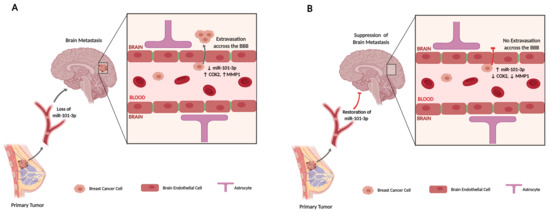
Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling
Abstract
1. Introduction
2. Results
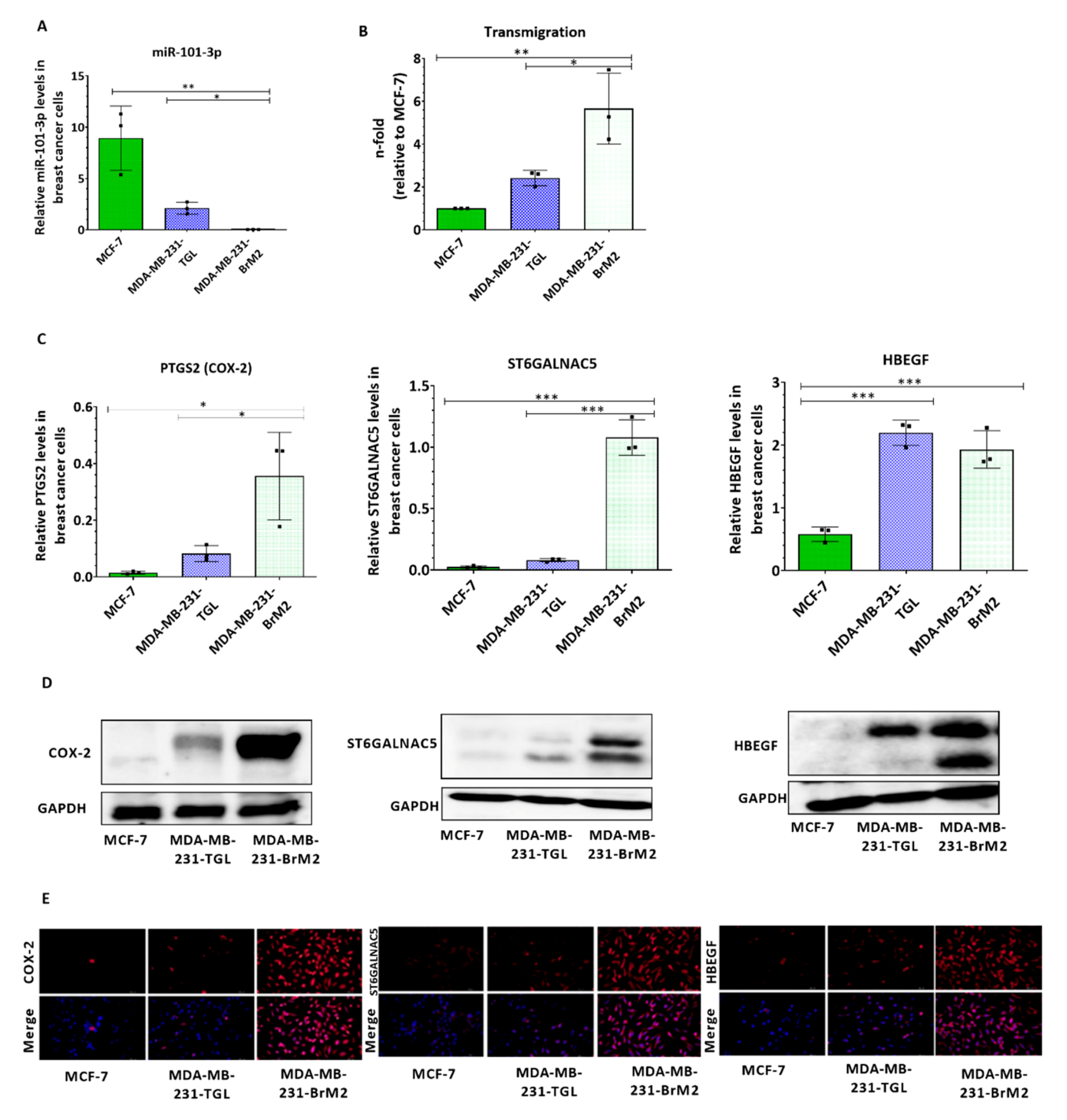
2.1. The Expression of mir-101-3p is Attenuated in Brain Metastatic Breast Cancer Cells
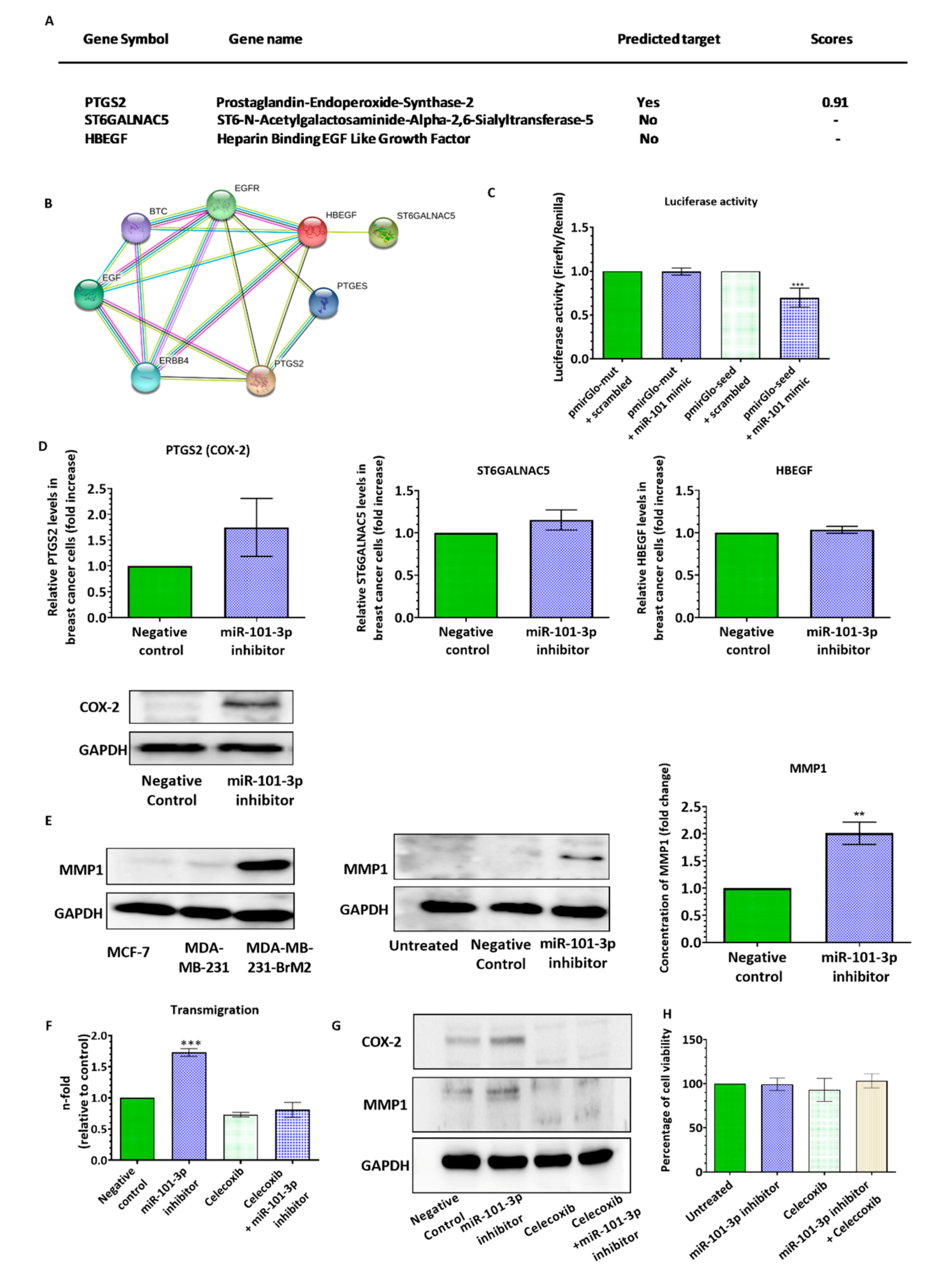
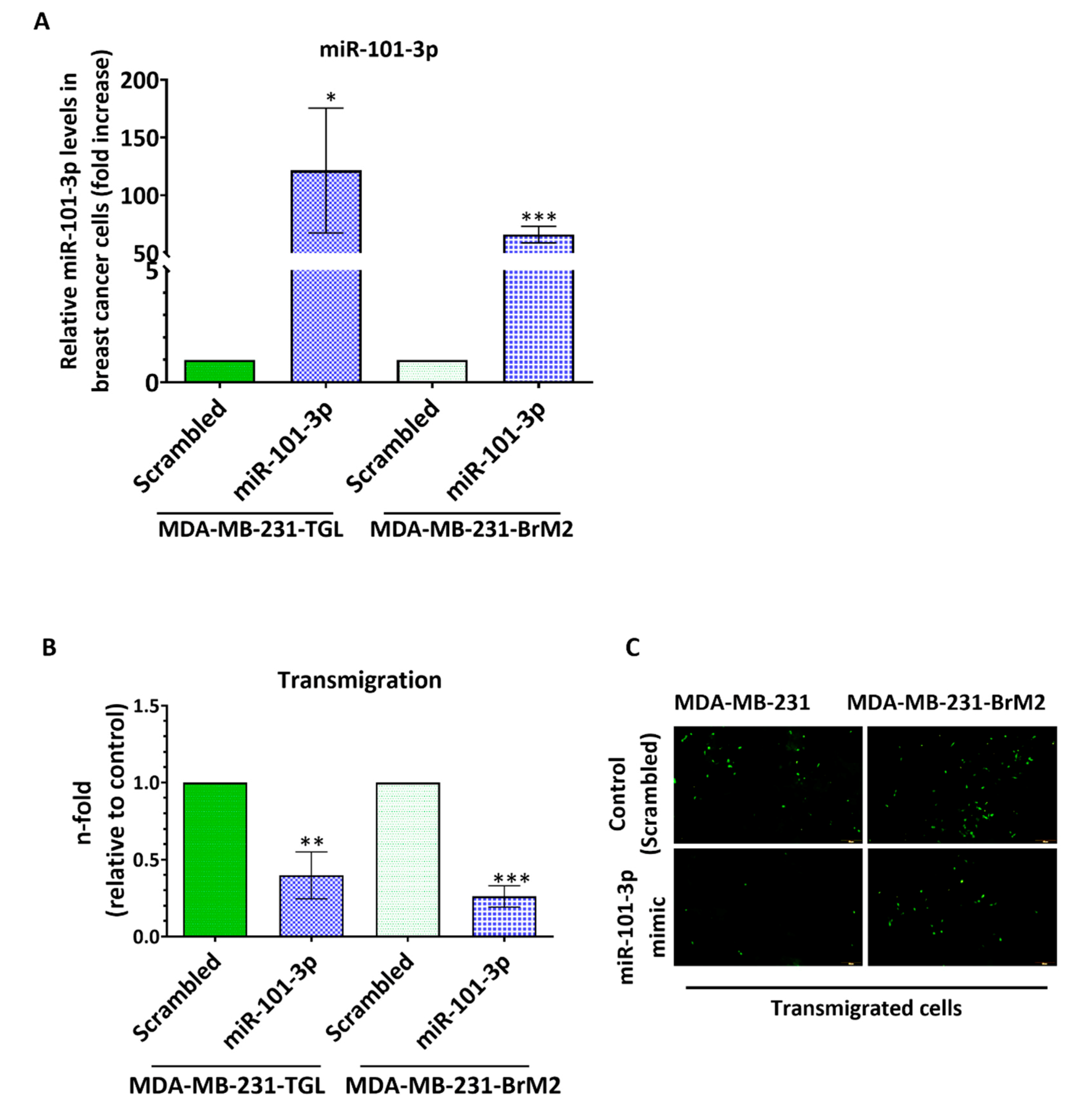
2.2. Loss of miR-101-3p Promotes Trans-Endothelial Cell Migration by Upregulating COX-2/MMP-1 Signaling Pathway and Reducing Expression of the Inter-Endothelial Junctions
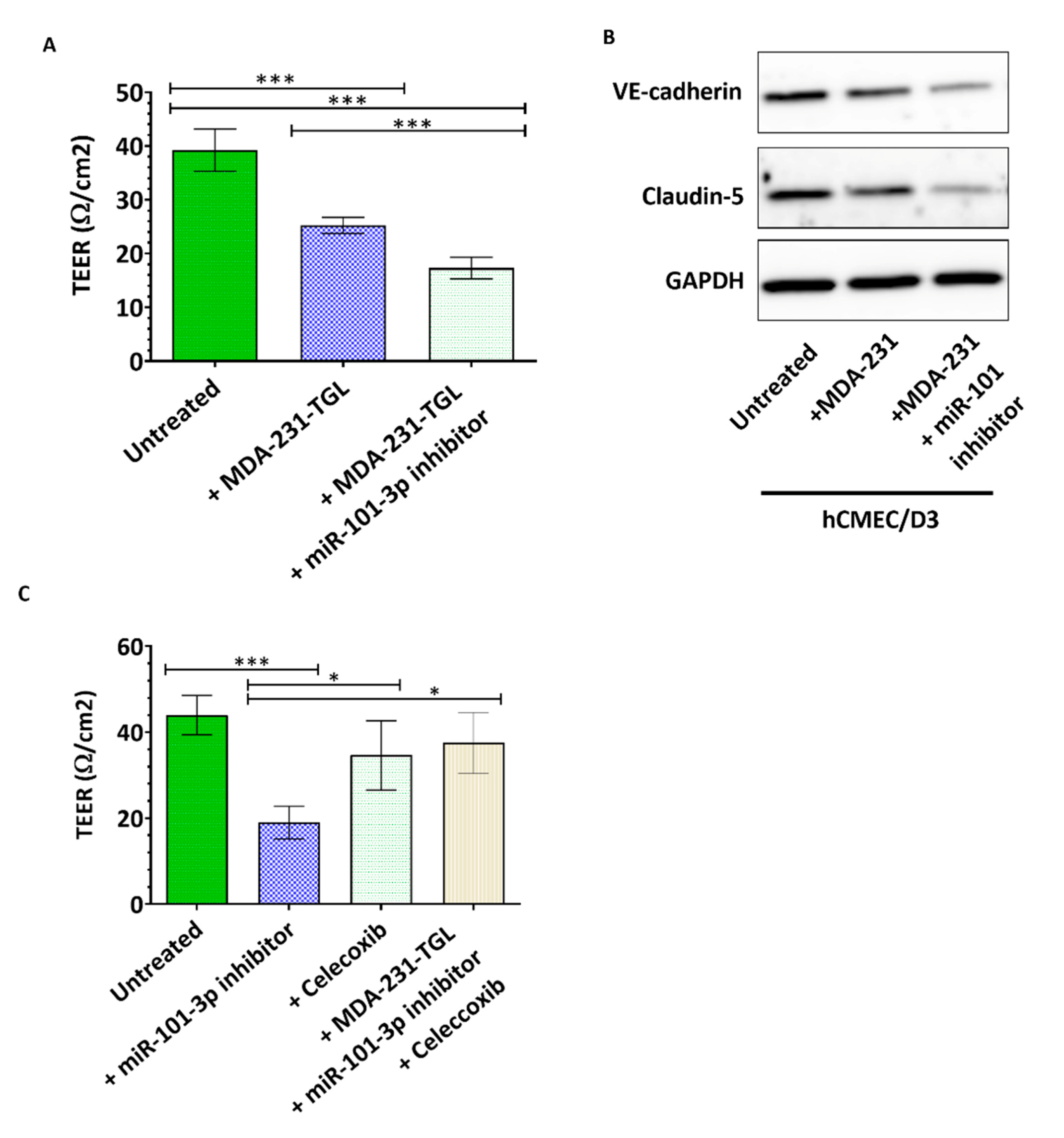
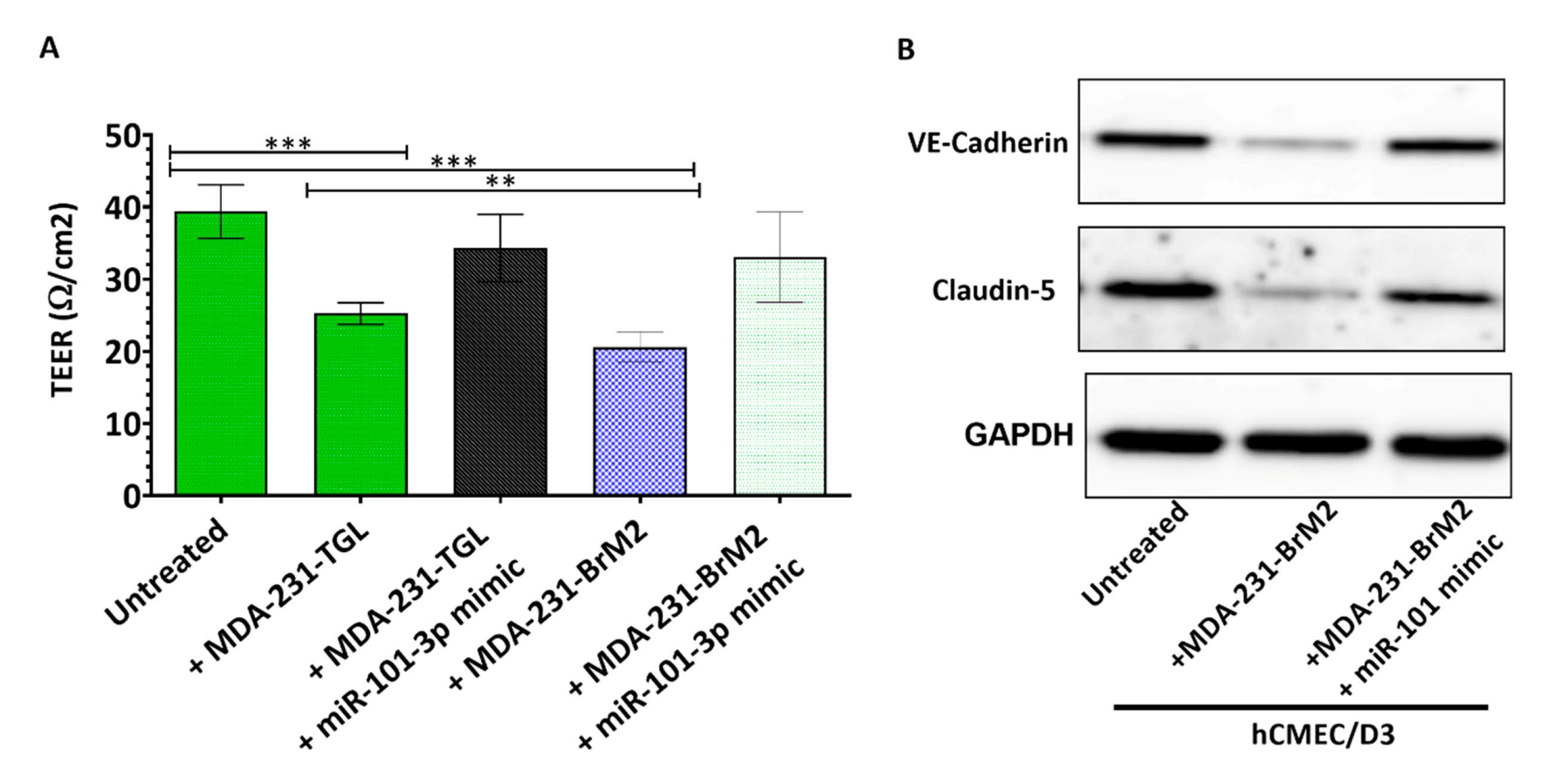
2.3. miR-101-3p Reduces Trans-Endothelial Cell Migration by Reducing COX-2/MMP-1 Expression and Protecting the Endthelial Barrier
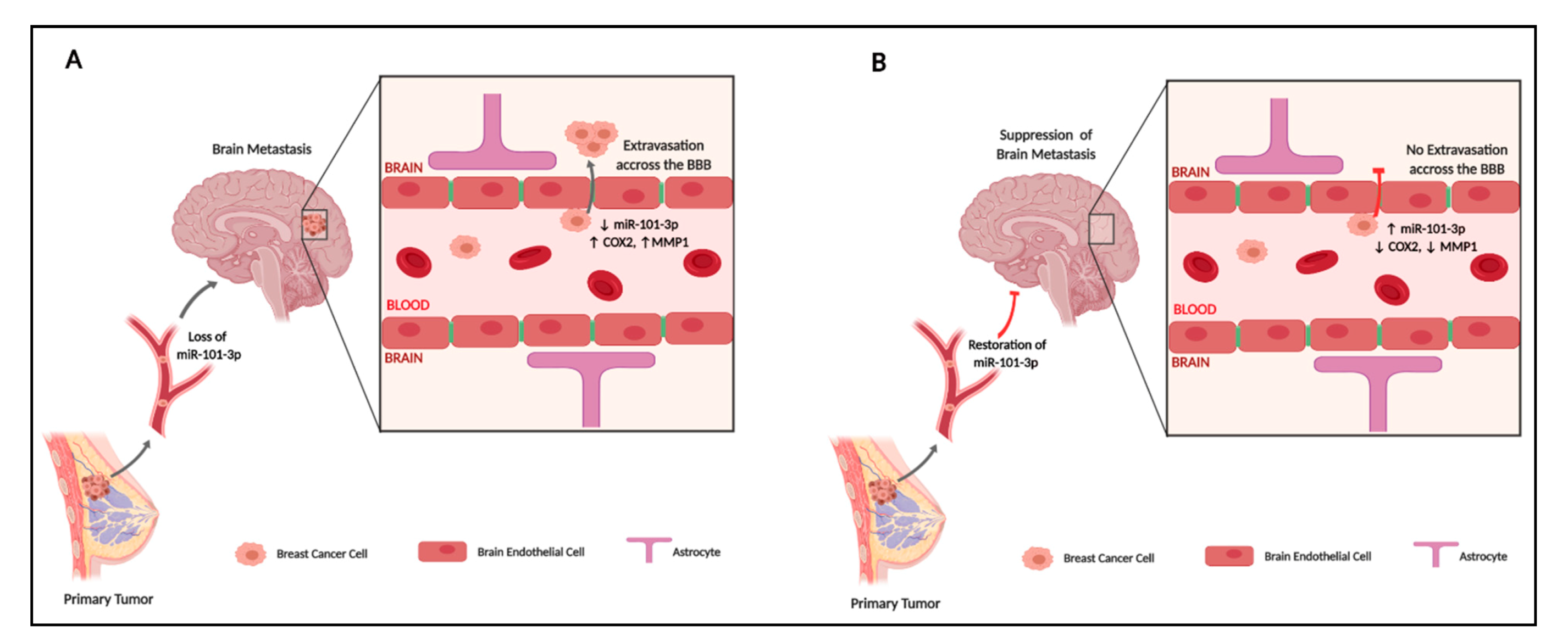
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
4.2. Establishment of the In Vitro Brain Endothelial Cells (BEC) Monolayer
4.3. Transendothelial Electrical Resistance (TEER) Measurement
4.4. In Silico Bioinformatics Analysis
4.5. MicroRNA Transfection
4.6. Pharmacological Treatment and Cell Viability Assay
4.7. Real-Time PCR
4.8. Cancer Cells Trans-Endothelial Migration Assay
4.9. Western Blot
4.10. Immunofluorescence
4.11. Luciferase Assay
4.12. Enzyme-Linked Immunosorbant Assay (ELISA)
4.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kodack, D.P.; Askoxylakis, V.; Ferraro, G.B.; Fukumura, D.; Jain, R.K. Emerging strategies for treating brain metastases from breast cancer. Cancer Cell 2015, 27, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Niewöhner, J.; Tiefenthaler, G. The Blood-Brain Barrier Challenge for the Treatment of Brain Cancer, Secondary Brain Metastases, and Neurological Diseases. Cancer Genom. Proteom. 2015, 12, 167–177. [Google Scholar]
- Pedrosa, R.M.; Mustafa, D.A.; Soffietti, R.; Kros, J.M. Breast cancer brain metastasis: Molecular mechanisms and directions for treatment. Neuro Oncol. 2018, 20, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Aversa, C.; Rossi, V.; Geuna, E.; Martinello, R.; Milani, A.; Redana, S.; Valabrega, G.; Aglietta, M.; Montemurro, F. Metastatic breast cancer subtypes and central nervous system metastases. Breast 2014, 23, 623–628. [Google Scholar] [CrossRef]
- Anderson, R.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2019, 16, 185–204. [Google Scholar] [CrossRef]
- Valiente, M.; Ahluwalia, M.S.; Boire, A.; Brastianos, P.K.; Goldberg, S.B.; Lee, E.Q.; Le Rhun, E.; Preusser, M.; Winkler, F.; Soffietti, R. The Evolving Landscape of Brain Metastasis. Trends Cancer 2018, 4, 176–196. [Google Scholar] [CrossRef]
- Ni, W.; Chen, W.; Lu, Y. Emerging findings into molecular mechanism of brain metastasis. Cancer Med. 2018, 7, 3820–3833. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Custódio-Santos, T.; Videira, M.A.; Brito, M.A. Brain metastasization of breast cancer. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1868, 132–147. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rüger, R. Dissection of the Process of Brain Metastasis Reveals Targets and Mechanisms for Molecular-based Intervention. Cancer Genom. Proteom. 2016, 13, 14. [Google Scholar]
- Paolillo, M.; Schinelli, S. Brain infiltration by cancer cells: Different roads to the same target? J. Cancer Metastasis Treat. 2015. [Google Scholar] [CrossRef][Green Version]
- Eichler, A.F.; Chung, E.; Kodack, D.P.; Loeffler, J.S.; Fukumura, D.; Jain, R.K. The biology of brain metastases—Translation to new therapies. Nat. Rev. Clin. Oncol. 2011, 8, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Brain Metastasis of Breast Cancer: Crossing the Blood-brain Barrier. J. Appl. Clin. Pathol. 2017, 1, 3. [Google Scholar] [CrossRef][Green Version]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I.A. Role of the Blood-Brain Barrier in the Formation of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Boil. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.-F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Watabe, K. Non-coding RNAs in cancer brain metastasis. Front. Biosci. 2016, 8, 187–202. [Google Scholar] [CrossRef]
- Alsidawi, S.; Malek, E.; Driscoll, J.J. MicroRNAs in Brain Metastases: Potential Role as Diagnostics and Therapeutics. Int. J. Mol. Sci. 2014, 15, 10508–10526. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J. MicroRNA-mediated breast cancer metastasis: From primary site to distant organs. Oncogene 2011, 31, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, N.; Aharonov, R.; Meiri, E.; Rosenwald, S.; Spector, Y.; Zepeniuk, M.; Benjamin, H.; Shabes, N.; Tabak, S.; Levy, A.; et al. MicroRNAs accurately identify cancer tissue origin. Nat. Biotechnol. 2008, 26, 462–469. [Google Scholar] [CrossRef]
- Li, C.-Y.; Xiong, D.-D.; Huang, C.-Q.; He, R.-Q.; Liang, H.-W.; Pan, D.-H.; Wang, H.-L.; Wang, Y.-W.; Zhu, H.-W.; Chen, G. Clinical Value of miR-101-3p and Biological Analysis of its Prospective Targets in Breast Cancer: A Study Based on The Cancer Genome Atlas (TCGA) and Bioinformatics. Med. Sci. Monit. 2017, 23, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Sharma, S.; Liu, Y.; Mo, Y.-Y.; Wu, K.; Zhang, Y.-Y.; Pochampally, R.; Martinez, L.A.; Lo, H.-W.; Watabe, K. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene 2015, 34, 4890–4900. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.-B.; Hao, C.J.; Cui, Y.; Han, X.-C.; Hu, Y.; Li, F.-F.; Ma, X.; Ma, X. MiR-101 Is Involved in Human Breast Carcinogenesis by Targeting Stathmin1. PLOS ONE 2012, 7, e46173. [Google Scholar] [CrossRef]
- Li, J.-T.; Jia, L.-T.; Liu, N.-N.; Zhu, X.-S.; Liu, Q.-Q.; Wang, X.-L.; Yu, F.; Liu, Y.-L.; Yang, A.; Gao, C.-F. MiRNA-101 inhibits breast cancer growth and metastasis by targeting CX chemokine receptor 7. Oncotarget 2015, 6, 30818–30830. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Li, L.; Guo, R.; Li, X.; Lu, Y.; Guan, X.; Gitau, S.C.; Wang, L.; Xu, C.; Yang, B.; et al. miR-101 Promotes Breast Cancer Cell Apoptosis by Targeting Janus Kinase 2. Cell. Physiol. Biochem. 2014, 34, 413–422. [Google Scholar] [CrossRef]
- Yoneda, T.; Williams, P.J.; Hiraga, T.; Niewolna, M.; Nishimura, R. A Bone-Seeking Clone Exhibits Different Biological Properties from the MDA-MB-231 Parental Human Breast Cancer Cells and a Brain-Seeking Clone In Vivo and In Vitro. J. Bone Miner. Res. 2001, 16, 1486–1495. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Zhou, S.-F.; He, S.-M.; Zeng, S.; Zhou, Z.-W.; He, Z.-X. Hsa-microRNA-181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: A bioinformatic and clinical study and the therapeutic implication. Drug Des. Dev. Ther. 2015, 9, 1103–1175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riffo-Campos, A.L.; Riquelme, I.; Brebi-Mieville, P. Tools for Sequence-Based miRNA Target Prediction: What to Choose? Int. J. Mol. Sci. 2016, 17, 1987. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, S.K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2014, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zheng, C.; Bai, E.; Yang, K. miR-101 inhibits glioma cell invasion via the downregulation of COX-2. Oncol. Lett. 2016, 12, 2538–2544. [Google Scholar] [CrossRef][Green Version]
- Chakrabarty, A.; Tranguch, S.; Daikoku, T.; Jensen, K.; Furneaux, H.; Dey, S.K. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc. Natl. Acad. Sci. USA 2007, 104, 15144–15149. [Google Scholar] [CrossRef]
- Strillacci, A.; Griffoni, C.; Sansone, P.; Paterini, P.; Piazzi, G.; Lazzarini, G.; Spisni, E.; Pantaleo, M.A.; Biasco, G.; Tomasi, V. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp. Cell Res. 2009, 315, 1439–1447. [Google Scholar] [CrossRef]
- Hao, Y.; Gu, X.; Zhao, Y.; Greene, S.; Sha, W.; Smoot, D.T.; Califano, J.; Wu, T.-C.; Pang, X. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev. Res. 2011, 4, 1073–1083. [Google Scholar] [CrossRef]
- Shao, Y.; Li, P.; Zhu, S.-T.; Yue, J.-P.; Ji, X.-J.; He, Z.; Ma, D.; Wang, L.; Wang, Y.-J.; Zong, Y.; et al. Cyclooxygenase-2, a Potential Therapeutic Target, Is Regulated by miR-101 in Esophageal Squamous Cell Carcinoma. PLOS ONE 2015, 10, e0140642. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Pang, Y.-Y.; Yang, H.; Li, J.; Lu, H.-X.; Wang, H.-L.; Mo, W.-J.; Huang, L.-S.; Feng, Z.-B.; Chen, G. Identification of miR-101-3p targets and functional features based on bioinformatics, meta-analysis and experimental verification in hepatocellular carcinoma. Am. J. Transl. Res. 2017, 9, 2088–2105. [Google Scholar] [PubMed]
- Wu, K.; Fukuda, K.; Xing, F.; Zhang, Y.; Sharma, S.; Liu, Y.; Chan, M.D.; Zhou, X.; Qasem, S.A.; Pochampally, R.; et al. Roles of the Cyclooxygenase 2 Matrix Metalloproteinase 1 Pathway in Brain Metastasis of Breast Cancer. J. Boil. Chem. 2015, 290, 9842–9854. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ma, X.-B.; Kang, H.-F.; Gao, J.; Min, W.-L.; Guan, H.-T.; Diao, Y.; Lu, W.-F.; Wang, X.-J. Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in Vitro and in Vivo. Cancer Cell Int. 2012, 12, 53. [Google Scholar] [CrossRef]
- Jia, W.; Lu, R.; Martin, T.A.; Jiang, W.G. The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (Review). Mol. Med. Rep. 2013, 9, 779–785. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Chin, I.; Chen, Z.; Dai, H. The Role of VE-cadherin in Blood-brain Barrier Integrity Under Central Nervous System Pathological Conditions. Curr. Neuropharmacol. 2018, 16, 1375–1384. [Google Scholar] [CrossRef]
- Bouchie, A. First microRNA mimic enters clinic. Nat. Biotechnol. 2013, 31, 577. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122 – A key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Png, K.J.; Halberg, N.; Yoshida, M.; Tavazoie, S.F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 2011, 481, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.F.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massagué, J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sullivan, P.S.; Goodman, J.C.; Gunaratne, P.H.; Marchetti, D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011, 71, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [PubMed]
- He, X.-P.; Shao, Y.; Li, X.-L.; Xu, W.; Chen, G.-S.; Sun, H.-H.; Xu, H.-C.; Xu, X.; Tang, D.; Zheng, X.-F.; et al. Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 2012, 279, 4201–4212. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, Y.-J.; Yoo, H.; Lee, S.H.; Park, J.B.; Kim, H.J. Human brain endothelial cell-derived COX-2 facilitates extravasation of breast cancer cells across the blood-brain barrier. Anticancer. Res. 2011, 31, 7. [Google Scholar]
- Wen, H.; Watry, D.D.; Marcondes, M.C.G.; Fox, H.S. Selective Decrease in Paracellular Conductance of Tight Junctions: Role of the First Extracellular Domain of Claudin-5. Mol. Cell. Boil. 2004, 24, 8408–8417. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. MMP-Mediated Disruption of Claudin-5 in the Blood–Brain Barrier of Rat Brain after Cerebral Ischemia. In Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin, Germany, 2011; Volume 762, pp. 333–345. [Google Scholar]
- Yang, J.-E.; Lu, Y.; Lin, Y.-Y.; Zheng, Z.-Y.; Fang, J.-H.; He, S.; Zhuang, S.-M. Vascular mimicry formation is promoted by paracrine TGF-β and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016, 383, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Singh, S.K. HIV-1 Tat C Modulates Expression of miRNA-101 to Suppress VE-Cadherin in Human Brain Microvascular Endothelial Cells. J. Neurosci. 2013, 33, 5992–6000. [Google Scholar] [CrossRef] [PubMed]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Miller, L.; Lo, H.-W. EGFR and HER2 signaling in breast cancer brain metastasis. Front. Biosci. 2016, 8, 245. [Google Scholar]


| Correlation Coefficient | miR-101-3p Expression |
|---|---|
| PTGS2 expression | r = −0.8059 |
| ST6GALNAC5 expression | r = −0.7150 |
| HBEGF expression | r = −0.9289 |
| Transmigrated cells | r = −0.8756 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harati, R.; Mohammad, M.G.; Tlili, A.; El-Awady, R.A.; Hamoudi, R. Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals 2020, 13, 144. https://doi.org/10.3390/ph13070144
Harati R, Mohammad MG, Tlili A, El-Awady RA, Hamoudi R. Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals. 2020; 13(7):144. https://doi.org/10.3390/ph13070144
Chicago/Turabian StyleHarati, Rania, Mohammad G. Mohammad, Abdelaziz Tlili, Raafat A. El-Awady, and Rifat Hamoudi. 2020. "Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling" Pharmaceuticals 13, no. 7: 144. https://doi.org/10.3390/ph13070144
APA StyleHarati, R., Mohammad, M. G., Tlili, A., El-Awady, R. A., & Hamoudi, R. (2020). Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals, 13(7), 144. https://doi.org/10.3390/ph13070144