondo, M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneim.-Forsch. 1993, 43, 363–366. [Google Scholar]
- Aihara, M.; Imagawa, K.; Funakoshi, Y.; Ohmoto, Y.; Kikuchi, M. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Digest. Dis. Sci. 1998, 43, 160S–166S. [Google Scholar]
- Kim, H.; Seo, J.Y.; Kim, K.H. Inhibition of lipid peroxidation, NF-κB activation and IL-8 production by rebamipide in Helicobacter pylori-stimulated gastric epithelial cells. Digest. Dis. Sci. 2000, 45, 621–628. [Google Scholar] [CrossRef]
- Ishihara, K.; Komuro, Y.; Nishiyama, N.; Yamasaki, K.; Hotta, K. Effect of rebamipide on mucus secretion by endogenous prostaglandin-independent mechanism in rat gastric mucosa. Arzneim.-Forsch. 1992, 42, 1462–1466. [Google Scholar]
- Kawata, K.; Hanawa, T.; Hanawa, K. Investigation of the effect of the rebamipide mouthwash on the crisis of the stomatitis induced by the cancer chemotherapy and/or radiotherapy. Shinyaku Rinsho 2001, 50, 273–280. [Google Scholar]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.T.; Margarit, M.V.; Salcedo, G.E. Characterization and solubility study of solid dispersions of flunarizine and polyvinylpyrrolidone. Il Farmaco 2002, 57, 723–727. [Google Scholar] [CrossRef]
- Li, Y.; Goto, S.; Shimada, Y.; Makino, K. Phase solution and solution recrystallization equilibrium constants of hydroxypropyl-β-cyclodextrin complexes with nifedipine and nicardipine hydrochloride. J. Pharm. Sci. Technol. Jpn. 2016, 76, 267–273. [Google Scholar] [CrossRef]
- García-Rodriguez, J.J.; de la Torre-Iglesias, P.M.; Vegas-Sánchez, M.C.; Torrado-Durán, S.; Bolás-Fernández, F.; Torrado-Santiago, S. Changed crystallinity of mebendazole solid dispersion: Improved anthelmintic activity. Int. J. Pharm. 2011, 403, 23–28. [Google Scholar] [CrossRef]
- Pongpeerapat, A.; Higashi, K.; Tozuka, Y.; Moribe, K.; Yamamoto, K. Molecular interaction among Probucol/PVP/SDS multicomponent system investigated by solid-state NMR. Pharm. Res. 2006, 23, 2566–2574. [Google Scholar] [CrossRef]
- Laske, S.; Paudel, A.; Scheibelhofer, O. A review of pat strategies in secondary solid oral dosage manufacturing of small molecules. J. Pharm. Sci. 2017, 106, 667–712. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; FDA-2003-D-0032; FDA: Washington, DC, USA, 2004.
- Luypaert, J.; Massart, D.L.; Heyden, Y.V. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta 2007, 72, 865–883. [Google Scholar] [CrossRef]
- Beer, T.R.M.D.; Bodson, C.; Dejaegher, B.; Walczak, B.; Vercruysse, P.; Burggraeve, A.; Lemos, A.; Delattre, L.; Vander Heyden, Y.; Remon, J.P.; et al. Raman spectroscopy as a process analytical technology (PAT) tool for the inline monitoring and understanding of a powder blending process. J. Pharm. Biomed. Anal. 2008, 48, 772–779. [Google Scholar] [CrossRef]
- Wu, H.; Heilweil, E.J.; Hussain, A.S.; Khan, M.A. Process analytical technology (pat): Quantification approaches in terahertz spectroscopy for pharmaceutical application**The views and opinions expressed in this article are only of the authors, and do not necessarily reflect the views or policies of the FDA. J. Pharm. Sci. 2008, 97, 970–984. [Google Scholar] [CrossRef]
- Pomerantsev, A.L.; Rodionova, O.Y. Process analytical technology: A critical view of the chemometricians. J. Chemom. 2012, 26, 299–310. [Google Scholar] [CrossRef]
- Blanco, M.; Coello, J.; Iturriaga, H.; Maspoch, S. Near-infrared spectroscopy in the pharmaceutical industry. Analyst 1998, 123, 16. [Google Scholar] [CrossRef]
- Yan, H.; Siesler, H.W. Quantitative analysis of a pharmaceutical formulation: Performance comparison of different handheld near-infrared spectrometers. J. Pharm. Biomed. Anal. 2018, 160, 179–186. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.H.; da Silva, J.J.; Pereira, C.F. Portable near-infrared instruments: Application for quality control of polymorphs in pharmaceutical raw materials and calibration transfer. J. Pharm. Biomed. Anal. 2017, 134, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. TrAC Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Norris, T.; Aldridge, P.K.; Sonja Sekulic, S. Determination of end-points for polymorph conversions of crystalline organic compounds using online near-infrared spectroscopy. Analyst 1997, 122, 549–552. [Google Scholar] [CrossRef]
- Blanco, M.; Alcalá, M. Simultaneous quantitation of five active principles in a pharmaceutical preparation: Development and validation of a near infrared spectroscopic method. Eur. J. Pharm. Sci. 2006, 27, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Donoso, M.; Ghaly, E.S. Use of near-infrared for quantitative measurement of viscosity and concentration of active ingredient in pharmaceutical gel. Pharm. Dev. Technol. 2006, 11, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Alcalà, M.; Blanco, M.; Bautista, M.; González, J.M. On-line monitoring of a granulation process by NIR spectroscopy. J. Pharm. Sci. 2010, 99, 336–345. [Google Scholar] [CrossRef]
- Zhou, G.X.; Ge, Z.; Dorwart, J.; Izzo, B.; Kukura, J.; Bicker, G.; Wyvratt, J. Determination and differentiation of surface and bound water in drug substances by near infrared spectroscopy. J. Pharm. Sci. 2003, 92, 1058–1065. [Google Scholar] [CrossRef]
- Wahl, P.R.; Fruhmann, G.; Sacher, S.; Straka, G.; Sowinski, S.; Khinast, J.G. PAT for tableting: Inline monitoring of API and excipients via NIR spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; He, H.; Zhang, J.; Martin, S.; Chen, R.; Repka, M.A. Application of ft-nir analysis for inline and real-time monitoring of pharmaceutical hot melt extrusion: A technical note. AAPS PharmSciTech 2018, 19, 3425–3429. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Takeuchi, M.; Otsuka, M.; Ben-Nissan, B.; Grossin, D.; Tanaka, H. Effect of carbon dioxide on self-setting apatite cement formation from tetracalcium phosphate and dicalcium phosphate dihydrate; ATR-IR and chemoinformatics analysis. Colloid Polym. Sci. 2015, 293, 2781–2788. [Google Scholar] [CrossRef]
- Lambert, D.; Muehlethaler, C.; Esseiva, P.; Massonnet, G. Combining spectroscopic data in the forensic analysis of paint: Application of a multiblock technique as chemometric tool. Forensic Sci. Int. 2016, 263, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, V.; Massart, D.L. Combining spectroscopic data (MS, IR): Exploratory chemometric analysis for characterizing similarity/diversity of chemical structures. J. Pharm. Biomed. Anal. 2001, 26, 225–239. [Google Scholar] [CrossRef]
- Alamprese, C.; Casale, M.; Sinelli, N.; Lanteri, S.; Casiraghi, E. Detection of minced beef adulteration with turkey meat by UV–vis, NIR and MIR spectroscopy. LWT Food. Sci. Technol. 2013, 53, 225–232. [Google Scholar] [CrossRef]
- Groot, P.J.D.; Swierenga, H.; Postma, G.J.; Melssen, W.J.; Buydens, L.M.C. Effect on the partial least-squares prediction of yarn properties combining raman and infrared measurements and applying wavelength selection. Appl. Spectrosc. 2003, 57, 642–648. [Google Scholar] [CrossRef]
- Kawano, Y.; Ishii, N.; Shimizu, Y.; Hanawa, T. Development and characterization of a suspension containing nanoparticulated rebamipide for a mouth wash for stomatitis. J. Pharm. Sci. Technol. Jpn. 2017, 77, 104–115. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Matsumura, S.; Takeuchi, M.; Tanaka, H. Effect of hydroxypropyl cellulose and hydroxypropyl methylcellulose on carbamazepine polymorphic transformation; attenuated total reflectance-infrared spectroscopy and chemoinformatics analysis. Colloid Polym. Sci. 2015, 293, 3471–3478. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Pal, S.; Mitome, H.; Takeuchi, M.; Tanaka, H. Prediction of differential scanning calorimetry curve of theophylline direct compression model tablet using Raman spectra. J. Drug Deliv. Sci. Technol. 2019, 49, 254–259. [Google Scholar] [CrossRef]
- Xiong, X.; Du, Q.; Zeng, X.; He, J.; Yang, H.; Li, H. Solvates and polymorphs of rebamipide: Preparation, characterization, and physicochemical analysis. RSC Adv. 2017, 7, 23279–23286. [Google Scholar] [CrossRef]
- Jeon, S.H.; Sohn, Y.T. The solid state of rebamipide: Preparation, characterization, and dissolution. Arch. Pharm. Res. 2016, 39, 508–515. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yasuji, T.; Yamamoto, H.; Kawashima, Y. Temperature- and moisture-induced crystallization of amorphous lactose in composite particles with sodium alginate prepared by spray-drying. Pharm. Dev. Technol. 2000, 5, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mahlin, D.; Berggren, J.; Alderborn, G.; Engström, S. Moisture-induced surface crystallization of spray-dried amorphous lactose particles studied by atomic force microscopy. J. Pharm. Sci. 2004, 93, 29–37. [Google Scholar] [CrossRef]
- Lust, A.; Strachan, C.J.; Veski, P.; Aaltonen, J.; Heinämäki, J.; Yliruusi, J.; Kogermann, K. Amorphous solid dispersions of piroxicam and Soluplus(®): Qualitative and quantitative analysis of piroxicam recrystallization during storage. Int. J. Pharm. 2015, 486, 306–314. [Google Scholar] [CrossRef] [PubMed]
- den Mooter, G.V.; Augustijns, P.; Blaton, N.; Kinget, R. Physico-chemical characterization of solid dispersions of temazepam with polyethylene glycol 6000 and PVP K30. Int. J. Pharm. 1998, 164, 67–80. [Google Scholar] [CrossRef]
- Falk, M.; Ford, T.A. Infrared spectrum and structure of liquid water. Can. J. Chem. 1966, 44, 1699–1707. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Marsac, P.J.; Stanford, L.A.; Taylor, L.S. Phase behavior of poly(vinylpyrrolidone) containing amorphous solid dispersions in the presence of moisture. Mol. Pharm. 2009, 6, 1492–1505. [Google Scholar] [CrossRef]
- Crowley, K.J.; Zografi, G. Water vapor absorption into amorphous hydrophobic drug/poly(vinylpyrrolidone) dispersions. J. Pharm. Sci. 2002, 91, 2150–2165. [Google Scholar] [CrossRef]
- Nair, R.; Nyamweya, N.; Gönen, S.; Martínez-Miranda, L.J.; Hoag, S.W. Influence of various drugs on the glass transition temperature of poly(vinylpyrrolidone): A thermodynamic and spectroscopic investigation. Int. J. Pharm. 2001, 225, 83–96. [Google Scholar] [CrossRef]
- Kreft, K.; Kozamernik, B.; Urleb, U. Qualitative determination of polyvinylpyrrolidone type by near-infrared spectrometry. Int. J. Pharm. 1999, 177, 1–6. [Google Scholar] [CrossRef]
- Karavas, E.; Ktistis, G.; Xenakis, A.; Georgarakis, E. Miscibility behavior and formation mechanism of stabilized felodipine-polyvinylpyrrolidone amorphous solid dispersions. Drug Dev. Ind. Pharm. 2005, 31, 473–489. [Google Scholar] [CrossRef]
- Ghosh, I.; Snyder, J.; Vippagunta, R.; Alvine, M.; Vakil, R.; Tong, W.Q.; Vippagunta, S. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int. J. Pharm. 2011, 419, 12–19. [Google Scholar] [CrossRef]
- Feng, D.; Peng, T.; Huang, Z.; Singh, V.; Shi, Y.; Wen, T.; Lu, M.; Quan, G.; Pan, X.; Wu, C. Polymer–surfactant system based amorphous solid dispersion: Precipitation inhibition and bioavailability enhancement of itraconazole. Pharmaceutics 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Krill, S.L.; Wang, Z.; Telang, C. Miscibility/stability considerations in binary solid dispersion systems composed of functional excipients towards the design of multi-component amorphous systems. J. Pharm. Sci. 2009, 98, 4711–4723. [Google Scholar] [CrossRef]
- Roscigno, P.; Asaro, F.; Pellizer, G.; Ortona, O.; Paduano, L. Complex Formation between Poly(vinylpyrrolidone) and Sodium Decyl Sulfate Studied through NMR. Langmuir 2003, 19, 9638–9644. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Shah, B.; Kakumanu, V.K.; Bansal, A.K. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J. Pharm. Sci. 2006, 95, 1641–1665. [Google Scholar] [CrossRef] [PubMed]
- Molecular Vibrations. Available online: https://books.google.com/books/about/Molecular_Vibrations.html?hl=ja&id=yKVPDwAAQBAJ (accessed on 10 December 2019).
- Sundaraganesan, N.; Saleem, H.; Mohan, S.; Ramalingam, M.; Sethuraman, V. FTIR, FT-Raman spectra and ab initio DFT vibrational analysis of 2-bromo-4-methyl-phenylamine. Spectrochim. Acta A Mol. Biomol. Spec. 2005, 62, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Berens, P.H.; Wilson, K.R. Molecular dynamics and spectra. I. Diatomic rotation and vibration. J. Chem. Phys. 1981, 74, 4872–4882. [Google Scholar] [CrossRef]
- Wall, M.E.; Rechtsteiner, A.; Rocha, L.M. Singular Value Decomposition and Principal Component Analysis. In A Practical Approach to Microarray Data Analysis; Berrar, D.P., Dubitzky, W., Granzow, M., Eds.; Springer: New York, NY, USA, 2003; pp. 91–109. [Google Scholar] [CrossRef]
- Materials Studio Materials Modeling & Simulation Application|Dassault Systèmes BIOVIA. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-materials-studio/ (accessed on 10 December 2019).
- Camo Analytics—The Leader in Industrial Analytics. Available online: https://www.camo.com/unscrambler/ (accessed on 10 December 2019).
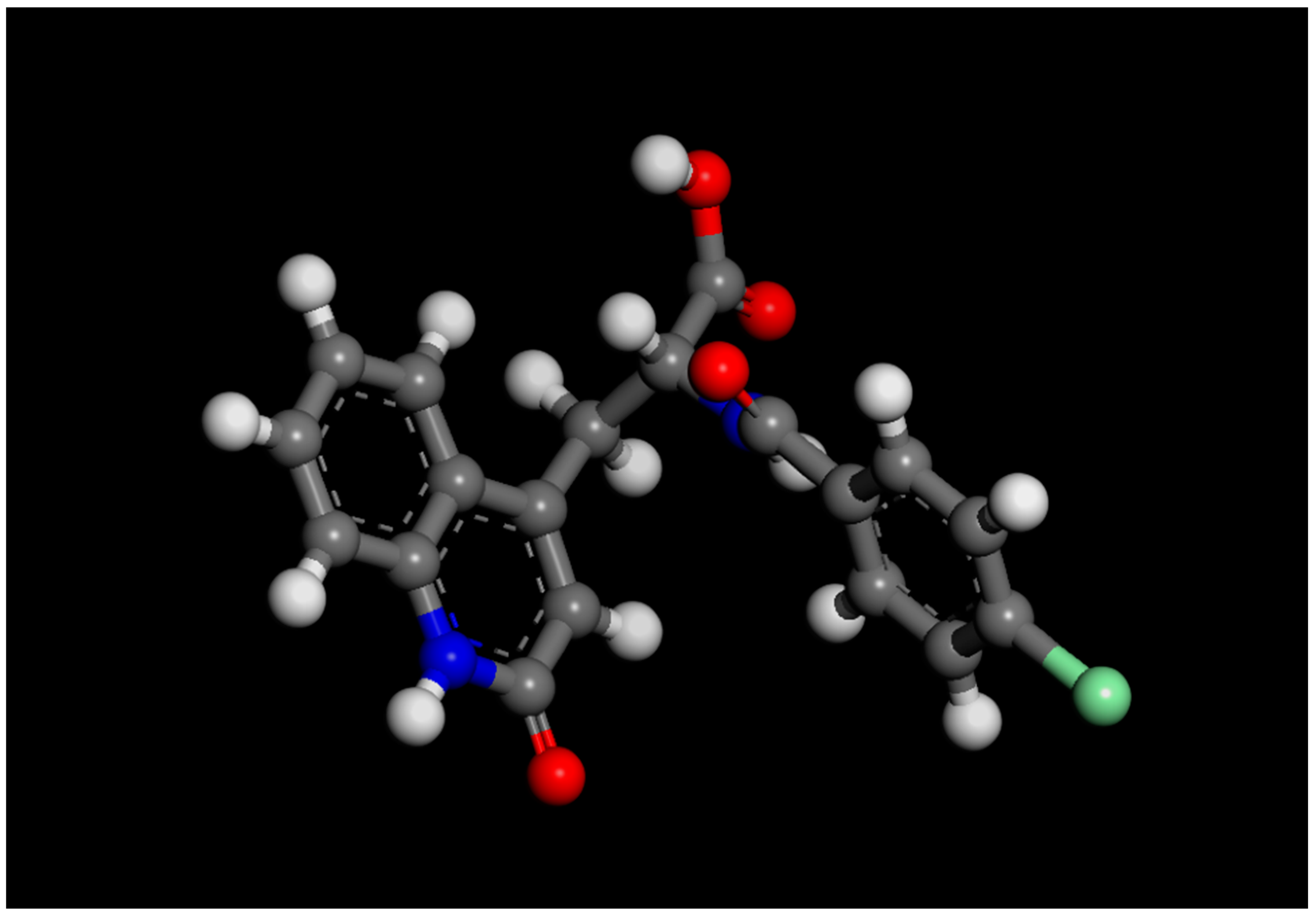


| MIR Spectra R2 Values | RB Form I | Amorphous RB | ||
|---|---|---|---|---|
| RH 30% | RH 75% | RH 30% | RH 75% | |
| RB form I | 0.990 | 0.992 | 0.846 | 0.981 |
| Amorphous RB | 0.774 | 0.808 | 0.920 | 0.836 |
| Component | Amorphous and RB form I | RSP Samples | RSH Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | Cal. | Val. | Dif. | Cal. | Val. | Dif. | Cal. | Val. | Dif. |
| PC1 | 88.8 | 87.8 | 87.8 | 77.86 | 74.78 | 74.78 | 83.9 | 81.9 | 81.9 |
| PC2 | 95.2 | 93.8 | 6.0 | 92.11 | 83.76 | 8.98 | 93.6 | 91.8 | 9.9 |
| PC3 | 96.3 | 94.4 | 0.6 | 94.95 | 83.87 | 0.11 | 95.9 | 93.7 | 1.9 |
| PC4 | 96.9 | 94.8 | 0.5 | 96.71 | 85.40 | 1.53 | 96.9 | 94.2 | 0.5 |
| PC5 | 97.3 | 94.9 | 0.02 | 97.66 | 86.17 | 0.77 | 97.6 | 94.6 | 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, Y.; Utsunomiya, Y.; Umeda, D.; Yonemochi, E.; Kawano, Y.; Hanawa, T. Effect of Polymers and Storage Relative Humidity on Amorphous Rebamipide and Its Solid Dispersion Transformation: Multiple Spectra Chemometrics of Powder X-Ray Diffraction and Near-Infrared Spectroscopy. Pharmaceuticals 2020, 13, 147. https://doi.org/10.3390/ph13070147
Otsuka Y, Utsunomiya Y, Umeda D, Yonemochi E, Kawano Y, Hanawa T. Effect of Polymers and Storage Relative Humidity on Amorphous Rebamipide and Its Solid Dispersion Transformation: Multiple Spectra Chemometrics of Powder X-Ray Diffraction and Near-Infrared Spectroscopy. Pharmaceuticals. 2020; 13(7):147. https://doi.org/10.3390/ph13070147
Chicago/Turabian StyleOtsuka, Yuta, Yuiko Utsunomiya, Daiki Umeda, Etsuo Yonemochi, Yayoi Kawano, and Takehisa Hanawa. 2020. "Effect of Polymers and Storage Relative Humidity on Amorphous Rebamipide and Its Solid Dispersion Transformation: Multiple Spectra Chemometrics of Powder X-Ray Diffraction and Near-Infrared Spectroscopy" Pharmaceuticals 13, no. 7: 147. https://doi.org/10.3390/ph13070147
APA StyleOtsuka, Y., Utsunomiya, Y., Umeda, D., Yonemochi, E., Kawano, Y., & Hanawa, T. (2020). Effect of Polymers and Storage Relative Humidity on Amorphous Rebamipide and Its Solid Dispersion Transformation: Multiple Spectra Chemometrics of Powder X-Ray Diffraction and Near-Infrared Spectroscopy. Pharmaceuticals, 13(7), 147. https://doi.org/10.3390/ph13070147








