Metformin Alleviates Obesity and Systemic Oxidative Stress in Obese Young Swine
Abstract
1. Introduction
2. Results
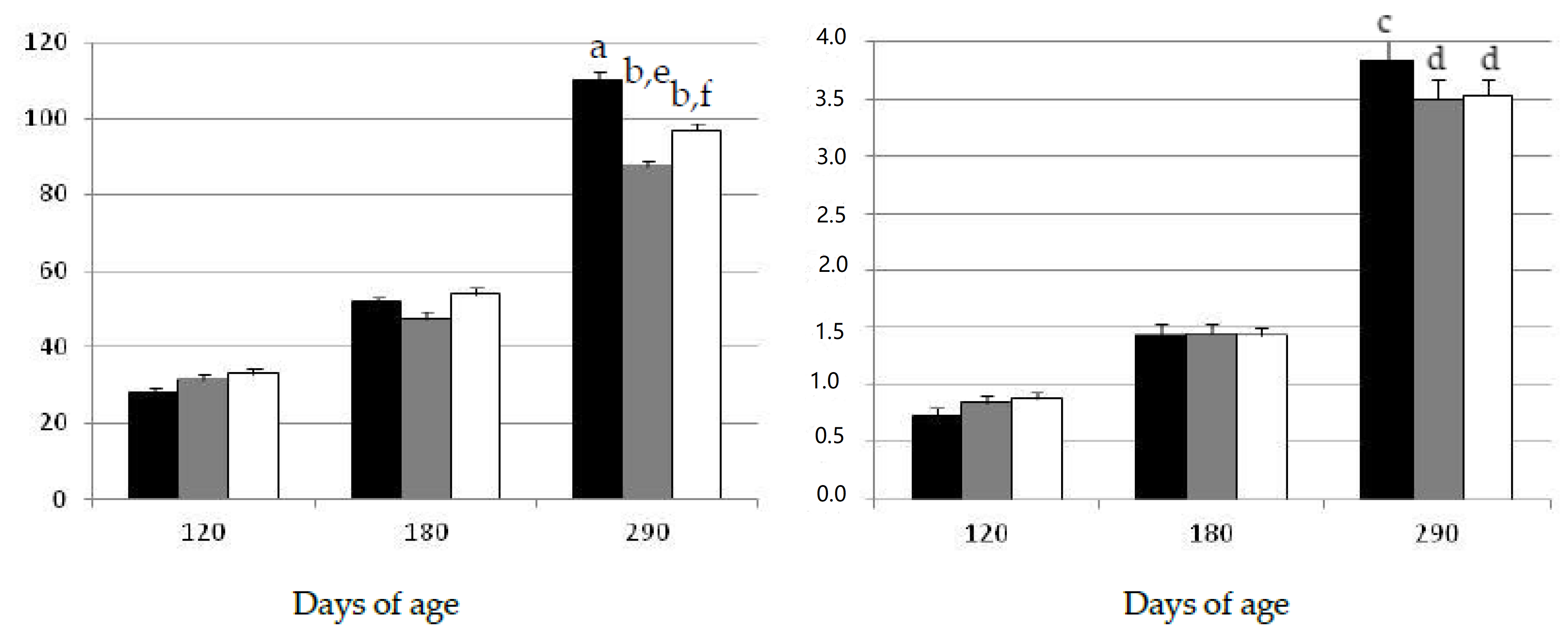
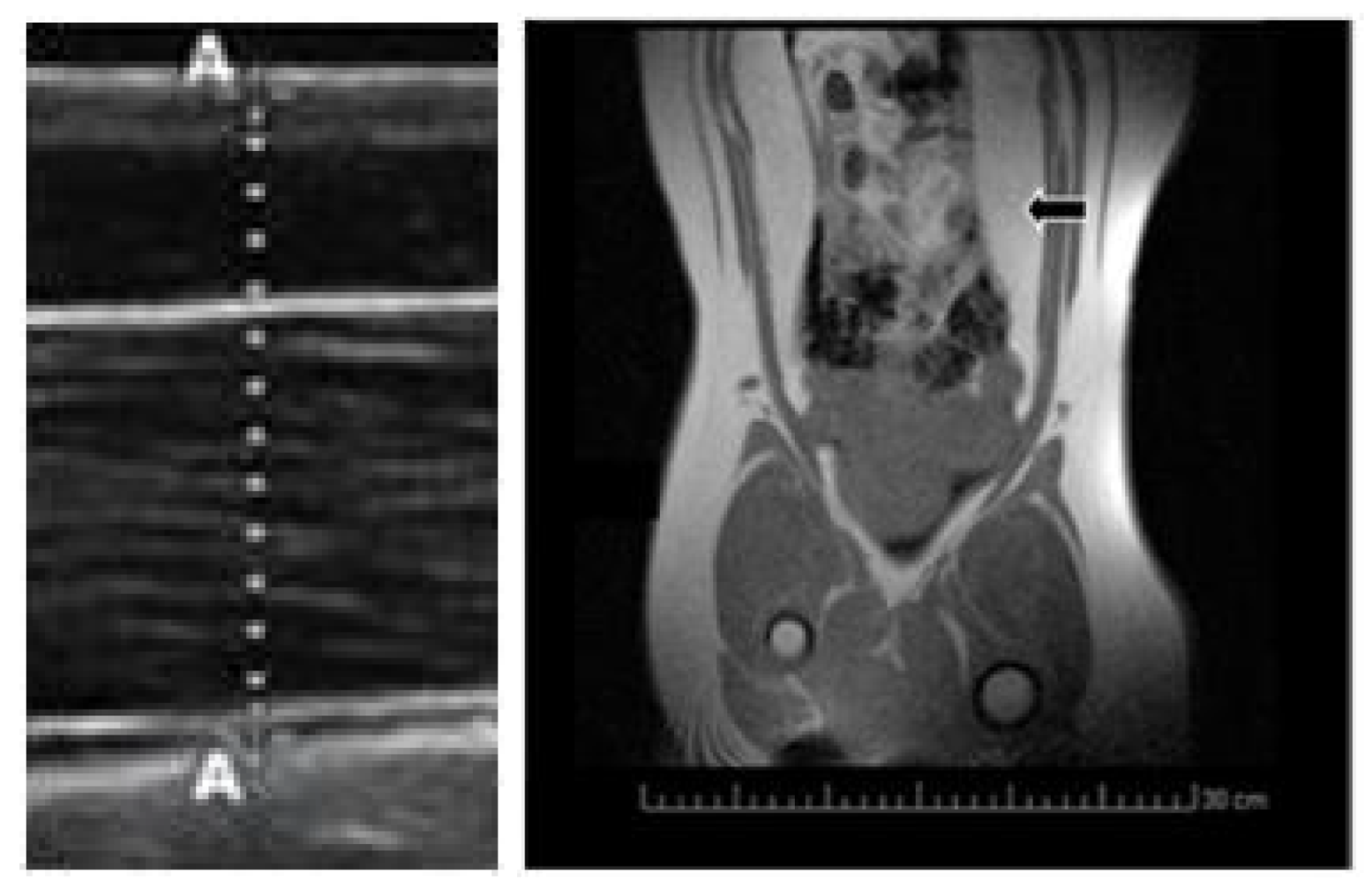
2.1. Changes in Growth Patterns and Adiposity
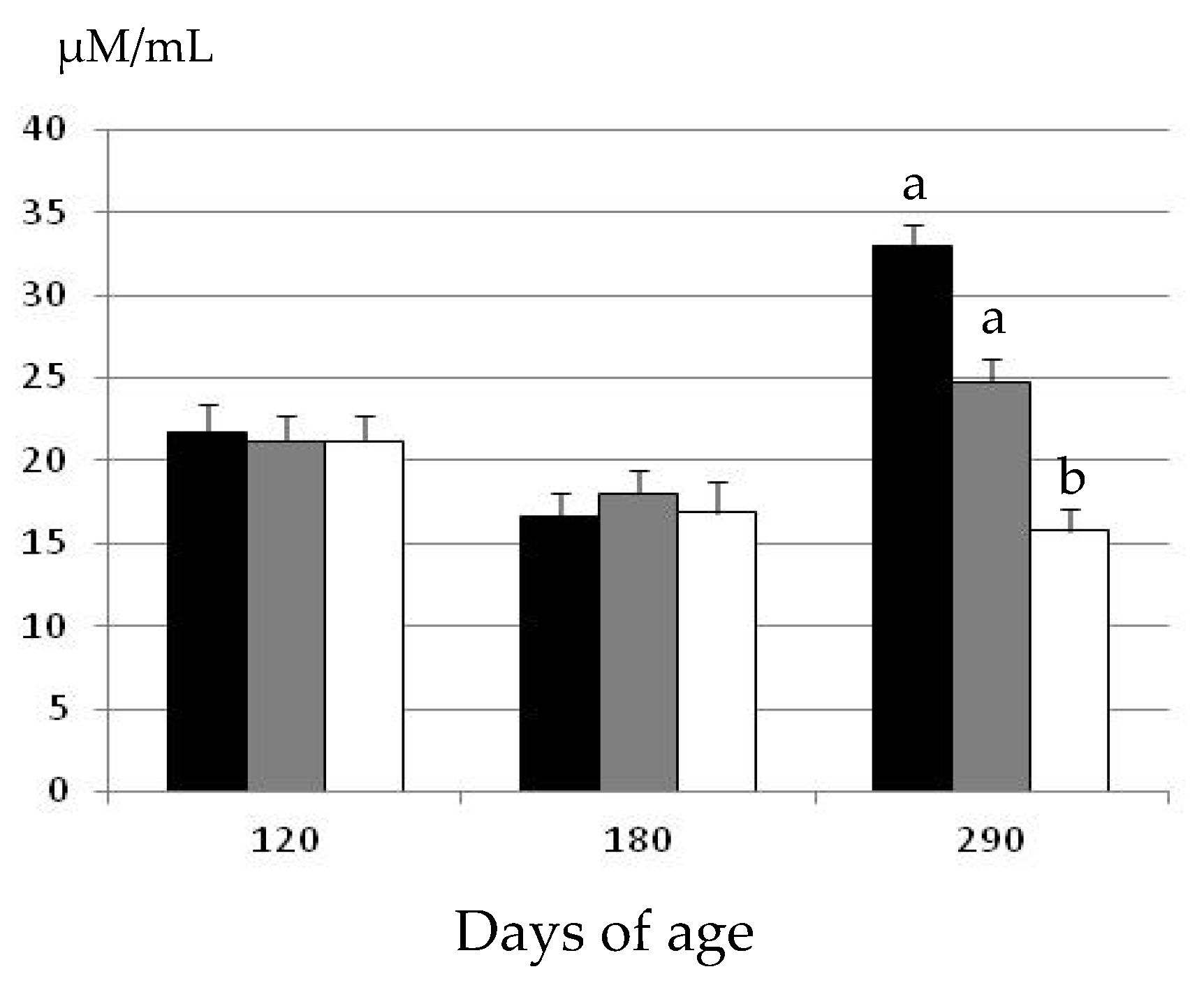
2.2. Changes in Oxidative Status
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Animals and Experimental Design
4.3. Evaluation of Growth Patterns, Adiposity and Oxidative Status
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christensen, M.L.; Rashed, S.M.; Sinclair, J.; Cowan, P.A.; Velasquez-Mieyer, P.; Burghen, G.A. Type 2 Diabetes Mellitus in Children and Adolescents: The New Challenge. J. Pediatr. Pharmacol. Ther. 2004, 9, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, V.; Wu, S.; Aguilar, A.; Bonner, R.; Suarez, E.; De Luca, F. Association between Oxidative Stress and Masked Hypertension in a Multi-Ethnic Population of Obese Children and Adolescents. J. Pediatr. 2011, 158, 628–633.e1. [Google Scholar] [CrossRef] [PubMed]
- Salman, Z.K.; Refaat, R.; Selima, E.; El Sarha, A.; Ismail, M.A. The combined effect of metformin and l-cysteine on inflammation, oxidative stress and insulin resistance in streptozotocin-induced type 2 diabetes in rats. Eur. J. Pharmacol. 2013, 714, 448–455. [Google Scholar] [CrossRef]
- Park, C.S.; Bang, B.-R.; Kwon, H.-S.; Moon, K.-A.; Kim, T.; Lee, K.-Y.; Moon, H.-B.; Cho, Y.S. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol. 2012, 84, 1660–1670. [Google Scholar] [CrossRef]
- Calixto, M.C.; Lintomen, L.; André, D.M.; Leiria, L.O.; Ferreira, D.; Lellis-Santos, C.; Anhê, G.F.; Bordin, S.A.; Landgraf, R.G.; Antunes, E. Metformin Attenuates the Exacerbation of the Allergic Eosinophilic Inflammation in High Fat-Diet-Induced Obesity in Mice. PLoS ONE 2013, 8, e76786. [Google Scholar] [CrossRef]
- Shetty, P. India faces growing breast cancer epidemic. Lancet 2012, 379, 992–993. [Google Scholar] [CrossRef]
- Kong, A.P.S.; Xu, G.; Brown, N.; So, W.-Y.; Ma, R.C.; Chan, J.C.N. Diabetes and its comorbidities—Where East meets West. Nat. Rev. Endocrinol. 2013, 9, 537–547. [Google Scholar] [CrossRef]
- Dulin, A.; Thind, H.; Affuso, O.; Baskin, M.L. The associations of perceived neighborhood disorder and physical activity with obesity among African American adolescents. BMC Public Health 2013, 13, 440. [Google Scholar] [CrossRef]
- Li, J.S.; Barnett, T.A.; Goodman, E.; Wasserman, R.C.; Kemper, A.R. Approaches to the Prevention and Management of Childhood Obesity: The Role of Social Networks and the Use of Social Media and Related Electronic Technologies. Circulation 2013, 127, 260–267. [Google Scholar] [CrossRef]
- Mendez, R.; Grissom, M. Disorders of childhood growth and development: Childhood obesity. FP Essent. 2013, 410, 20–24. [Google Scholar] [PubMed]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Bähr, A.; Wolf, E. Domestic Animal Models for Biomedical Research. Reprod. Domest. Anim. 2012, 47, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Houpt, K.A.; Houpt, T.R.; Pond, W.G. The Pig as a Model for the Study of Obesity and of Control of Food Intake: A Review. Yale J. Boil. Med. 1979, 52, 307–329. [Google Scholar]
- Torres-Rovira, L.; Gonzalez-Anover, P.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Óvilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Effect of an obesogenic diet during the juvenile period on growth pattern, fatness and metabolic, cardiovascular and reproductive features of Swine with obesity/leptin resistance. Endocr. Metab. Immune Disord. Drug Targ. 2013, 13, 143–151. [Google Scholar] [CrossRef]
- Torres-Rovira, L.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Óvilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Diet-Induced Swine Model with Obesity/Leptin Resistance for the Study of Metabolic Syndrome and Type 2 Diabetes. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Gonzalez-Añover, P.; Encinas, T.; Gomez-Izquierdo, E.; Sanz, E.; Letelier, C.; Torres-Rovira, L.; Pallares, P.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Advanced Onset of Puberty in Gilts ofThrifty Genotype(Iberian Pig). Reprod. Domest. Anim. 2009, 45, 1003–1007. [Google Scholar] [CrossRef]
- Gonzalez-Añover, P.; Vigo, E.; Encinas, T.; Torres-Rovira, L.; Pallares, P.; Gomez-Izquierdo, E.; Sanchez-Sanchez, R.; Mallo, F.; Gonzalez-Bulnes, A. Prepuberal evolution of plasma leptin levels in gilts of thrifty genotype (Iberian pig) and lean commercial crosses (LargeWhite × Landrace). Res. Vet. Sci. 2012, 93, 100–102. [Google Scholar] [CrossRef]
- Astiz, S.; Gonzalez-Bulnes, A.; Astiz, I.; Barbero, A.; Garcia-Real, I.; Perez-Solana, M. Advanced onset of puberty after metformin therapy in swine with thrifty genotype. Exp. Physiol. 2014, 99, 1241–1252. [Google Scholar] [CrossRef]
- Wabitsch, M.; Hauner, H.; Heinze, E.; Bockmann, A.; Benz, R.; Mayer, H.; Teller, W. Body fat distribution and steroid hormone concentrations in obese adolescent girls before and after weight reduction. J. Clin. Endocrinol. Metab. 1995, 80, 3469–3475. [Google Scholar]
- Reinehr, T.; De Sousa, G.; Roth, C.L.; Andler, W. Androgens before and after Weight Loss in Obese Children. J. Clin. Endocrinol. Metab. 2005, 90, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; López-Bermejo, A.; Díaz, M.; Marcos, M.V.; De Zegher, F. Metformin Treatment for Four Years to Reduce Total and Visceral Fat in Low Birth Weight Girls with Precocious Pubarche. J. Clin. Endocrinol. Metab. 2008, 93, 1841–1845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malin, S.K.; Gerber, R.; Chipkin, S.R.; Braun, B. Independent and Combined Effects of Exercise Training and Metformin on Insulin Sensitivity in Individuals with Prediabetes. Diabetes Care 2011, 35, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.; de Zegher, F.; Valls, C.; Dunger, D.B.; Ibañez, L. Persisting benefits 12–18 months after discontinuation of pubertal metformin therapy in low birth-weight girls. Clin. Endocrinol. (Oxf.) 2007, 67, 468–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibáñez, L.; Lopez-Bermejo, A.; Díaz, M.; Marcos, M.V.; De Zegher, F. Pubertal Metformin Therapy to Reduce Total, Visceral, and Hepatic Adiposity. J. Pediatr. 2010, 156, 98–102.e1. [Google Scholar] [CrossRef]
- Ibañez, L.; Lopez-Bermejo, A.; Dıaz, M.; Marcos, M.V.; de Zegher, F. Early metformin therapy (age 8–12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J. Clin. Endocrinol. Metab. 2011, 96, 1262–1267. [Google Scholar] [CrossRef]
- Ibáñez, L.; Ong, K.; Valls, C.; Marcos, M.V.; Dunger, D.B.; De Zegher, F. Metformin Treatment to Prevent Early Puberty in Girls with Precocious Pubarche. J. Clin. Endocrinol. Metab. 2006, 91, 2888–2891. [Google Scholar] [CrossRef]
- Ibáñez, L.; Valls, C.; Marcos, M.V.; Ong, K.; Dunger, D.B.; De Zegher, F. Insulin Sensitization for Girls with Precocious Pubarche and with Risk for Polycystic Ovary Syndrome: Effects of Prepubertal Initiation and Postpubertal Discontinuation of Metformin Treatment. J. Clin. Endocrinol. Metab. 2004, 89, 4331–4337. [Google Scholar] [CrossRef]
- Reinehr, T. Lifestyle intervention in childhood obesity: Changes and challenges. Nat. Rev. Endocrinol. 2013, 9, 607–614. [Google Scholar] [CrossRef]
- Gore, D.C.; Wolf, S.E.; Sanford, A.; Herndon, D.N.; Wolfe, R.R. Influence of Metformin on Glucose Intolerance and Muscle Catabolism Following Severe Burn Injury. Ann. Surg. 2005, 241, 334–342. [Google Scholar] [CrossRef]
- Lee, C.G.; Boyko, E.J.; Barrett-Connor, E.; Miljkovic, I.; Hoffman, A.R.; Everson-Rose, S.A.; Lewis, C.E.; Cawthon, P.M.; Strotmeyer, E.S.; Orwoll, E.S. Insulin Sensitizers May Attenuate Lean Mass Loss in Older Men With Diabetes. Diabetes Care 2011, 34, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic Effects of Metformin in Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef]
- Scarpello, J.H.; Howlett, H.C. Metformin therapy and clinical uses. Diabetes Vasc. Dis. Res. 2008, 5, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Galuska, D.; Zierath, J.; Thörne, A.; Sonnenfeld, T.; Wallberg-Henriksson, H. Metformin increases insulin-stimulated glucose transport in insulin-resistant human skeletal muscle. Diabete Metab. 1991, 17, 159–163. [Google Scholar]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef]
- Suwa, M.; Egashira, T.; Nakano, H.; Sasaki, H.; Kumagai, S. Metformin increases the PGC-1α protein and oxidative enzyme activities possibly via AMPK phosphorylationin skeletal muscle in vivo. J. Appl. Physiol. 2006, 101, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, A.; Wahli, W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Med. Wkly. 2010, 140, 13071. [Google Scholar] [CrossRef]
- Bonala, S.; Lokireddy, S.; Arigela, H.; Teng, S.; Wahli, W.; Sharma, I.; McFarlane, C.; Kambadur, R. Peroxisome Proliferator-activated Receptor β/δ Induces Myogenesis by Modulating Myostatin Activity. J. Boil. Chem. 2012, 287, 12935–12951. [Google Scholar] [CrossRef]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Ben Sahra, I.; Le Marchand-Brustel, Y.; Tanti, J.-F. Metformin and cancer therapy. Curr. Opin. Oncol. 2012, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Astiz, S.; Lopez-Bote, C.; Perez-Solana, M.L.; Ayuso, M.; García-Real, I.; Gonzalez-Bulnes, A. Maternal Malnutrition and Offspring Sex Determine Juvenile Obesity and Metabolic Disorders in a Swine Model of Leptin Resistance. PLoS ONE 2013, 8, e78424. [Google Scholar] [CrossRef] [PubMed]
| Group OB | Group EXE | Group MET | |
|---|---|---|---|
| Visceral Fat Depot (cm2) | 31.2 ± 5.3a | 18.3 ± 3.4b | 17.2 ± 4.1b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astiz, S.; Gonzalez-Bulnes, A.; Astiz, I.; Barbero, A.; Pesantez-Pacheco, J.L.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Heras-Molina, A. Metformin Alleviates Obesity and Systemic Oxidative Stress in Obese Young Swine. Pharmaceuticals 2020, 13, 142. https://doi.org/10.3390/ph13070142
Astiz S, Gonzalez-Bulnes A, Astiz I, Barbero A, Pesantez-Pacheco JL, Garcia-Contreras C, Vazquez-Gomez M, Heras-Molina A. Metformin Alleviates Obesity and Systemic Oxidative Stress in Obese Young Swine. Pharmaceuticals. 2020; 13(7):142. https://doi.org/10.3390/ph13070142
Chicago/Turabian StyleAstiz, Susana, Antonio Gonzalez-Bulnes, Isabel Astiz, Alicia Barbero, Jose Luis Pesantez-Pacheco, Consolacion Garcia-Contreras, Marta Vazquez-Gomez, and Ana Heras-Molina. 2020. "Metformin Alleviates Obesity and Systemic Oxidative Stress in Obese Young Swine" Pharmaceuticals 13, no. 7: 142. https://doi.org/10.3390/ph13070142
APA StyleAstiz, S., Gonzalez-Bulnes, A., Astiz, I., Barbero, A., Pesantez-Pacheco, J. L., Garcia-Contreras, C., Vazquez-Gomez, M., & Heras-Molina, A. (2020). Metformin Alleviates Obesity and Systemic Oxidative Stress in Obese Young Swine. Pharmaceuticals, 13(7), 142. https://doi.org/10.3390/ph13070142





