A New Phage Lysin Isolated from the Oral Microbiome Targeting Streptococcus pneumoniae
Abstract
1. Introduction
2. Results
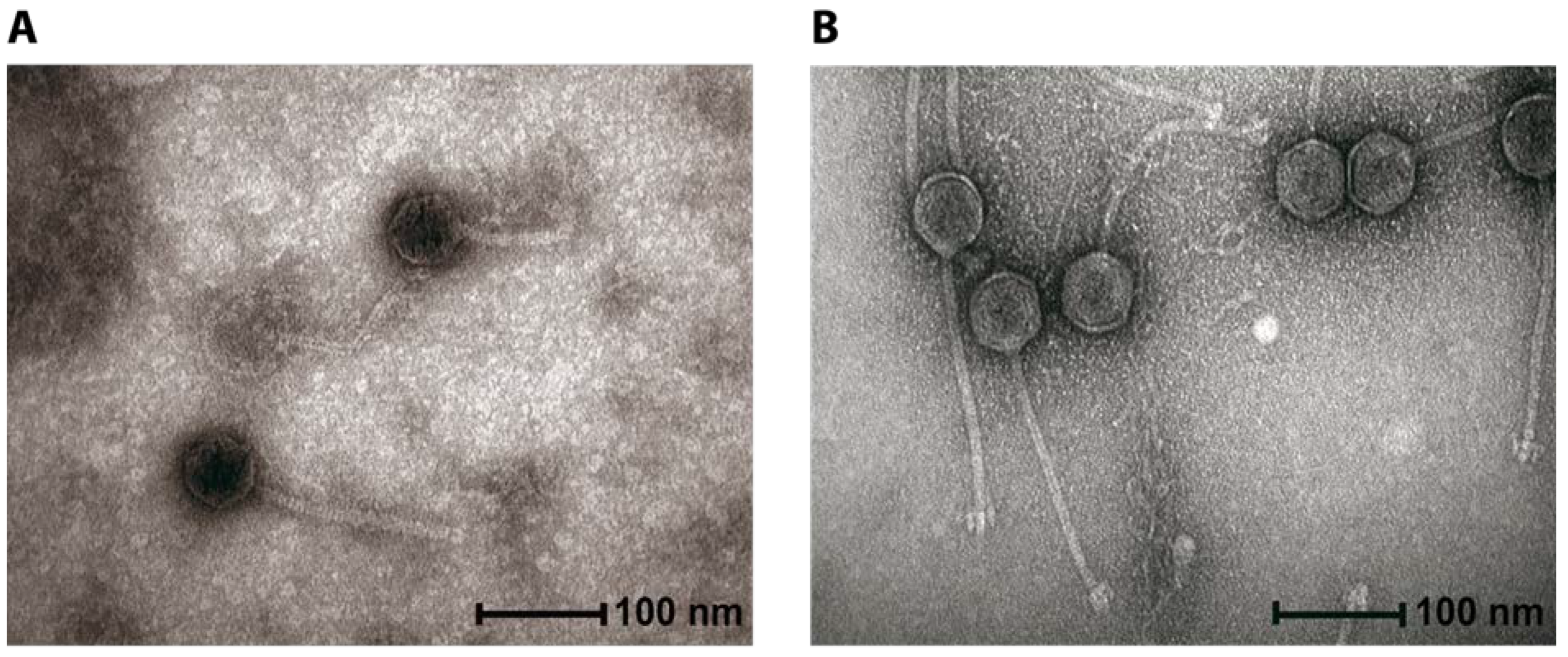
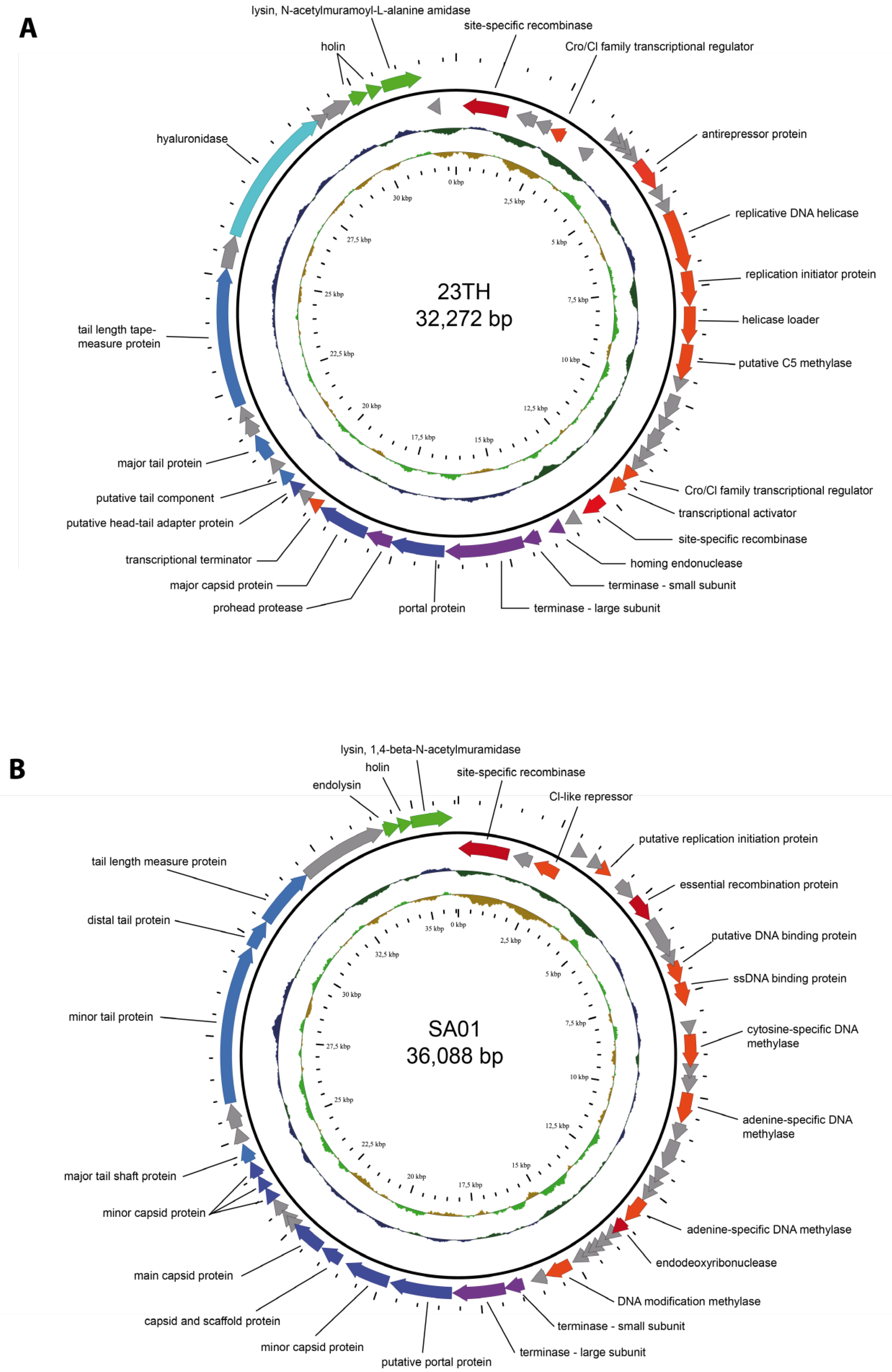
2.1. General Phage Characteristics, Genomic and Phylogenetic Analysis
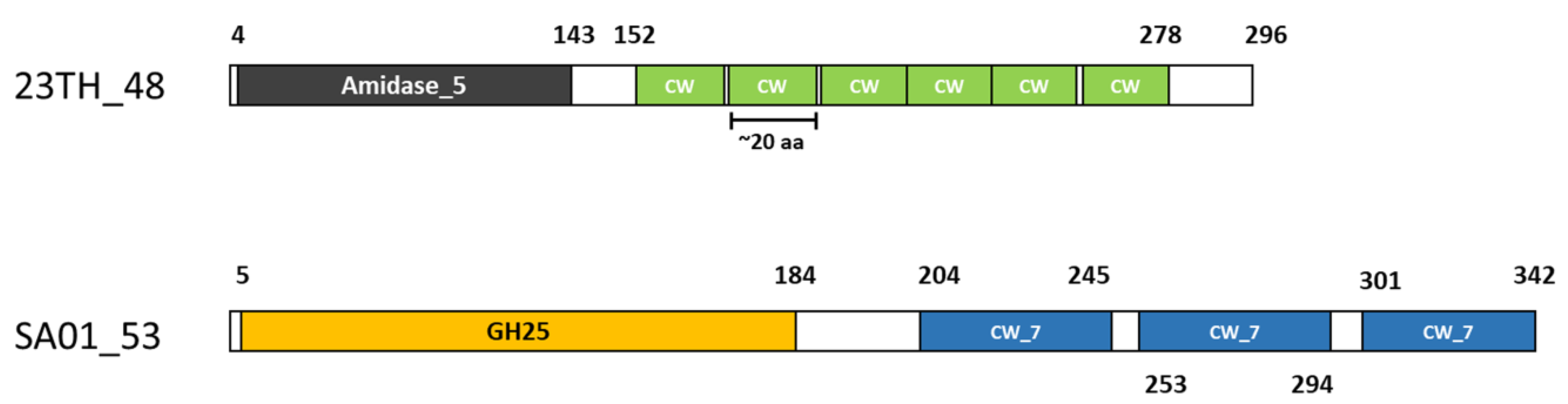
2.2. Endolysins of Phages 23TH and SA01
2.3. Cloning and Expression of Lysins
2.4. Endolysin Host Range
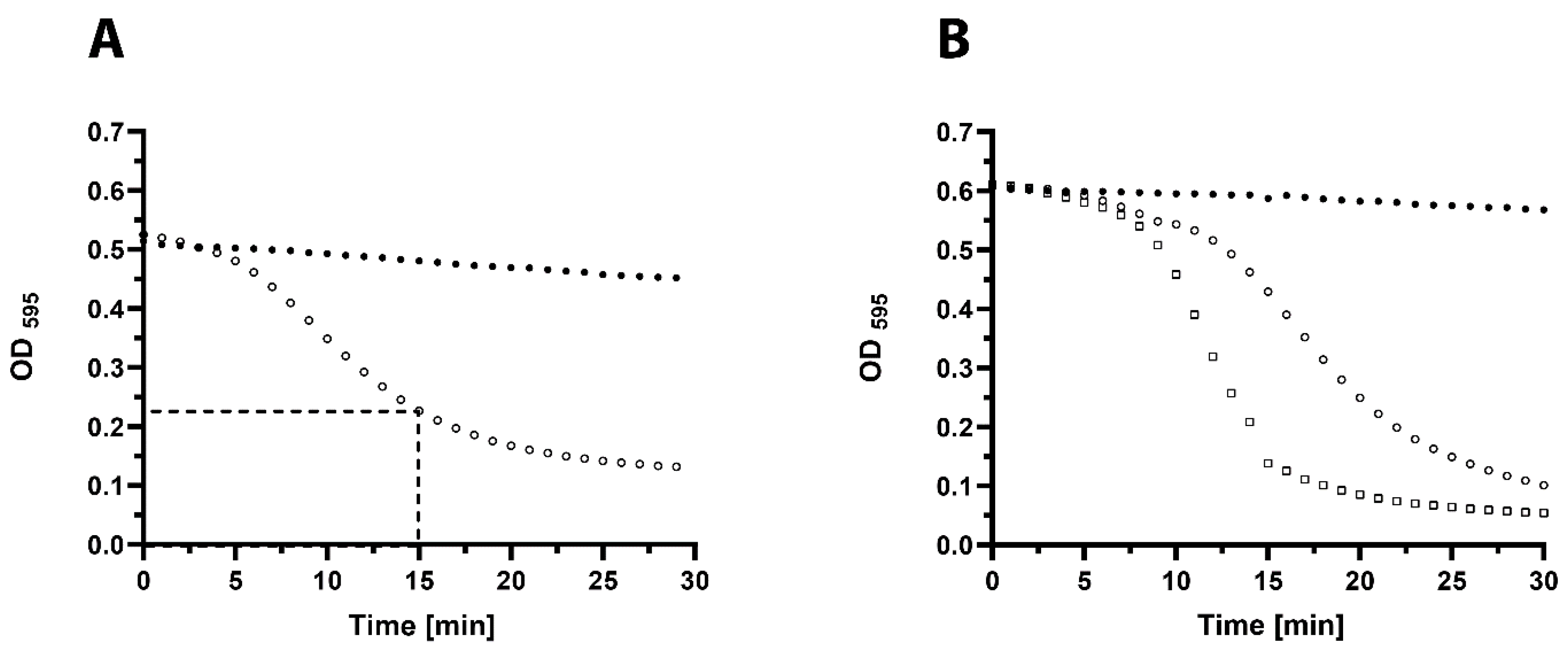
2.5. In Vitro Activity of 23TH_48
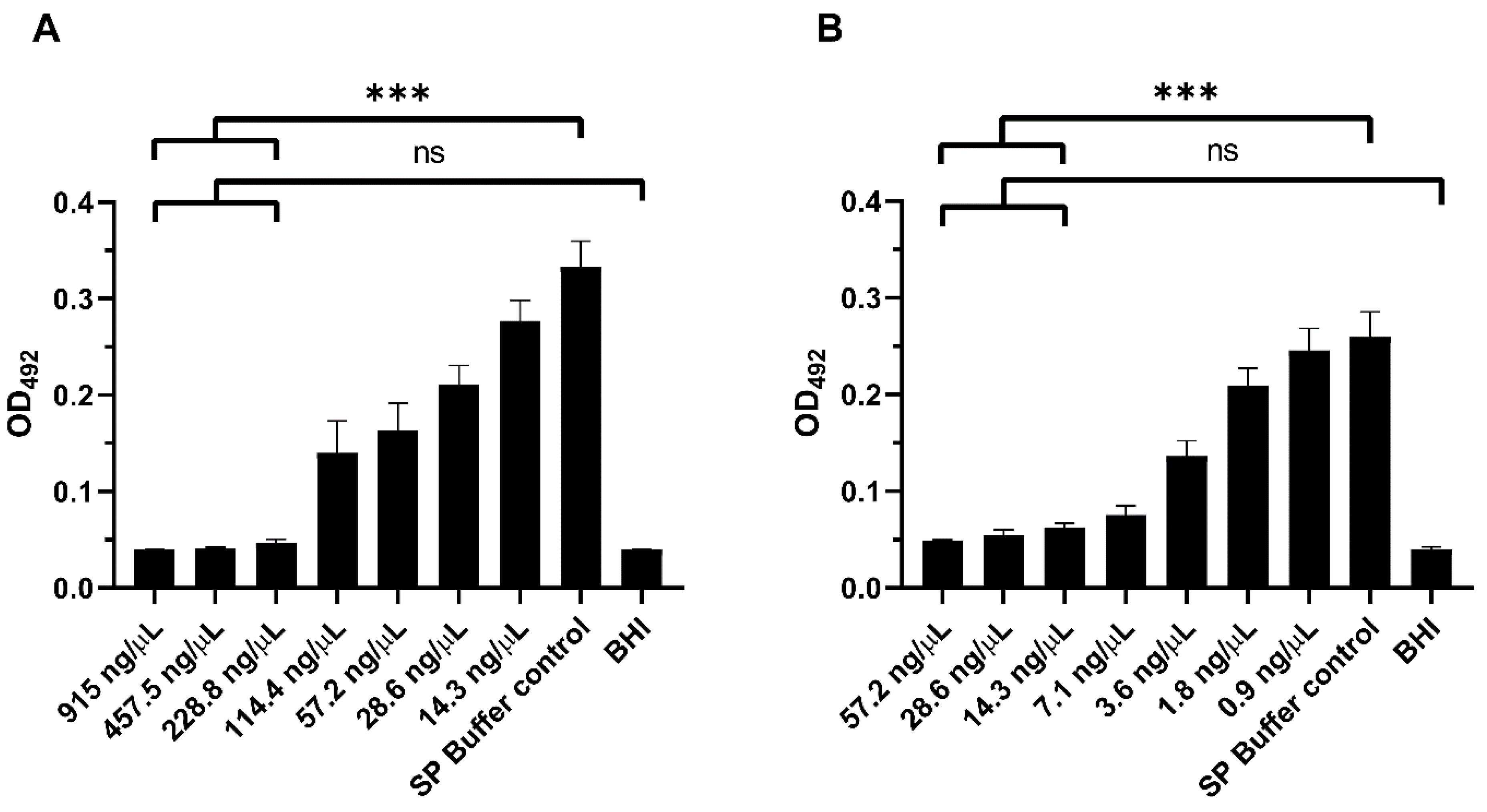
2.6. In Vitro Biofilm Assays
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Plaque Assays and Phage Propagation
4.3. Efficiency of Lysogeny
4.4. CsCl Purification of Phages for Transmission Electron Microscopy
4.5. Viral DNA Extraction, Amplification, Library Preparation and Sequencing
4.6. Bioinformatic Analysis of Genomes
4.7. Cloning of Lysin Genes
4.8. Expression and Purification
4.9. Screening for Lytic Activity and Host Range of Lysins
4.10. In Vitro Quantification of Lysin 23TH_48 Activity Against S. pneumoniae
4.11. Biofilm Assays S. pneumoniae R6
4.12. Statistical Analysis
4.13. Accession Number
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global Burden of Childhood Pneumonia and Diarrhoea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef]
- World Health Organization. Top 10 Causes of Death. Available online: https://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/ (accessed on 16 March 2020).
- Rodrigues, C.M.C.; Groves, H. Community-Acquired Pneumonia in Children: The Challenges of Microbiological Diagnosis. J. Clin. Microbiol. 2017, 56. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Klugman, K.P. The Significance of Serotype Replacement for Pneumococcal Disease and Antibiotic Resistance. In Hot Topics in Infection and Immunity in Children V; Springer: New York, NY, USA, 2009; pp. 121–128. [Google Scholar] [CrossRef]
- Keller, L.E.; Robinson, D.A.; McDaniel, L.S. Nonencapsulated Streptococcus Pneumoniae: Emergence and Pathogenesis. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Cherazard, R.; Epstein, M.; Doan, T.-L.; Salim, T.; Bharti, S.; Smith, M.A. Antimicrobial Resistant Streptococcus Pneumoniae. Am. J. Ther. 2017, 24, e361–e369. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V. Development of Phage Lysins as Novel Therapeutics: A Historical Perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. The Recombinant Phage Lysin LysK Has a Broad Spectrum of Lytic Activity against Clinically Relevant Staphylococci, Including Methicillin-Resistant Staphylococcus Aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef]
- Nelson, D.; Schuch, R.; Chahales, P.; Zhu, S.; Fischetti, V.A. PlyC: A Multimeric Bacteriophage Lysin. Proc. Natl. Acad. Sci. USA 2006, 103, 10765–10770. [Google Scholar] [CrossRef]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In Vitro Characterization of PlySK1249, a Novel Phage Lysin, and Assessment of Its Antibacterial Activity in a Mouse Model of Streptococcus Agalactiae Bacteremia. Antimicrob. Agents Chemother. 2013, 6276–6283. [Google Scholar] [CrossRef]
- Briers, Y.; Volckaert, G.; Cornelissen, A.; Lagaert, S.; Michiels, C.W.; Hertveldt, K.; Lavigne, R. Muralytic Activity and Modular Structure of the Endolysins of Pseudomonas Aeruginosa Bacteriophages? KZ and EL. Mol. Microbiol. 2007, 65, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel Phage Lysin Capable of Killing the Multidrug-Resistant Gram-Negative Bacterium Acinetobacter Baumannii in a Mouse Bacteremia Model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.; García, P. Synergy Between Two Chimeric Lysins to Kill Streptococcus Pneumoniae. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, N.; Campillo, N.E.; García, E.; Gallego, C.; Pera, B.; Diakun, G.P.; Sáiz, J.L.; García, P.; Fernando Díaz, J.; Menéndez, M. Cpl-7, a Lysozyme Encoded by a Pneumococcal Bacteriophage with a Novel Cell Wall-Binding Motif. J. Biol. Chem. 2010, 285, 33184–33196. [Google Scholar] [CrossRef]
- Bustamante, N.; Iglesias-Bexiga, M.; Bernardo-García, N.; Silva-Martín, N.; García, G.; Campanero-Rhodes, M.A.; García, E.; Usón, I.; Buey, R.M.; García, P.; et al. Deciphering How Cpl-7 Cell Wall-Binding Repeats Recognize the Bacterial Peptidoglycan. Sci. Rep. 2017, 7, 16494. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Djurkovic, S.; Fischetti, V.A. Phage Lytic Enzyme Cpl-1 as a Novel Antimicrobial for Pneumococcal Bacteremia. Infect. Immun. 2003, 71, 6199–6204. [Google Scholar] [CrossRef]
- Domenech, M.; García, E.; Moscoso, M.; Garciá, E.; Moscoso, M. In Vitro Destruction of Streptococcus Pneumoniae Biofilms with Bacterial and Phage Peptidoglycan Hydrolases. Antimicrob. Agents Chemother. 2011, 55, 4144–4148. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid Killing of Streptococcus Pneumoniae with a Bacteriophage Cell Wall Hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef]
- Vázquez, R.; García, E.; García, P. Phage Lysins for Fighting Bacterial Respiratory Infections: A New Generation of Antimicrobials. Front. Immunol. 2018. [Google Scholar] [CrossRef]
- NCBI Genome Information. S. anginosus. Available online: https://www.ncbi.nlm.nih.gov/genome/browse/#!/prokaryotes/2604/ (accessed on 4 September 2020).
- Brueggemann, A.B.; Harrold, C.L.; Rezaei Javan, R.; Van Tonder, A.J.; McDonnell, A.J.; Edwards, B.A. Pneumococcal Prophages Are Diverse, but Not without Structure or History. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- NCBI Genome Information. S. infantis. Available online: https://www.ncbi.nlm.nih.gov/genome/browse/#!/prokaryotes/3041/ (accessed on 4 September 2020).
- Dalmasso, M.; de Haas, E.; Neve, H.; Strain, R.; Cousin, F.J.; Stockdale, S.R.; Ross, R.P.; Hill, C. Isolation of a Novel Phage with Activity against Streptococcus Mutans Biofilms. PLoS ONE 2015, 10, e0138651. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Fischetti, V.A. Mutagenesis of a Bacteriophage Lytic Enzyme PlyGBS Significantly Increases Its Antibacterial Activity against Group B Streptococci. Appl. Microbiol. Biotechnol. 2007, 74, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Gaeng, S.; Scherer, S.; Neve, H.; Loessner, M.J. Gene Cloning and Expression and Secretion of Listeria Monocytogenes Bacteriophage-Lytic Enzymes in Lactococcus Lactis. Appl. Environ. Microbiol. 2000, 66, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R.C. Structure and Lytic Activity of a Bacillus Anthracis Prophage Endolysin. J. Biol. Chem. 2005, 280, 35433–35439. [Google Scholar] [CrossRef] [PubMed]
- Sass, P.; Bierbaum, G. Lytic Activity of Recombinant Bacteriophage Φ11 and Φ12 Endolysins on Whole Cells and Biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007, 73, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Kramer, K.; Ebel, F.; Scherer, S. C-Terminal Domains of Listeria Monocytogenes Bacteriophage Murein Hydrolases Determine Specific Recognition and High-Affinity Binding to Bacterial Cell Wall Carbohydrates. Mol. Microbiol. 2002, 44, 335–349. [Google Scholar] [CrossRef]
- Zimmer, M.; Vukov, N.; Scherer, S.; Loessner, M.J. The Murein Hydrolase of the Bacteriophage Φ3626 Dual Lysis System Is Active against All Tested Clostridium Perfringens Strains. Appl. Environ. Microbiol. 2002, 68, 5311–5317. [Google Scholar] [CrossRef]
- Maestro, B.; Sanz, J.M. Choline Binding Proteins from Streptococcus Pneumoniae: A Dual Role as Enzybiotics and Targets for the Design of New Antimicrobials. Antibiotics 2016, 5, 21. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct Detection of Bacterial Biofilms on the Middle-Ear Mucosa of Children with Chronic Otitis Media. J. Am. Med. Assoc. 2006, 296, 202–211. [Google Scholar] [CrossRef]
- Coates, H.; Thornton, R.; Langlands, J.; Filion, P.; Keil, A.D.; Vijayasekaran, S.; Richmond, P. The Role of Chronic Infection in Children with Otitis Media with Effusion: Evidence for Intracellular Persistence of Bacteria. Otolaryngol. Head Neck Surg. 2008, 138, 778–781. [Google Scholar] [CrossRef]
- Sanderson, A.R.; Leid, J.G.; Hunsaker, D. Bacterial Biofilms on the Sinus Mucosa of Human Subjects with Chronic Rhinosinusitis. Laryngoscope 2006, 116, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; García, E.; Moscoso, M. Biofilm Formation in Streptococcus Pneumoniae. Microbial Biotechnol. 2012, 455–465. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; Karlström, Å.; Iverson, A.R.; Loeffler, J.M.; Fischetti, V.A. Novel Strategy to Prevent Otitis Media Caused by Colonizing Streptococcus Pneumoniae. PLoS Pathog. 2007, 3, e28. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to Virus Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Díez-Martínez, R.; De Paz, H.; Bustamante, N.; García, E.; Menéndez, M.; García, P. Improving the Lethal Effect of Cpl-7, a Pneumococcal Phage Lysozyme with Broad Bactericidal Activity, by Inverting the Net Charge of Its Cell Wall-Binding Module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef] [PubMed]
- Pohane, A.A.; Patidar, N.D.; Jain, V. Modulation of Domain-Domain Interaction and Protein Function by a Charged Linker: A Case Study of Mycobacteriophage D29 Endolysin. FEBS Lett. 2015, 589, 695–701. [Google Scholar] [CrossRef]
- Schmelcher, M.; Tchang, V.S.; Loessner, M.J. Domain Shuffling and Module Engineering of Listeria Phage Endolysins for Enhanced Lytic Activity and Binding Affinity. Microb. Biotechnol. 2011, 4, 651–662. [Google Scholar] [CrossRef]
- Gräslund, S.; Nordlund, P.; Weigelt, J.; Hallberg, B.M.; Bray, J.; Gileadi, O.; Knapp, S.; Oppermann, U.; Arrowsmith, C.; Hui, R.; et al. Protein Production and Purification. Nat. Methods 2008, 5, 135–146. [Google Scholar] [CrossRef]
- Pimenta, F.; Gertz, R.E.; Park, S.H.; Kim, E.; Moura, I.; Milucky, J.; Rouphael, N.; Farley, M.M.; Harrison, L.H.; Bennett, N.M.; et al. Streptococcus infantis, Streptococcus Mitis, and Streptococcus Oralis Strains with Highly Similar Cps5 Loci and Antigenic Relatedness to Serotype 5 Pneumococci. Front. Microbiol. 2019, 9, 3199. [Google Scholar] [CrossRef]
- Garcia, J.L.; Diaz, E.; Romero, A.; Garcia, P. Carboxy-Terminal Deletion Analysis of the Major Pneumococcal Autolysin. J. Bacteriol. 1994, 176, 4066–4072. [Google Scholar] [CrossRef]
- Romero, P.; López, R.; García, E. Key Role of Amino Acid Residues in the Dimerization and Catalytic Activation of the Autolysin LytA, an Important Virulence Factor in Streptococcus Pneumoniae. J. Biol. Chem. 2007, 282, 17729–17737. [Google Scholar] [CrossRef] [PubMed]
- Díez-Martínez, R.; De Paz, H.D.; García-Fernández, E.; Bustamante, N.; Euler, C.W.; Fischetti, V.A.; Menendez, M.; García, P. A Novel Chimeric Phage Lysin with High in Vitro and in Vivo Bactericidal Activity against Streptococcus Pneumoniae. J. Antimicrob. Chemother. 2014, 70, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, B.; Fresco-Taboada, A.; Iglesias-Bexiga, M.; Menéndez, M.; García, P. PL3 Amidase, a Tailor-Made Lysin Constructed by Domain Shuffling with Potent Killing Activity against Pneumococci and Related Species. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.M.; Fischetti, V.A. Synergistic Lethal Effect of a Combination of Phage Lytic Enzymes with Different Activities on Penicillin-Sensitive and -Resistant Streptococcus Pneumoniae Strains. Antimicrob. Agents Chemother. 2003, 47, 375–377. [Google Scholar] [CrossRef]
- Jado, I. Phage Lytic Enzymes as Therapy for Antibiotic-Resistant Streptococcus Pneumoniae Infection in a Murine Sepsis Model. J. Antimicrob. Chemother. 2003, 52, 967–973. [Google Scholar] [CrossRef]
- Rodríguez-Cerrato, V.; García, P.; del Prado, G.; García, E.; Gracia, M.; Huelves, L.; Ponte, C.; López, R.; Soriano, F. In Vitro Interactions of LytA, the Major Pneumococcal Autolysin, with Two Bacteriophage Lytic Enzymes (Cpl-1 and Pal), Cefotaxime and Moxifloxacin against Antibiotic-Susceptible and -Resistant Streptococcus Pneumoniae Strains. J. Antimicrob. Chemother. 2007, 60, 1159–1162. [Google Scholar] [CrossRef]
- Vouillamoz, J.; Entenza, J.M.; Giddey, M.; Fischetti, V.A.; Moreillon, P.; Resch, G. Bactericidal Synergism between Daptomycin and the Phage Lysin Cpl-1 in a Mouse Model of Pneumococcal Bacteraemia. Int. J. Antimicrob. Agents 2013, 42, 416–421. [Google Scholar] [CrossRef]
- Diaz, E.; Lopez, R.; Garcia, J.L. Chimeric Pneumococcal Cell Wall Lytic Enzymes Reveal Important Physiological and Evolutionary Traits. J. Biol. Chem. 1991, 266, 5464–5471. [Google Scholar]
- Corsini, B.; Díez-Martínez, R.; Aguinagalde, L.; González-Camacho, F.; García-Fernández, E.; Letrado, P.; García, P.; Yuste, J. Chemotherapy with Phage Lysins Reduces Pneumococcal Colonization of the Respiratory Tract. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. MetaSPAdes: A New Versatile Metagenomic Assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A Modular and Extensible Implementation of the RAST Algorithm for Building Custom Annotation Pipelines and Annotating Batches of Genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam Protein Families Database: Towards a More Sustainable Future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Chang, H.-Y.; Daugherty, L.; Fraser, M.; Hunter, S.; Lopez, R.; McAnulla, C.; McMenamin, C.; Nuka, G.; Pesseat, S.; et al. The InterPro Protein Families Database: The Classification Resource after 15 Years. Nucleic Acids Res. 2015, 43, D213–D221. [Google Scholar] [CrossRef] [PubMed]
- Soding, J.; Biegert, A.; Lupas, A.N. The HHpred Interactive Server for Protein Homology Detection and Structure Prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef]
- Grazziotin, A.L.; Koonin, E.V.; Kristensen, D.M. Prokaryotic Virus Orthologous Groups (PVOGs): A Resource for Comparative Genomics and Protein Family Annotation. Nucleic Acids Res. 2017, 45, D491–D498. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Juncker, A.S.; Willenbrock, H.; von Heijne, G.; Brunak, S.; Nielsen, H.; Krogh, A. Prediction of Lipoprotein Signal Peptides in Gram-Negative Bacteria. Protein Sci. 2003, 12, 1652–1662. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. TRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 0955–0964. [Google Scholar] [CrossRef]
- Laslett, D. ARAGORN, a Program to Detect TRNA Genes and TmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Tunney, M.M.; Ramage, G.; Field, T.R.; Moriarty, T.F.; Storey, D.G. Rapid Colorimetric Assay for Antimicrobial Susceptibility Testing of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004, 48, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
| Genus | Species | Tested Isolates | 23TH_48 | |
|---|---|---|---|---|
| Streptococcus | agalactiae | 2 | - |  |
| Streptococcus | lutentiensis | 10 | - |  |
| Streptococcus | infantis | 1 | + |  |
| Streptococcus | anginosus | 1 | - |  |
| Streptococcus | pneumoniae | 6 | + |  |
| Streptococcus | sanguinis | 1 | - |  |
| Streptococcus | hyointestinalis | 1 | - |  |
| Streptococcus | pyogenes | 2 | - |  |
| Streptococcus | dysgalactiae | 4 | - |  |
| Streptococcus | bovis | 3 | - |  |
| Streptococcus | mutans | 2 | - |  |
| Streptococcus | uberis | 6 | - |  |
| Streptococcus | infantarius | 1 | - |  |
| Streptococcus | salivarius | 5 | - |  |
| Lactococcus | lactis | 1 | - |  |
| Staphylococcus | aureus | 1 | - |  |
| Enterococcus | faecalis | 1 | - |  |
| Bacillus | cereus | 1 | - |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Kamp, I.; Draper, L.A.; Smith, M.K.; Buttimer, C.; Ross, R.P.; Hill, C. A New Phage Lysin Isolated from the Oral Microbiome Targeting Streptococcus pneumoniae. Pharmaceuticals 2020, 13, 478. https://doi.org/10.3390/ph13120478
van der Kamp I, Draper LA, Smith MK, Buttimer C, Ross RP, Hill C. A New Phage Lysin Isolated from the Oral Microbiome Targeting Streptococcus pneumoniae. Pharmaceuticals. 2020; 13(12):478. https://doi.org/10.3390/ph13120478
Chicago/Turabian Stylevan der Kamp, Imme, Lorraine A. Draper, Muireann K. Smith, Colin Buttimer, R. Paul Ross, and Colin Hill. 2020. "A New Phage Lysin Isolated from the Oral Microbiome Targeting Streptococcus pneumoniae" Pharmaceuticals 13, no. 12: 478. https://doi.org/10.3390/ph13120478
APA Stylevan der Kamp, I., Draper, L. A., Smith, M. K., Buttimer, C., Ross, R. P., & Hill, C. (2020). A New Phage Lysin Isolated from the Oral Microbiome Targeting Streptococcus pneumoniae. Pharmaceuticals, 13(12), 478. https://doi.org/10.3390/ph13120478






