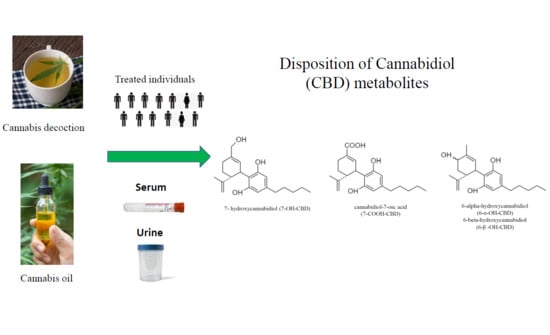
Disposition of Cannabidiol Metabolites in Serum and Urine from Healthy Individuals Treated with Pharmaceutical Preparations of Medical Cannabis
Abstract
1. Introduction
2. Results
2.1. Subjects and Study Design
2.2. Concentration–Time Profiles and Pharmacokinetics of CBD Metabolites in Serum after Decoction and Oil Administration
2.3. Urinary Excretion of CBD Metabolites after Decoction and Oil Administration
3. Discussion
4. Materials and Method
4.1. Subjects’ Enrolment
4.2. Cannabis Decoction and Oil Preparation
4.3. Study Design
4.4. Biological Samples’ Collection
4.5. Determination of Cannabinoids in Herbal Preparations and CDB Metabolites in Serum and Urine Samples
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018, 56, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Pichini, S.; Pellegrini, M.; Rotolo, M.C.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. THC and CBD concentrations in blood, oral fluid and urine following a single and repeated administration of “light cannabis”. Clin. Chem. Lab. Med. 2019, 58, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Stability of cannabinoids in cannabis FM1 flowering tops and oil preparation evaluated by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2019, 57, e165–e168. [Google Scholar] [CrossRef] [PubMed]
- Marchei, E.; Tittarelli, R.; Pellegrini, M.; Rotolo, M.C.; Pacifici, R.; Pichini, S. Is “light cannabis” really light? Determination of cannabinoids content in commercial products. Clin. Chem. Lab. Med. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. Ist. Super. Sanita 2020, 56. [Google Scholar]
- Pérez-Acevedo, A.P.; Pacifici, R.; Mannocchi, G.; Gottardi, M.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Busardò, F.P.; Pichini, S.; et al. Disposition of cannabinoids and their metabolites in serum, oral fluid, sweat patch and urine from healthy individuals treated with pharmaceutical preparations of medical cannabis. Phyther. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Fucile, C.; Manfredini, L.; De Grandis, E.; Gherzi, M.; Martelli, A.; Tripodi, G.; Mattioli, F.; Cangemi, G. A UHPLC-MS/MS method for the quantification of Δ9-tetrahydrocannabinol and cannabidiol in decoctions and in plasma samples for therapeutic monitoring of medical cannabis. Bioanalysis 2018, 10, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Gherzi, M.; Milano, G.; Fucile, C.; Calevo, M.G.; Mancardi, M.M.; Nobili, L.; Astuni, P.; Marini, V.; Barco, S.; Cangemi, G.; et al. Safety and pharmacokinetics of medical cannabis preparation in a monocentric series of young patients with drug resistant epilepsy. Complement. Ther. Med. 2020, 51. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Licata, M.; Verri, P.; Vandelli, D.; Palazzoli, F.; Marchesi, F.; Cainazzo, M.M.; Pini, L.A.; Guerzoni, S. Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH)—A pilot study. Eur. J. Clin. Pharmacol. 2018, 74, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I.; Hanuš, L. Human metabolites of cannabidiol: A review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.; Brine, D.; Perez-Reyes, M. Metabolism of cannabinoids in man. In Tea Pharmacology of Marijuana; Braude, M.C., Szara, S., Eds.; Raven Press: New York, NY, USA, 1976; pp. 93–113. [Google Scholar]
- Pichini, S.; Malaca, S.; Gottardi, M.; Pérez-Acevedo, A.P.; Papaseit, E.; Perez-Maña, C.; Farré, M.; Pacifici, R.; Tagliabracci, A.; Mannocchi, G.; et al. UHPLC-MS/MS analysis of cannabidiol metabolites in serum and urine samples. Application to an individual treated with medical cannabis. Talanta 2021, 223, 121772. [Google Scholar] [CrossRef] [PubMed]
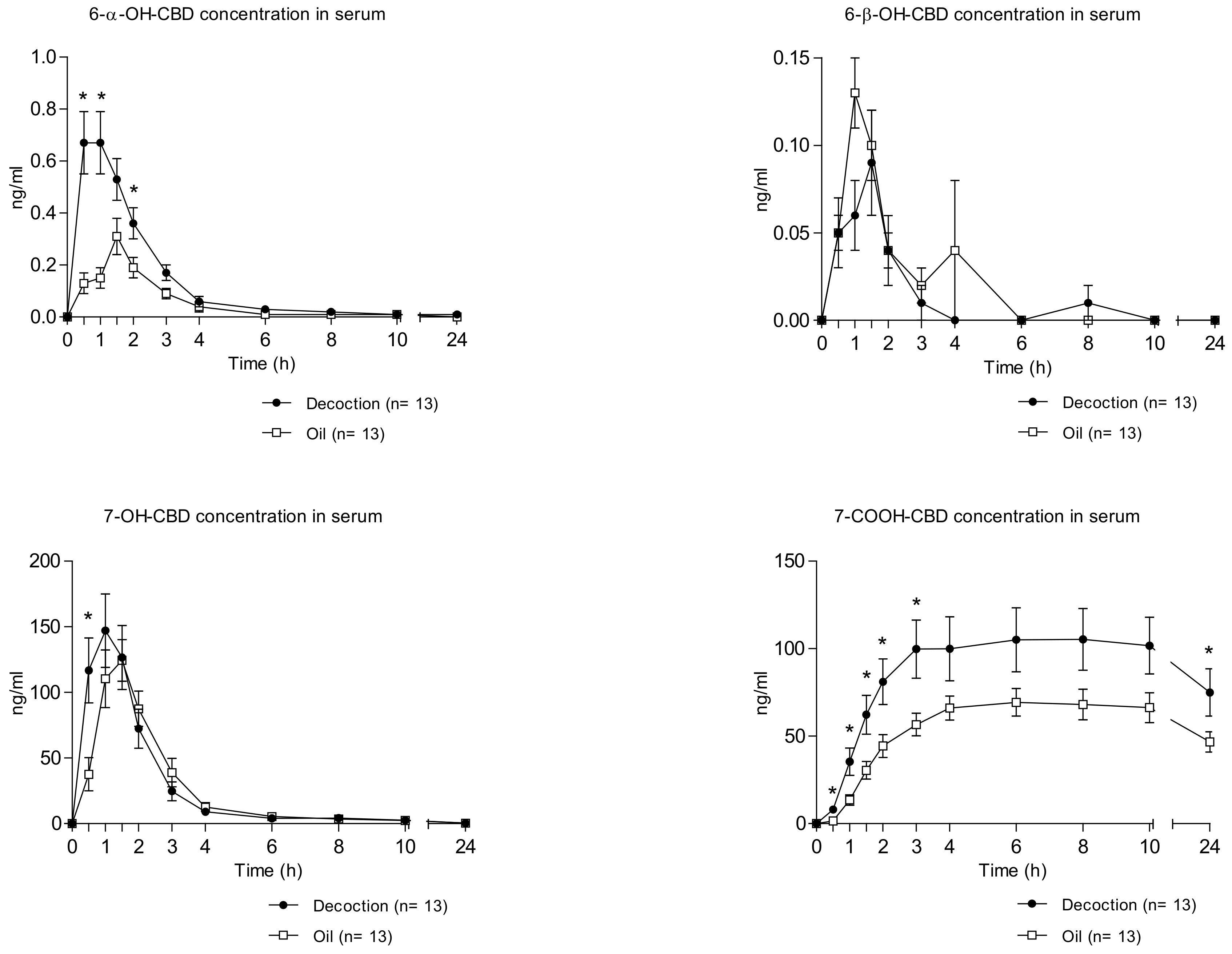

| Parameters | Cannabis Decoction (Mean ± SD) | Cannabis Oil (Mean ± SD) | p Value |
|---|---|---|---|
| 6-α-OH-CBD | |||
| Cmax (ng/mL) | 0.80 ± 0.41 | 0.42 ± 0.18 | 0.004 |
| Tmax (h) | 1.0 (0.5–2) | 1.5 (0.5–3) | 0.115 |
| AUC0–10h (ng/mL·h) | 1.59 ± 0.85 | 0.68 ± 0.33 | 0.005 |
| AUC0–24h (ng/mL·h) | 1.69 ± 0.84 | 0.78 ± 0.47 | 0.006 |
| Ke (h−1) | 0.39 ± 0.35 | 0.55 ± 0.34 | 0.421 |
| t1/2 (h) | 4.62 ± 5.37 | 2.37 ± 2.65 | 0.349 |
| 6-β-OH-CBD § | |||
| Cmax (ng/mL) | 0.12 ± 0.08 | 0.17 ± 0.11 | 0.067 |
| Tmax (hour) | 1.5 (0.5–3) | 1.0 (0.5–4) | 1.000 |
| AUC0–10h (ng/mL·h) | 0.16 ± 0.10 | 0.25 ± 0.29 | 0.223 |
| AUC0–24h (ng/mL·h) | 0.17 ± 0.11 | 0.26 ± 0.29 | 0.217 |
| 7-OH-CBD | |||
| Cmax (ng/mL) | 159.93 ± 101.75 | 151.45 ± 58.81 | 0.727 |
| Tmax (hour) | 1.0 (0.5–2) | 1.5 (1–2) | 0.194 |
| AUC0–10h (ng/mL·h) | 306.73 ± 204.52 | 280.00 ± 128.62 | 0.587 |
| AUC0–24h (ng/mL·h) | 327.54 ± 217.36 | 299.37 ± 140.64 | 0.586 |
| Ke (h−1) | 0.21 ± 0.06 | 0.26 ± 0.09 | 0.170 |
| t1/2 (h) | 3.51 ± 0.94 | 2.89 ± 0.74 | 0.118 |
| 7-COOH-CBD § | |||
| Cmax (ng/mL) | 118.03 ± 64.94 | 74.73 ± 31.84 | 0.031 |
| Tmax (hour) | 8.0 (3–24) | 6.0 (4–24) | 0.272 |
| AUC0–10h (ng/mL·h) | 885.94 ± 531.41 | 552.89 ± 225.26 | 0.036 |
| AUC0–24h (ng/mL·h) | 2122.73 ± 1257.15 | 1343.35 ± 569.39 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Acevedo, A.P.; Busardò, F.P.; Pacifici, R.; Mannocchi, G.; Gottardi, M.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Di Trana, A.; et al. Disposition of Cannabidiol Metabolites in Serum and Urine from Healthy Individuals Treated with Pharmaceutical Preparations of Medical Cannabis. Pharmaceuticals 2020, 13, 459. https://doi.org/10.3390/ph13120459
Pérez-Acevedo AP, Busardò FP, Pacifici R, Mannocchi G, Gottardi M, Poyatos L, Papaseit E, Pérez-Mañá C, Martin S, Di Trana A, et al. Disposition of Cannabidiol Metabolites in Serum and Urine from Healthy Individuals Treated with Pharmaceutical Preparations of Medical Cannabis. Pharmaceuticals. 2020; 13(12):459. https://doi.org/10.3390/ph13120459
Chicago/Turabian StylePérez-Acevedo, Ana Pilar, Francesco Paolo Busardò, Roberta Pacifici, Giulio Mannocchi, Massimo Gottardi, Lourdes Poyatos, Esther Papaseit, Clara Pérez-Mañá, Soraya Martin, Annagiulia Di Trana, and et al. 2020. "Disposition of Cannabidiol Metabolites in Serum and Urine from Healthy Individuals Treated with Pharmaceutical Preparations of Medical Cannabis" Pharmaceuticals 13, no. 12: 459. https://doi.org/10.3390/ph13120459
APA StylePérez-Acevedo, A. P., Busardò, F. P., Pacifici, R., Mannocchi, G., Gottardi, M., Poyatos, L., Papaseit, E., Pérez-Mañá, C., Martin, S., Di Trana, A., Pichini, S., & Farré, M. (2020). Disposition of Cannabidiol Metabolites in Serum and Urine from Healthy Individuals Treated with Pharmaceutical Preparations of Medical Cannabis. Pharmaceuticals, 13(12), 459. https://doi.org/10.3390/ph13120459