Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review
Abstract
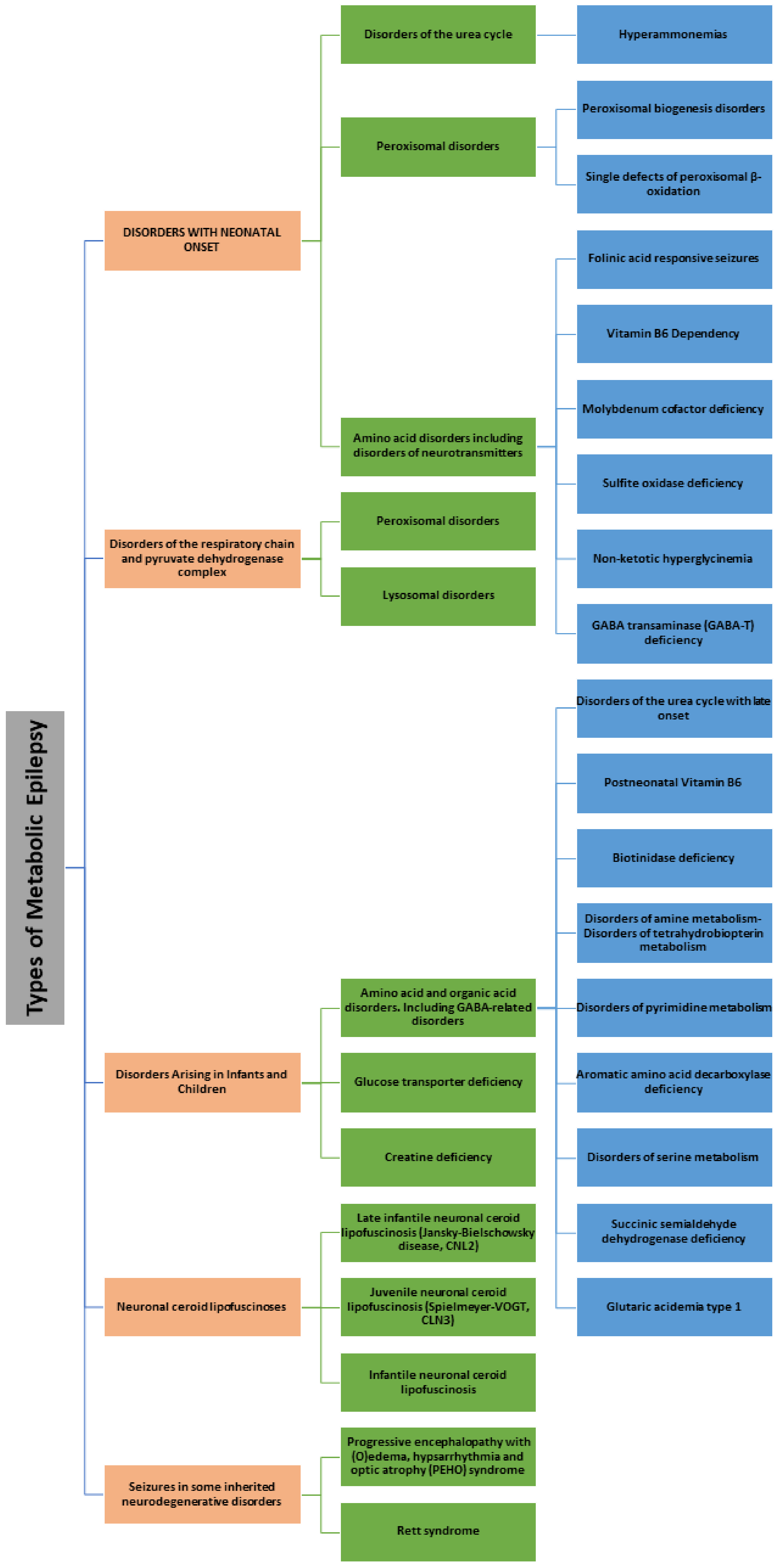
1. Introduction
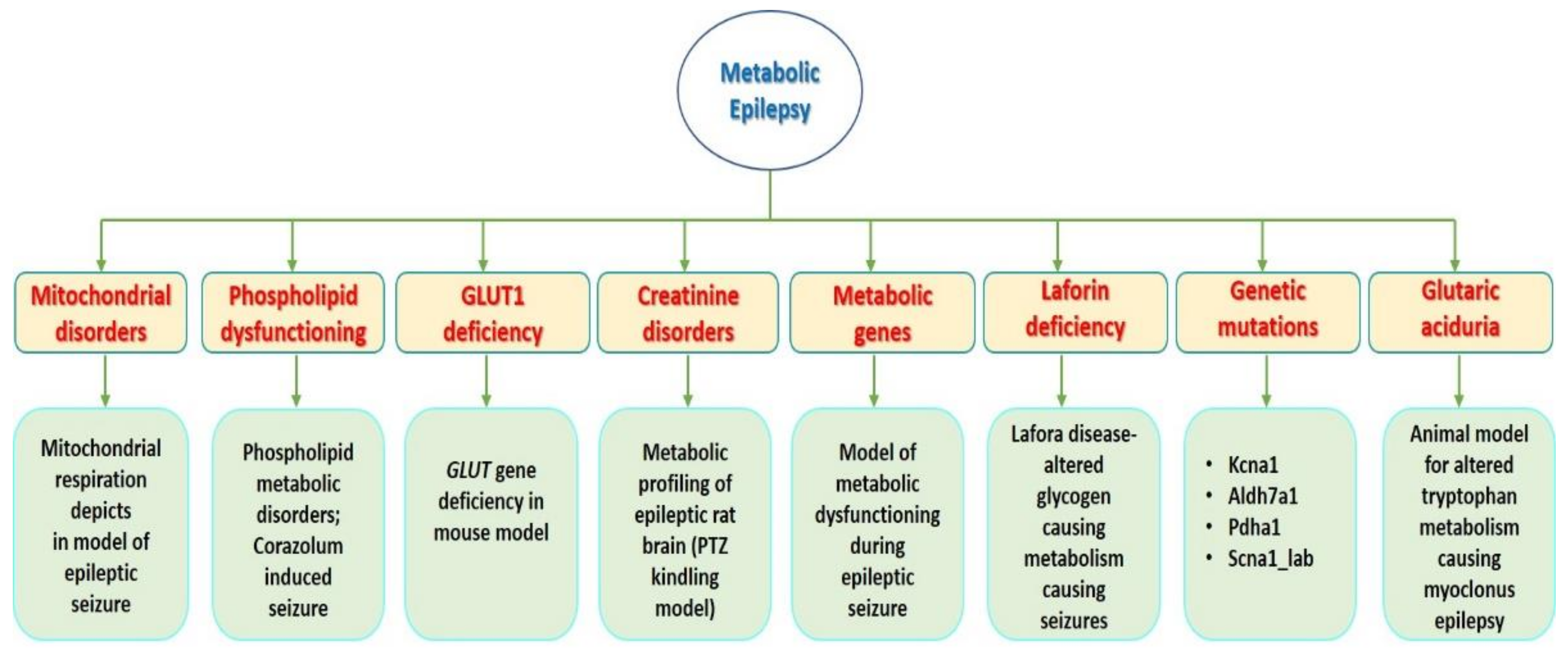
2. Results and Discussion
2.1. Metabolic Genes Responsible for Epilepsy in the Obese Rat
2.2. Metabolic Profiling of Epileptic Rat Brain in PTZ Kindling Model
2.3. Mutation of Ubiquitin Ligase (Ube3a Phenotype) Causes Angelman Syndrome in Mice-a Rare Genetic Epileptic Neurodegeneration
2.4. Metabolic Features in Repetitive Seizures
2.5. Epilepsy and Metabolic Dysfunction in a Mouse Model-Glut Deficiency (G1D)
2.6. Mitochondrial Respiration Deficits in Rat Epilepsy Model
2.7. A Rat Model of Pilocarpine-Induced Epilepsy with an Abnormality in Metabolic Connectivity
2.8. Epilepsy Due to Metabolic Dysfunction via Adiponectin Deficiency
2.9. Myoclonus Epilepsy Model: Impairment of Serotonin (5HT) and 3-Hydroxyanthranilic Acid Metabolism
2.10. Model for Metabolic Dysfunction during an Epileptic Seizure in Pilocarpine-Treated Rats
2.11. Lafora Disease Model-Altered Glycogen Metabolism Causing Epilepsy
2.12. Animal Model for Phospholipid Metabolic Disorders: Corazolum Induced Seizures
2.13. An Animal Model for Altered Tryptophan Metabolism Causing Myoclonus Epilepsy
2.14. Long Noncoding RNAs (lncRNA) Cancer Susceptibility Candidate 2 (lncRNA CASC2) Inhibits Astrocytic Activation and Adenosine Metabolism
2.15. HMGB1 Modulates Glutamate Metabolism in KA-Induced Seizures
2.16. Lipid Metabolism Altered in Rat Model of Post-Traumatic Epilepsy (PTE)
2.17. Altered Glycolysis and Mitochondrial Respiration in a Zebrafish Model of Dravet Syndrome (DS)
2.18. Alterations in Cytosolic and Mitochondrial [U-13C] Glucose Metabolism in a Chronic Epilepsy Mouse Model
2.19. BAD KO Provides Metabolic Seizure Resistance in a Genetic Model of Epilepsy with Sudden Unexplained Death in Epilepsy
2.20. Metabolic Perturbations Associated with the Consumption of a Ketogenic Medium-Chain TAG Diet in Dogs with Idiopathic Epilepsy
2.21. A Novel Metabolism-Based Zebrafish Model to Uncovers HDACs 1 and 3 as a Potential Combined Anti-Seizure Drug Target
2.22. Pyridoxine-Dependent Epilepsy (PDE) in Zebrafish Caused by Aldh7a1 Deficiency
2.23. PDH Deficiency in Mouse Model
3. Materials and Methods
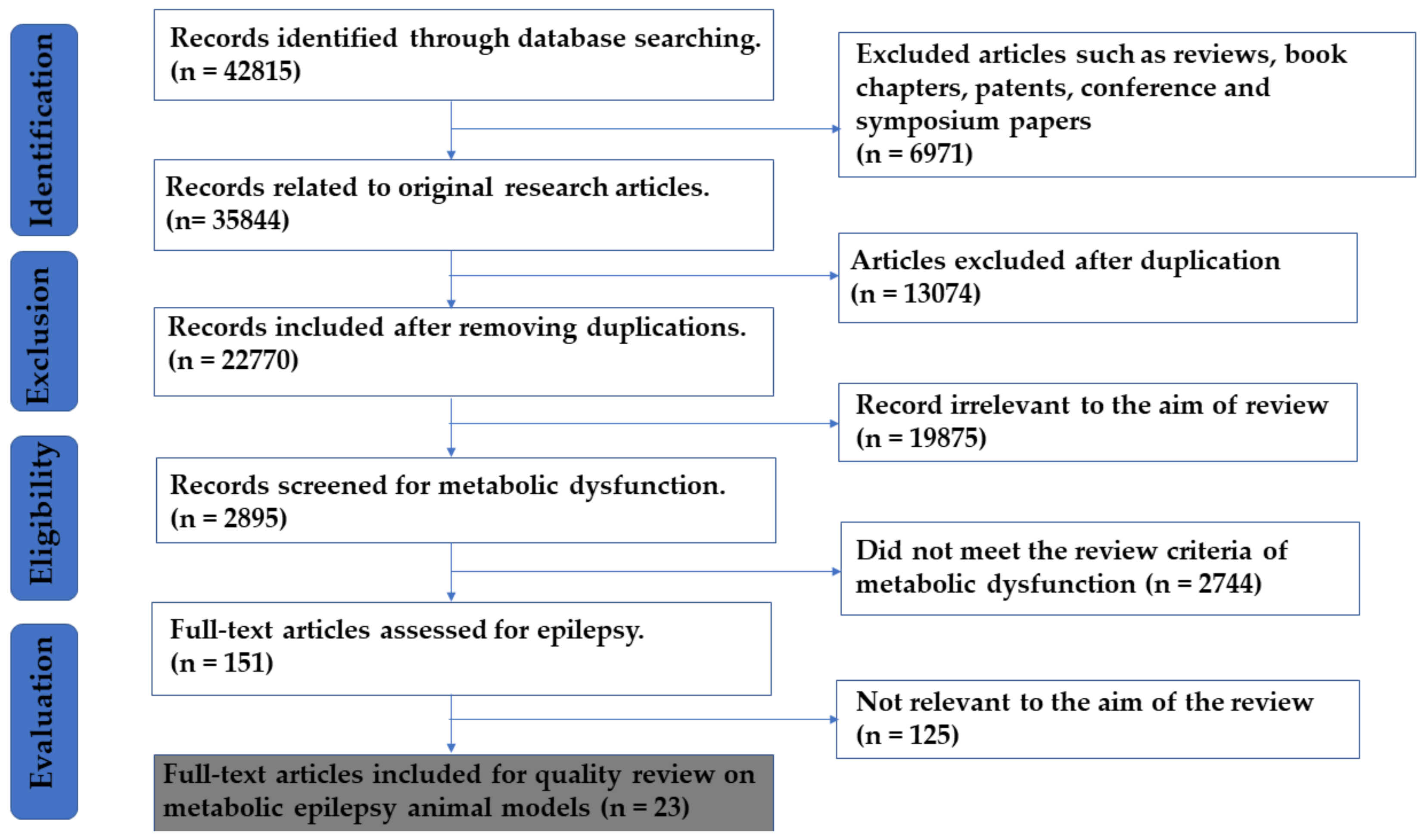
3.1. Search Methods
3.2. Study Exclusion/Inclusion and Selection Criteria
3.3. Data Extraction and Analysis
4. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| CNS | Central Nervous System |
| ILAE | International League Against Epilepsy |
| 5MTHF | 5-Methyltetrahydrofolate |
| CFD | Cerebral Folate Deficiency |
| GAMT | Guanidinoacetate Methyltransferase |
| AGAT | Arginine Glycine Amidino Transferase |
| PNPO | Pyridoxamine Phosphate Oxidase |
| CFD | Cerebral Folate Deficiency |
| FOLR | Folate Receptor 1 |
| GLUT1 | Glucose Transporter 1 |
| MERRF | Myoclonic Epilepsy with Ragged Red Fibers |
| MELAS | Mitochondrial Encephalopathy with Lactic Acidosis and Stroke-like Episodes |
| BDNF | Brain Derived Neurotropic Factor |
| TCA | Tricarboxylic Acid Cycle |
| ATP | Adenosine Triphosphate |
| HNMR | Proton Nuclear Magnetic Resonance |
| PTZ | Pentylenetetrazol |
| KA | Kainic Acid |
| LTP | Long Term Potentiation |
| ROS | Reactive Oxygen Species |
| fMRI | Functional Magnetic Resonance Imaging |
| HFD | High Fat Diet |
| CSTB | Cystatin B Gene |
| LD | Lafora Disease |
| TCA | Tricarboxylic acid |
| 5HIAA | 5-Hydroxyindole Acetic Acid |
| Ube3A | Ubiquitin-protein ligase E3A |
| PED | Pyridoxine-Dependent Epilepsy |
| HMGB1 | High Mobility Group Box 1 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| Cas9 | CRISPR Associated Endonuclease 9 |
| HDAC 1-3 | Histone Deacetylases |
| Aldh7a | Aldehyde Dehydrogenase (ALDH) 7 Family Member A1 gene |
| Scn1a | Sodium Voltage-Gated Channel Alpha Subunit 1 |
| α-AASAD | α-Aminoadipic-Semialdehyde Dehydrogenase |
| PSC | Piperidine-6-Carboxylic Acid |
| PCDHA1 | Pyruvate Dehydrogenase α-Subunit Gene |
| GABA | α-Aminobutyric Acid |
| KCNA1 | Potassium Voltage-Gated Channel Subfamily A Member1 |
| EEG | Electroencephalogram |
| TBI | Traumatic Brain Injury |
| KD | Ketogenic Diet |
| SMEI | Severe Myoclonic Epilepsy of Infancy |
| RAGE | Receptor For Advanced Glycation End-products |
| TLR4 | Toll-Like Receptor- 4 |
| PRNCs | Primary Rat Neural Cells |
| SLC2A1 | Solute Carrier Family 2 Member1 |
| SD | Sprague Dawley |
References
- Lin Lin Lee, V.; Kar Meng Choo, B.; Chung, Y.-S.; P Kundap, U.; Kumari, Y.; Shaikh, M.F. Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 871. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-C.; Lee, Y.-M.; Kim, H.D. Mitochondrial disease and epilepsy. Brain Dev. 2013, 35, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Smith, J.; Patel, M. Metabolic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Hassan, Y.I.; Wijeratne, S.S. Biotin and biotinidase deficiency. Expert Rev. Endocrinol. Metab. 2008, 3, 715–724. [Google Scholar] [CrossRef]
- Wolf, B.; Grier, R.; McVoy, J.S.; Heard, G. Biotinidase deficiency: A novel vitamin recycling defect. J. Inherit. Metab. Dis. 1985, 8, 53–58. [Google Scholar] [CrossRef]
- Hyland, K.; Shoffner, J.; Heales, S.J. Cerebral folate deficiency. J. Inherit. Metab. Dis. 2010, 33, 563–570. [Google Scholar] [CrossRef]
- Scaglia, F. Cerebral Folate Deficiency and Epilepsy. Inherit. Metab. Epilepsies 2012, 261–266. [Google Scholar]
- Ramaekers, V.T.; Blau, N. Cerebral folate deficiency. Dev. Med. Child Neurol. 2004, 46, 843–851. [Google Scholar] [CrossRef]
- Pérez-Duenas, B.; Ormazábal, A.; Toma, C.; Torrico, B.; Cormand, B.; Serrano, M.; Sierra, C.; De Grandis, E.; Marfa, M.P.; García-Cazorla, A. Cerebral folate deficiency syndromes in childhood: Clinical, analytical, and etiologic aspects. Arch. Neurol. 2011, 68, 615–621. [Google Scholar] [CrossRef]
- Leuzzi, V. Inborn errors of creatine metabolism and epilepsy: Clinical features, diagnosis, and treatment. J. Child Neurol. 2002, 17, S89–S97. [Google Scholar]
- Allen, P.J. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci. Biobehav. Rev. 2012, 36, 1442–1462. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Donner, E.; Golja, A.; Rooney, C.M. Folinic Acid—Responsive Seizures Presenting as Breakthrough Seizures in a 3-Month-Old Boy. J. Child Neurol. 2003, 18, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.C.; Van Hove, J.L.; Scharer, G.; Hyland, K.; Plecko, B.; Waters, P.J.; Mercimek-Mahmutoglu, S.; Stockler-Ipsiroglu, S.; Salomons, G.S.; Rosenberg, E.H. Folinic acid–responsive seizures are identical to pyridoxine-dependent epilepsy. Ann. Neurol. 2009, 65, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Klepper, J.; Verbeek, M.M.; Leferink, M.; Hofste, T.; van Engelen, B.G.; Wevers, R.A.; Arthur, T.; Bahi-Buisson, N.; Ballhausen, D. Glucose transporter-1 deficiency syndrome: The expanding clinical and genetic spectrum of a treatable disorder. Brain 2010, 133, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S. Mitochondrial disease and epilepsy. Dev. Med. Child Neurol. 2012, 54, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Mahjoub, S.Z. Epilepsy in mitochondrial disorders. Seizure 2012, 21, 316–321. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A.; Fiorini, A.C.; Scorza, C.A.; de Almeida, A.C. Mitochondrial tRNA Glutamic Acid Variant 14709T> C Manifesting as Myoclonic Epilepsy with Ragged Red Fibers. Chin. Med. J. 2018, 131, 2518. [Google Scholar] [CrossRef]
- Wanders, R.J.; Waterham, H.R. Peroxisomal disorders: The single peroxisomal enzyme deficiencies. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 1707–1720. [Google Scholar] [CrossRef]
- Schmitt, B.; Baumgartner, M.; Mills, P.B.; Clayton, P.T.; Jakobs, C.; Keller, E.; Wohlrab, G. Seizures and paroxysmal events: Symptoms pointing to the diagnosis of pyridoxine-dependent epilepsy and pyridoxine phosphate oxidase deficiency. Dev. Med. Child Neurol. 2010, 52, e133–e142. [Google Scholar] [CrossRef]
- Goyal, M.; Fequiere, P.R.; McGrath, T.M.; Hyland, K. Seizures with decreased levels of pyridoxal phosphate in cerebrospinal fluid. Pediatr. Neurol. 2013, 48, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Dachet, F.; Ghoddoussi, F.; Bagla, S.; Fuerst, D.; Stanley, J.A.; Galloway, M.P.; Loeb, J.A. Altered metabolomic–genomic signature: A potential noninvasive biomarker of epilepsy. Epilepsia 2017, 58, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Szot, P.; White, S.S.; McCarthy, E.B.; Turella, A.; Rejniak, S.X.; Schwartzkroin, P.A. Behavioral and metabolic features of repetitive seizures in immature and mature rats. Epilepsy Res. 2001, 46, 191–203. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Grone, B.P.; Baraban, S.C. Animal models in epilepsy research: Legacies and new directions. Nat. Neurosci. 2015, 18, 339. [Google Scholar] [CrossRef] [PubMed]
- Zsurka, G.; Kunz, W.S. Mitochondrial dysfunction and seizures: The neuronal energy crisis. Lancet Neurol. 2015, 14, 956–966. [Google Scholar] [CrossRef]
- Schiavone, S.; Trabace, L. The use of antioxidant compounds in the treatment of first psychotic episode: Highlights from preclinical studies. Cns Neurosci. Ther. 2018. [Google Scholar] [CrossRef]
- Pecorelli, A.; Natrella, F.; Belmonte, G.; Miracco, C.; Cervellati, F.; Ciccoli, L.; Mariottini, A.; Rocchi, R.; Vatti, G.; Bua, A. NADPH oxidase activation and 4-hydroxy-2-nonenal/aquaporin-4 adducts as possible new players in oxidative neuronal damage presents in drug-resistant epilepsy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 507–519. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, K.; Bai, Y.; Zhang, A.; Xia, Z.; Chao, J.; Yao, H. NADPH oxidase activation is required for pentylenetetrazole kindling-induced hippocampal autophagy. Free Radic. Biol. Med. 2016, 94, 230–242. [Google Scholar] [CrossRef]
- Campistol, J.; Plecko, B. Treatable newborn and infant seizures due to inborn errors of metabolism. Epileptic Disord. 2015, 17, 229–242. [Google Scholar] [CrossRef]
- Dulac, O.; Plecko, B.; Gataullina, S.; Wolf, N.I. Occasional seizures, epilepsy, and inborn errors of metabolism. Lancet Neurol. 2014, 13, 727–739. [Google Scholar] [CrossRef]
- Wallace, S.J.; Farrell, K. Epilepsy in Children, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ruiz, N.; Pacheco, L.F.; Farrell, B.; Cox, C.B.; Ermolinsky, B.S.; Garrido-Sanabria, E.R.; Nair, S. Metabolic gene expression changes in the hippocampus of obese epileptic male rats in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2011, 1426, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Carmody, S.; Brennan, L. Effects of pentylenetetrazole-induced seizures on metabolomic profiles of rat brain. Neurochem. Int. 2010, 56, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ciarlone, S.L.; Grieco, J.C.; D’Agostino, D.P.; Weeber, E.J. Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol. Dis. 2016, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-h.; Armstrong, D.; Albrecht, U.; Atkins, C.M.; Noebels, J.L.; Eichele, G.; Sweatt, J.D.; Beaudet, A.L. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 1998, 21, 799–811. [Google Scholar] [CrossRef]
- Marin-Valencia, I.; Good, L.B.; Ma, Q.; Duarte, J.; Bottiglieri, T.; Sinton, C.M.; Heilig, C.W.; Pascual, J.M. Glut1 deficiency (G1D): Epilepsy and metabolic dysfunction in a mouse model of the most common human phenotype. Neurobiol. Dis. 2012, 48, 92–101. [Google Scholar] [CrossRef]
- Rowley, S.; Liang, L.-P.; Fulton, R.; Shimizu, T.; Day, B.; Patel, M. Mitochondrial respiration deficits driven by reactive oxygen species in experimental temporal lobe epilepsy. Neurobiol. Dis. 2015, 75, 151–158. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.K.; Kang, H.; Lee, H.; Im, H.-J.; Kim, E.E.; Chung, J.-K.; Lee, D.S. Abnormal metabolic connectivity in the pilocarpine-induced epilepsy rat model: A multiscale network analysis based on persistent homology. NeuroImage 2014, 99, 226–236. [Google Scholar] [CrossRef]
- Lee, E.B.; Warmann, G.; Dhir, R.; Ahima, R.S. Metabolic dysfunction associated with adiponectin deficiency enhances kainic acid-induced seizure severity. J. Neurosci. 2011, 31, 14361–14366. [Google Scholar] [CrossRef]
- Arbatova, J.; D’amato, E.; Vaarmann, A.; Zharkovsky, A.; Reeben, M. Reduced Serotonin and 3-Hydroxyanthranilic Acid Levels in Serum of Cystatin B-Deficient Mice, a Model System for Progressive Myoclonus Epilepsy. Epilepsia 2005, 46, 49–51. [Google Scholar] [CrossRef]
- Kann, O.; Kovács, R.; Njunting, M.; Behrens, C.J.; Otáhal, J.; Lehmann, T.-N.; Gabriel, S.; Heinemann, U. Metabolic dysfunction during neuronal activation in the ex vivo hippocampus from chronic epileptic rats and humans. Brain 2005, 128, 2396–2407. [Google Scholar] [CrossRef] [PubMed]
- Pederson, B.A.; Turnbull, J.; Epp, J.R.; Weaver, S.A.; Zhao, X.; Pencea, N.; Roach, P.J.; Frankland, P.W.; Ackerley, C.A.; Minassian, B.A. Inhibiting glycogen synthesis prevents Lafora disease in a mouse model. Ann. Neurol. 2013, 74, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Karagezyan, K.; Simonyan, L.; Karagezyan, M.; Ovsepyan, L.; Simonyan, A.; Ovakimyan, S. Characteristic Features of Phospholipid Metabolic Disorders in Membranes of Erythrocytes of White Rats in Corazolum-Induced Epileptoid Seizures. Doklady Biochem. Biophys. 2005, 404, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Vaarmann, A.; Kaasik, A.; Zharkovsky, A. Altered Tryptophan Metabolism in the Brain of Cystatin B-Deficient Mice: A Model System for Progressive Myoclonus Epilepsy. Epilepsia 2006, 47, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, H.; Ma, H.; Luo, L.; Yang, L.; Chen, F.; Qu, X.; Liu, H.; Zhang, R. LncRNA CASC2 inhibits astrocytic activation and adenosine metabolism by regulating PTEN in pentylenetetrazol-induced epilepsy model. J. Chem. Neuroanat. 2020, 101749. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Pappas, C.; Malapira, T.; Vale, F.Ĺ.; Tajiri, N.; Borlongan, C.V. Extracellular HMGB1 Modulates Glutamate Metabolism Associated with Kainic Acid-Induced Epilepsy-Like Hyperactivity in Primary Rat Neural Cells. Cell. Physiol. Biochem. 2017, 41, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.K.; Mukherjee, S.; Sharma, R.; Das, J.; Sharma, R.; Kumar, V.; Sinha, N.; Sharma, D. Altered lipid metabolism in post-traumatic epileptic rat model: One proposed pathway. Mol. Biol. Rep. 2019, 46, 1757–1773. [Google Scholar] [CrossRef]
- Kumar, M.G.; Rowley, S.; Fulton, R.; Dinday, M.T.; Baraban, S.C.; Patel, M. Altered glycolysis and mitochondrial respiration in a zebrafish model of Dravet syndrome. ENeuro 2016, 3. [Google Scholar] [CrossRef]
- McDonald, T.S.; Carrasco-Pozo, C.; Hodson, M.P.; Borges, K. Alterations in cytosolic and mitochondrial [U-13c] glucose metabolism in a chronic epilepsy mouse model. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Foley, J.; Burnham, V.; Tedoldi, M.; Danial, N.N.; Yellen, G. BAD knockout provides metabolic seizure resistance in a genetic model of epilepsy with sudden unexplained death in epilepsy. Epilepsia 2018, 59, e1–e4. [Google Scholar] [CrossRef]
- Law, T.H.; Volk, H.A.; Pan, Y.; Zanghi, B.; Want, E.J. Metabolic perturbations associated with the consumption of a ketogenic medium-chain TAG diet in dogs with idiopathic epilepsy. Br. J. Nutr. 2018, 120, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Ibhazehiebo, K.; Gavrilovici, C.; de la Hoz, C.L.; Ma, S.-C.; Rehak, R.; Kaushik, G.; Meza Santoscoy, P.L.; Scott, L.; Nath, N.; Kim, D.-Y. A novel metabolism-based phenotypic drug discovery platform in zebrafish uncovers HDACs 1 and 3 as a potential combined anti-seizure drug target. Brain 2018, 141, 744–761. [Google Scholar] [CrossRef] [PubMed]
- Pena, I.A.; Roussel, Y.; Daniel, K.; Mongeon, K.; Johnstone, D.; Mendes, H.W.; Bosma, M.; Saxena, V.; Lepage, N.; Chakraborty, P. Pyridoxine-dependent epilepsy in zebrafish caused by Aldh7a1 deficiency. Genetics 2017, 207, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Jakkamsetti, V.; Marin-Valencia, I.; Ma, Q.; Good, L.B.; Terrill, T.; Rajasekaran, K.; Pichumani, K.; Khemtong, C.; Hooshyar, M.A.; Sundarrajan, C. Brain metabolism modulates neuronal excitability in a mouse model of pyruvate dehydrogenase deficiency. Sci. Transl. Med. 2019, 11, eaan0457. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Keller, J.N.; Morrison, C.D. Obesity and vulnerability of the CNS. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2009, 1792, 395–400. [Google Scholar] [CrossRef]
- Jordan, S.D.; Könner, A.C.; Brüning, J.C. Sensing the fuels: Glucose and lipid signaling in the CNS controlling energy homeostasis. Cell. Mol. Life Sci. 2010, 67, 3255–3273. [Google Scholar] [CrossRef]
- Daniels, Z.; Nick, T.; Liu, C.; Cassedy, A.; Glauser, T. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology 2009, 73, 658–664. [Google Scholar] [CrossRef]
- St-Pierre, L.; Bubenik, G.; Parker, G.; Persinger, M. Insidious weight gain in prepubertal seized rats treated with an atypical neuroleptic: The role of food consumption, fluid consumption, and spontaneous ambulatory activity. Epilepsy Behav. 2009, 14, 288–292. [Google Scholar] [CrossRef]
- Verrotti, A.; Manco, R.; Agostinelli, S.; Coppola, G.; Chiarelli, F. The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia 2010, 51, 268–273. [Google Scholar] [CrossRef]
- Petty, S.J.; Pack, A.M. Obesity and Epilepsy. Epilepsy Interictal State Co-Morb. Qual. Life 2015, 193–202. [Google Scholar]
- Yau, J.; Seckl, J. 11 [beta]-hydroxysteroid dehydrogenase type I in the brain; thickening the glucocorticoid soup. Mol. Psychiatry 2001, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, S.; Campana, G.; Vella, S.; Fortuna, A.; Galietta, G.; Guarino, I.; Costa, L.; Capasso, A.; Renzi, P.; Frajese, G.V. Post-natal stress-induced endocrine and metabolic alterations in mice at adulthood involve different pro-opiomelanocortin-derived peptides. Peptides 2010, 31, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Vargas, H.; Martínez-Ezquerro, J.D.; Bienvenu, T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch. Med Res. 2011, 42, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11β-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef]
- Singh, A.; Trevick, S. The epidemiology of global epilepsy. Neurol. Clin. 2016, 34, 837–847. [Google Scholar] [CrossRef]
- Remy, S.; Beck, H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2005, 129, 18–35. [Google Scholar] [CrossRef]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef]
- Holtzman, D.; Mulkern, R.; Meyers, R.; Cook, C.; Allred, E.; Khait, I.; Jensen, F.; Tsuji, M.; Laussen, P. In vivo phosphocreatine and ATP in piglet cerebral gray and white matter during seizures. Brain Res. 1998, 783, 19–27. [Google Scholar] [CrossRef]
- Young, R.S.; Osbakken, M.D.; Briggs, R.W.; Yagel, S.K.; Rice, D.W.; Goldberg, S. 31P NMR study of cerebral metabolism during prolonged seizures in the neonatal dog. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1985, 18, 14–20. [Google Scholar] [CrossRef]
- Holtzman, D.; Meyers, R.; Khait, I.; Jensen, F. Brain creatine kinase reaction rates and reactant concentrations during seizures in developing rats. Epilepsy Res. 1997, 27, 7–11. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Horyn, O.; Lazarow, A.; Nissim, I. Metabolism of brain amino acids following pentylenetetrazole treatment. Epilepsy Res. 2003, 53, 151–162. [Google Scholar] [CrossRef]
- Eid, T.; Thomas, M.; Spencer, D.; Runden-Pran, E.; Lai, J.; Malthankar, G.; Kim, J.; Danbolt, N.; Ottersen, O.; De Lanerolle, N. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 2004, 363, 28–37. [Google Scholar] [CrossRef]
- Petroff, O.A.; Errante, L.D.; Rothman, D.L.; Kim, J.H.; Spencer, D.D. Glutamate–glutamine cycling in the epileptic human hippocampus. Epilepsia 2002, 43, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Alvestad, S.; Hammer, J.; Eyjolfsson, E.; Qu, H.; Ottersen, O.P.; Sonnewald, U. Limbic structures show altered glial–neuronal metabolism in the chronic phase of kainate induced epilepsy. Neurochem. Res. 2008, 33, 257–266. [Google Scholar] [CrossRef]
- Mao, H.; Toufexis, D.; Wang, X.; Lacreuse, A.; Wu, S. Changes of metabolite profile in kainic acid induced hippocampal injury in rats measured by HRMAS NMR. Exp. Brain Res. 2007, 183, 477–485. [Google Scholar] [CrossRef]
- Greer, P.L.; Hanayama, R.; Bloodgood, B.L.; Mardinly, A.R.; Lipton, D.M.; Flavell, S.W.; Kim, T.-K.; Griffith, E.C.; Waldon, Z.; Maehr, R. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 2010, 140, 704–716. [Google Scholar] [CrossRef]
- Williams, C.A.; Driscoll, D.J.; Dagli, A.I. Clinical and genetic aspects of Angelman syndrome. Genet. Med. 2010, 12, 385–395. [Google Scholar] [CrossRef]
- Williams, C.A.; Beaudet, A.L.; Clayton-Smith, J.; Knoll, J.H.; Kyllerman, M.; Laan, L.A.; Magenis, R.E.; Moncla, A.; Schinzel, A.A.; Summers, J.A. Angelman syndrome 2005: Updated consensus for diagnostic criteria. Am. J. Med. Genet. Part A 2006, 140, 413–418. [Google Scholar] [CrossRef]
- Lossie, A.; Whitney, M.; Amidon, D.; Dong, H.; Chen, P.; Theriaque, D.; Hutson, A.; Nicholls, R.; Zori, R.; Williams, C. Distinct phenotypes distinguish the molecular classes of Angelman syndrome. J. Med. Genet. 2001, 38, 834–845. [Google Scholar] [CrossRef]
- Dunn, D.W.; Austin, J.K.; Harezlak, J.; Ambrosius, W.T. ADHD and epilepsy in childhood. Dev. Med. Child Neurol. 2003, 45, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Amelioration of cognitive deficit by embelin in a scopolamine-induced Alzheimer’s disease-like condition in a rat model. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J.; Leiendecker, B. GLUT1 deficiency syndrome–2007 update. Dev. Med. Child Neurol. 2007, 49, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J. GLUT1 deficiency syndrome in clinical practice. Epilepsy Res. 2012, 100, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.M.; Campistol, J.; Antonio Gil-Nagel, M. Epilepsy in inherited metabolic disorders. Neurology 2008, 14, S2–S14. [Google Scholar] [CrossRef]
- Kudin, A.P.; Zsurka, G.; Elger, C.E.; Kunz, W.S. Mitochondrial involvement in temporal lobe epilepsy. Exp. Neurol. 2009, 218, 326–332. [Google Scholar] [CrossRef]
- Ryan, K.; Backos, D.S.; Reigan, P.; Patel, M. Post-translational oxidative modification and inactivation of mitochondrial complex I in epileptogenesis. J. Neurosci. 2012, 32, 11250–11258. [Google Scholar] [CrossRef]
- Sorce, S.; Krause, K.-H. NOX enzymes in the central nervous system: From signaling to disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef]
- Chrissobolis, S.; Faraci, F.M. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol. Med. 2008, 14, 495–502. [Google Scholar] [CrossRef]
- Chung, S.; Wang, N.; Hank, N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure 2007, 16, 296–304. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.-i.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880. [Google Scholar] [CrossRef] [PubMed]
- He, R.Q.; Zeng, Q.Y.; Zhu, P.; Bao, Y.X.; Zheng, R.Y.; Xu, H.Q. Risk of seizure relapse after antiepileptic drug withdrawal in adult patients with focal epilepsy. Epilepsy Behav. EB 2016, 64, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, H.; McNally, K.A.; Vanderhill, S.D.; Paige, A.L.; Chung, R.; Davis, K.; Norden, A.D.; Stokking, R.; Studholme, C.; Novotny Jr, E.J. Positive and negative network correlations in temporal lobe epilepsy. Cereb. Cortex 2004, 14, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Manzo, L.; Castoldia, A.F.; Coccinia, T.; Rossia, A.D.; Nicoteraa, P.; Costaa, L.G. Mechanisms of neurotoxicity: Applications to human biomonitoring. Toxicol. Lett. 1995, 77, 63–72. [Google Scholar] [CrossRef]
- Visioli, F.; de Turco, E.R.; Bazan, N.G. Daily electroconvulsive shock treatment alters the inositol lipid system response in the rat hippocampus. Neurochem. Res. 1994, 19, 705–708. [Google Scholar] [CrossRef]
- Eraković, V.; Župan, G.; Varljen, J.; Laginja, J.; Simonić, A. Lithium plus pilocarpine induced status epilepticus—biochemical changes. Neurosci. Res. 2000, 36, 157–166. [Google Scholar] [CrossRef]
- Eraković, V.; Župan, G.; Varljen, J.; Simonić, A. Pentylenetetrazol-induced seizures and kindling: Changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem. Int. 2003, 42, 173–178. [Google Scholar] [CrossRef]
- Dubé, C.; Boyet, S.; Marescaux, C.; Nehlig, A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp. Neurol. 2001, 167, 227–241. [Google Scholar] [CrossRef]
- Volkow, N.; Tomasi, D.; Kojori, E.S.; Wiers, C.; Cabrera, E.; Lindgren, E.; Miller, G.; Kim, S.; Wang, G.-J. Metabolic functional connectivity. J. Nucl. Med. 2016, 57, 1796. [Google Scholar]
- Clifford, D.; Olney, J.; Maniotis, A.; Collins, R.; Zorumski, C. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience 1987, 23, 953–968. [Google Scholar] [CrossRef]
- Papetti, L.; Parisi, P.; Leuzzi, V.; Nardecchia, F.; Nicita, F.; Ursitti, F.; Marra, F.; Paolino, M.C.; Spalice, A. Metabolic epilepsy: An update. Brain Dev. 2013, 35, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.A.; Singh, M.A.F. Effects of exercise on adiponectin: A systematic review. Obesity 2008, 16, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.I.; Bast, T.; Surtees, R. Epilepsy in inborn errors of metabolism. Epileptic Disord. 2005, 7, 67–81. [Google Scholar] [PubMed]
- Ahima, R.S. Metabolic actions of adipocyte hormones: Focus on adiponectin. Obesity 2006, 14. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castro, C.; Fu, Y.; Chung, B.H.; Garvey, W.T. Adiponectin and the metabolic syndrome: Mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007, 18, 263–270. [Google Scholar] [CrossRef]
- Lehesjoki, A.E. Molecular background of progressive myoclonus epilepsy. Embo J. 2003, 22, 3473–3478. [Google Scholar] [CrossRef]
- Minassian, B.A.; Lee, J.R.; Herbrick, J.-A.; Huizenga, J.; Soder, S.; Mungall, A.J.; Dunham, I.; Gardner, R.; Chung-yan, G.F.; Carpenter, S. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat. Genet. 1998, 20, 171–174. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Tryptophan–kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: The serotonin hypothesis revisited 40 years later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56. [Google Scholar]
- Pennacchio, L.A.; Bouley, D.M.; Higgins, K.M.; Scott, M.P.; Noebels, J.L.; Myers, R.M. Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nat. Genet. 1998, 20, 251–258. [Google Scholar] [CrossRef]
- Thom, M.; Bertram, E.H. Temporal lobe epilepsy. Handb. Clin. Neurol. 2012, 107, 225–240. [Google Scholar]
- Tagliabracci, V.S.; Girard, J.M.; Segvich, D.; Meyer, C.; Turnbull, J.; Zhao, X.; Minassian, B.A.; DePaoli-Roach, A.A.; Roach, P.J. Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J. Biol. Chem. 2008, 283, 33816–33825. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, F.; Wang, P.; Schmieder, P.; Girard, J.-M.; Awrey, D.E.; Wang, T.; Israelian, J.; Zhao, X.; Turnbull, J.; Heydenreich, M. Hyperphosphorylation of glucosyl C6 carbons and altered structure of glycogen in the neurodegenerative epilepsy Lafora disease. Cell Metab. 2013, 17, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C. Chemistry and function of phospholipids. Food Lipids Chem. Nutr. Biotechnol. 2008, 39–62. [Google Scholar]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef]
- Klein, J. Membrane breakdown in acute and chronic neurodegeneration: Focus on choline-containing phospholipids. J. Neural Transm. 2000, 107, 1027–1063. [Google Scholar] [CrossRef]
- Danner, N.; Julkunen, P.; Khyuppenen, J.; Hukkanen, T.; Könönen, M.; Säisänen, L.; Koskenkorva, P.; Vanninen, R.; Lehesjoki, A.-E.; Kälviäinen, R. Altered cortical inhibition in Unverricht–Lundborg type progressive myoclonus epilepsy (EPM1). Epilepsy Res. 2009, 85, 81–88. [Google Scholar] [CrossRef]
- Berkovic, S.F.; Mazarib, A.; Walid, S.; Neufeld, M.Y.; Manelis, J.; Nevo, Y.; Korczyn, A.D.; Yin, J.; Xiong, L.; Pandolfo, M. A new clinical and molecular form of Unverricht–Lundborg disease localized by homozygosity mapping. Brain 2005, 128, 652–658. [Google Scholar] [CrossRef]
- Chew, N.K.; Mir, P.; Edwards, M.J.; Cordivari, C.; Martino, D.; Schneider, S.A.; Kim, H.T.; Quinn, N.P.; Bhatia, K.P. The natural history of Unverricht-Lundborg disease: A report of eight genetically proven cases. Mov. Disord. 2008, 23, 107–113. [Google Scholar] [CrossRef]
- Shannon, P.; Pennacchio, L.A.; Houseweart, M.K.; Minassian, B.A.; Myers, R.M. Neuropathological changes in a mouse model of progressive myoclonus epilepsy: Cystatin B deficiency and Unverricht-Lundborg disease. J. Neuropathol. Exp. Neurol. 2002, 61, 1085–1091. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47. [Google Scholar] [CrossRef]
- Jiang, M.-C.; Ni, J.-J.; Cui, W.-Y.; Wang, B.-Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354. [Google Scholar] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Semple, B.D.; Jones, N.C.; Othman, I.; Shaikh, M.F. High mobility group box 1 (HMGB 1) as a novel frontier in epileptogenesis: From pathogenesis to therapeutic approaches. J. Neurochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M.F. Implication of HMGB1 signaling pathways in Amyotrophic lateral sclerosis (ALS): From molecular mechanisms to pre-clinical results. Pharmacol. Res. 2020, 104792. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 172487. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Shaikh, M.F.; Shah, S.; Kumari, Y.; Othman, I. Role of inflammation in epilepsy and neurobehavioral comorbidities: Implication for therapy. Eur. J. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Webster, K.M.; Sun, M.; Crack, P.; O’Brien, T.J.; Shultz, S.R.; Semple, B.D. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflammation 2017, 14, 10. [Google Scholar] [CrossRef]
- Brady, R.D.; Casillas-Espinosa, P.M.; Agoston, D.V.; Bertram, E.H.; Kamnaksh, A.; Semple, B.D.; Shultz, S.R. Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol. Dis. 2019, 123, 8–19. [Google Scholar] [CrossRef]
- Englander, J.; Cifu, D.X.; Diaz-Arrastia, R.; Center, M.S.K.T. Seizures after traumatic brain injury. Arch. Phys. Med. Rehabil. 2014, 95, 1223. [Google Scholar] [CrossRef]
- Dravet, C. Dravet syndrome history. Dev. Med. Child Neurol. 2011, 53, 1–6. [Google Scholar] [CrossRef]
- Marini, C.; Scheffer, I.E.; Nabbout, R.; Suls, A.; De Jonghe, P.; Zara, F.; Guerrini, R. The genetics of Dravet syndrome. Epilepsia 2011, 52, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.S.; Hallböök, T.; Reilly, C. Intellectual functioning and behavior in Dravet syndrome: A systematic review. Epilepsy Behav. 2020, 108, 107079. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain energy and oxygen metabolism: Emerging role in normal function and disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C.; Dinday, M.T.; Hortopan, G.A. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Lutas, A.; Yellen, G. The ketogenic diet: Metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013, 36, 32–40. [Google Scholar] [CrossRef]
- Engel Jr, J. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996, 26, 141–150. [Google Scholar] [CrossRef]
- Téllez-Zenteno, J.F.; Hernández-Ronquillo, L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res. Treat. 2012. [Google Scholar] [CrossRef]
- Smeland, O.B.; Hadera, M.G.; McDonald, T.S.; Sonnewald, U.; Borges, K. Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J. Cereb. Blood Flow Metab. 2013, 33, 1090–1097. [Google Scholar] [CrossRef]
- Strasser, A.; O’Connor, L.; Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 2000, 69, 217–245. [Google Scholar] [CrossRef]
- Martínez-François, J.R.; Fernández-Agüera, M.C.; Nathwani, N.; Lahmann, C.; Burnham, V.L.; Danial, N.N.; Yellen, G. BAD and KATP channels regulate neuron excitability and epileptiform activity. Elife 2018, 7, e32721. [Google Scholar] [CrossRef]
- Verdura, E.; Fons, C.; Schlüter, A.; Ruiz, M.; Fourcade, S.; Casasnovas, C.; Castellano, A.; Pujol, A. Complete loss of KCNA1 activity causes neonatal epileptic encephalopathy and dyskinesia. J. Med. Genet. 2020, 57, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R. Antiepileptic drugs and developmental neuroendocrine dysfunction: Every why has A Wherefore. Arch. Med. 2017, 9. [Google Scholar] [CrossRef]
- Schachter, S.C. Antiseizure Drugs: Mechanism of Action, Pharmacology, and Adverse Effects. Available online: http://www.uptodate.com (accessed on 21 October 2017).
- Canevini, M.P.; De Sarro, G.; Galimberti, C.A.; Gatti, G.; Licchetta, L.; Malerba, A.; Muscas, G.; La Neve, A.; Striano, P.; Perucca, E. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia 2010, 51, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Mercimek-Mahmutoglu, S.; Cordeiro, D.; Cruz, V.; Hyland, K.; Struys, E.A.; Kyriakopoulou, L.; Mamak, E. Novel therapy for pyridoxine dependent epilepsy due to ALDH7A1 genetic defect: L-arginine supplementation alternative to lysine-restricted diet. Eur. J. Paediatr. Neurol. 2014, 18, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P. Pyridoxine-dependent and pyridoxine-responsive seizures. Dev. Med. Child Neurol. 2001, 43, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
| S.N. | Model Type | Study Type | Study Sample | Animal/Subject Used for Model Design | A Drug or Technique Used for Modeling Metabolic Epilepsy (ME) | Outcomes | No. of Citations | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Metabolic gene responsible for epilepsy in obese rat | Pre-clinical (animal model) | n = 45 Groups—24 h (n = 5), 10 days (n = 5), 1month (n = 5) and 2 months (n = 5). | SD rats, 30–35 days old (125–150 g), male rats. | 4% Pilocarpine hydrochloride (350 mg/kg in saline, i.p.), methyl-scopolamine prior to Pilocarpine Injection. |
| 6 | [33] |
| 2 | Metabolic profiling of epileptic rat brain (PTZ kindling induced seizures) Type: Creatine disorders and Succinic semialdehyde dehydrogenase deficiency | Pre-clinical (animal model) | n = 10 | Male Wistar rats, group (a and b) were 4 weeks old and group (c) were 9 weeks. | PTZ (37 mg/kg body weight) every 48 h or every 72 h at the weekend over a 5-week period |
| 25 | [34] |
| 3 | Mutation of ubiquitin ligase (Ube3a phenotype) causes Angelman syndrome in mice—a rare genetic epileptic neurodegeneration. Type: disruption of UBE3A, Mitochondrial disorders | Pre-clinical (animal model) |
|
| 8 and 12 weeks of age 12–16 weeks of age. |
| 608 | [35,36] |
| 4 | Metabolic features in repetitive seizures. Type: Mitochondrial disorders | Pre-clinical (animal model) | (n = 7 immature; n = 6 mature) | Immature animals: SD rat pups (P15) Mature animals: male Sprague–Dawley rats (P60). |
|
| 10 | [23] |
| 5 | Glut1 gene deficiency in mouse model. Type: GLUT-1 deficiency | Pre-clinical (animal model) | n = 104 | G1D transgenic antisense mice 3 and 5 months of age | G1D gene knockdown to produce Glut1 deficiency |
| 16 | [37] |
| 6 | Mitochondrial respiration deficits in rat epilepsy model Type: Mitochondrial disorders | Pre-clinical (animal model) | n = 4–8 in each group | Adult male SD rats (300–350 g) | KA (11 mg/kg, s.c.). |
| 16 | [38] |
| 7 | Abnormal metabolic function in the Pilocarpine-induced epilepsy rat model. Type: Mitochondrial disorders | Pre-clinical (animal model) | Adult male SD rats (7 weeks old), weighing 180–200 g |
|
| 13 | [39] | |
| 8 | Metabolic dysfunction via adiponectin deficiency. Type: adiponectin -responsive seizures (Mitochondrial disorders) | Pre-clinical (animal model) |
|
| KA-Induced Seizure |
| 26 | [40] |
| 9 | Myoclonus Epilepsy: impairment of serotonin (5HT) and 3-Hydroxyanthranilic Acid metabolism. Type: adiponectin-Responsive seizures (Mitochondrial disorders) | Pre-clinical (animal model) and Clinical study (Human Subjects) | n = 4 (mice) Unverricht- Lundborg type (EPM1) diagnosed human patients n = 2 |
| Valporic acid induced metabolic disturbances in myclonus epilepsy |
| 8 | [41] |
| 10 | Model for metabolic dysfunction during epileptic seizure in Pilocarpine treated rats Type: Mitochondrial disorders | Pre-clinical (animal model) and Clinical study (Human subjects) |
|
|
|
| 104 | [42] |
| 11 | Lafora disease—altered glycogen metabolism causing epilepsy. Type: Laforin or malin deficency | Pre-clinical (animal model) In-vitro study | n = 3–8 genotype |
| Genetic knock down Epm2a−/−/Gys1+/+ are labeled as LKO mice model and Epm2a−/−/Gys1+/+ knock down are labelled as DKO experimental mice |
| 34 | [43] |
| 12 | Phospholipid metabolic disorders- corazolum- induced seizures. Type: Phospholipid dysfunctioning | Pre-clinical (animal model) | n = 50 | Male albino rats weighing 180–200 g, | Single intramuscularinjections of corazolum (dose, 8–9 mg peranimal), sodium thiosulfate (1 mg per animal), and vitamin E (0.4 mg per animal) to produce corazolum- induced seizures. |
| 0 | [44] |
| 13 | Animal model for altered tryptophan metabolismin causing myoclonus Epilepsy. Type: Glutaric Aciduria | Pre-clinical (animal model) | n = 3 | 5-month-old mice homozygous. for a disruption in the Cstb gene (Cstb−/−, 129SvJ strain | By disruption in the Cstb gene |
| 14 | [45] |
| 14 | long noncoding RNAs cancer susceptibility candidate 2 (lncRNA CASC2) inhibits astrocytic activation and adenosine metabolism | Pre-clinical (animal model) | 5 group n = 12 | Male SD rats (200−220 g). | LncRNA CASC2 suppression in PTZ induced rats. |
| 1 | [46] |
| 15 | HMGB1 modulates glutamate metabolism in KA induced seizures | Pre-clinical (animal model) | Neuronal cell culture plate—Cells (4 × 104 cells/well) | Primary rat neural cells (PRNCs)—BrainBit (E18 rat cortex) | KA—10 μM |
| 13 | [47] |
| 16 | Lipid metabolism altered in post-traumatic epileptic rat model | Pre-clinical (animal model) | 2 groups (n = 10; n represents the number) | Six months old male Wistar rats, weighing 350–400 g | ferric chloride (FeCl3) to cause post-traumatic epilepsy (PTE). |
| 4 | [48] |
| 17 | Altered glycolysis and mitochondrial respiration in a zebrafish model of Dravet Syndrome | Pre-clinical (animal model) | 96 plate well | Scn1Lab mutant zebrafish (HM/WT), 5dfp | voltage-gated sodium channel-1A_Lab mutation (SCN1A_Lab) |
| 24 | [49] |
| 18 | Alterations in cytosolic and mitochondrial [U-13C] glucose metabolism in a chronic epilepsy mouse model | Pre-clinical (animal model) | n = 10–12 group -2 | Male CD1 mice | Pilocarpine induced status epilepticus (SE) model |
| 9 | [50] |
| 19 | BAD KO provides metabolic seizure resistance in a genetic model of epilepsy with SUDEP | Pre-clinical (animal model) | Male and female Kcna1−/− (n = 29; 10 female, 19 male) and Kcna1−/− Bad−/− (n = 15; 10 female, 5 male) mice | Kcna1−/− mice | BCL2-associated agonist of cell death (BAD)—Kcna1−/− mice |
| 6 | [51] |
| 20 | Metabolic perturbations associated with the consumption of a ketogenic medium-chain TAG diet in dogs with idiopathic epilepsy | Pre-clinical (animal model) | Male n = 10 and female n = 6 dogs Avg. weight 29.3 kg Avg. year 4.59 years old | 21 dogs with idiopathic epilepsy of different breed | Idiopathic epilepsy in dogs |
| 8 | [52] |
| 21 | A novel metabolism-based zebrafish model to uncovers HDACs 1 and 3 as a potential combined anti-seizure drug target: | Pre-clinical (animal model) | Zebrafish larvae, Kcna1-null mice | 5–7dpf 96 plate well, wild-type zebrafish (TL strain) Kcna1-null mice | Kcna1-null mice, PTZ induced zebrafish model. |
| 15 | [53] |
| 22 | Pyridoxine-dependent epilepsy in zebrafish caused by Aldh7a1 deficiency | Pre-clinical (animal model) | Zebrafish larvae | Zebrafish larvae 5–14dpf | Aldh7a1-null mutation, pyridoxin dependent epilepsy |
| 32 | [54] |
| 23 | Pyruvate dehydrogenase deficiency in mouse model | Pre-clinical (animal model) Clinical data | Human blood sample mouse model of (PDHD) | Zebrafish larvae and Pdha1 KO mouse 2–3 months old | Pdha1 knockdown mouse model (PDHD) |
| 3 | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundap, U.P.; Paudel, Y.N.; Shaikh, M.F. Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review. Pharmaceuticals 2020, 13, 106. https://doi.org/10.3390/ph13060106
Kundap UP, Paudel YN, Shaikh MF. Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review. Pharmaceuticals. 2020; 13(6):106. https://doi.org/10.3390/ph13060106
Chicago/Turabian StyleKundap, Uday Praful, Yam Nath Paudel, and Mohd. Farooq Shaikh. 2020. "Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review" Pharmaceuticals 13, no. 6: 106. https://doi.org/10.3390/ph13060106
APA StyleKundap, U. P., Paudel, Y. N., & Shaikh, M. F. (2020). Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review. Pharmaceuticals, 13(6), 106. https://doi.org/10.3390/ph13060106







