Abstract
Preparations with essential oils and their dosages applied in the therapy of children’s infectious diseases are well documented. In contrast, information is only sparingly available about uses of isolated pure essential oil compounds for the treatment of such infections. To find out safe antimicrobials from essential oils, microbiological inhibitory data of children pathogens were combined with oral and dermal acute toxicity data to calculate oral and dermal therapeutical indices (TI). The superiority of antibiotic drugs became obvious following calculating oral TIs of antimicrobials from higher plants, which suggests that oral administrations of essential oil compounds are not suitable to cure severe infections. A few selected compounds from higher plants show moderate effectiveness against gram-positive bacteria, yeast and fungi, but not gram-negative bacteria. Topical application or inhalation of selected compounds for the treatment or additional treatment of mild infections is reasonable.
1. Introduction
Essential oils possess a wide spectrum of pharmacological activities, e.g. anti-inflammatory, hyperemic, spasmolytic, expectorant, diuretic, choleretic, carminative or antiseptic effects [1]. For this reason, essential oils became officinal drugs in many countries (Table 1), which is documented in their respective pharmacopoeias [2].
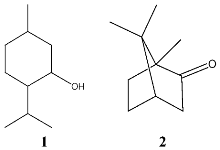
However, essential oils are not free of side effects and skin, respiratory- and gastrointestinal tract irritations, allergic reactions, phototoxicity, abortive as well as mutagenic and carcinogenic properties may be caused [1]. Even cases of poisoning of children by volatile oils, such as eucalyptus, sassafras, turpentine, wintergreen, chenopodium and citronella oil had occurred [3]. Moreover, care has to be taken with essential oils or preparations containing menthol 1 or camphor 2.
In infants both compounds may cause respiratory depression or even death (Kratschmer Reflex), when given directly into the nose or close to the nose onto skin [4]. Adverse effects similar to the ‘Kratschmer Reflex’ may occur also with other compounds from essential oils and can not be predicted from animal experiments.
The use of essential oils for healing purposes is recommended especially in the treatment of catarrhal diseases. Their administration to children presupposes the selection of safe oils and the determination of appropriate doses to avoid side effects. Among officinal essential oils only a few oils come into question. Table 2 shows their appropriate doses that are calculated from adult doses and respect body weight, size and surface area of children [5].

Table 1.
Officinal Essential Oils Listed in Various Pharmacopoeias.
| Country | Austria | Europe | France | Germany | Italy | Japan | Switzerland | United Kingdom | USA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Essential Oil | Botanical Origin | |||||||||
| Anise (Staranise) | Pimpinella anisum L.; Illicium verum Hook | X | X | X | X | X | ||||
| Ambrose | Chenopodium ambrosioides L. | X | ||||||||
| Bitter-Orange-Flower | Citrus aurantium L. subspecies aurantium | X | X | X | X | |||||
| Camellia | Camellia japonica L. | X | ||||||||
| Caraway | Carum carvi L. | X | X | X | X | |||||
| Cardamom | Elettaria cardamomum Maton var. minuscula Burkill | X | ||||||||
| Chamomile | Matricaria chamomilla L. | X | X | |||||||
| Chia | Salvia lavandulifolia Vahl (Salvia hispanica) | X | X | |||||||
| Cinnamon | Cinnamomum cassia Blume; C. ceylanicum Nees | X | X | X | X | X | ||||
| Citronella | Cymbopogon winterianus Jovitt | X | X | |||||||
| Clove | Syzygium aromaticum Merril et L.M. Perry | X | X | X | X | X | X | |||
| Coriander | Coriandrum sativum L. | X | ||||||||
| Dill | Anethum graveolens L. | X | X | |||||||
| Eucalyptus | Eucalyptus globulus Labillardiere etc. | X | X | X | X | X | X | |||
| Fennel | Foeniculum vulgare Miller var. vulgare | X | X | X | X | |||||
| Juniper | Juniperus communis L. | X | X | |||||||
| Laurel | Laurus nobilis L. | X | ||||||||
| Lavender | Lavandula angustifolia Miller | X | X | X | X | |||||
| Lemon | Citrus limon (L.) Burman filius | X | X | X | X | X | ||||
| Mentha | Mentha arvensis L. var. piperascens Holmes ex Christy | X | ||||||||
| Mint dementholized | X | X | X | |||||||
| Norway spruce | Picea abies (L.) Karsten; Abies sibirica Ledebour | X | X | |||||||
| Nutmeg | Myristica fragrans Houttuyn | X | X | X | X | |||||
| Orange | Citrus sinensis Osbeck | X | X | X | X | |||||
| Peppermint | Mentha piperita L. | X | X | X | X | X | X | |||
| Pine needle | Pinus silvestris L. | X | ||||||||
| Pumilio Pine | Pinus mugo Turra var. pumilio Zenari | X | X | |||||||
| Rose | Rosa gallica L. etc. | X | ||||||||
| Rosemary | Rosmarinus officinalis L. | X | X | X | ||||||
| Sage | Salvia officinalis L. | X | X | |||||||
| Spearmint | Mentha spicata L.; Mentha cardiaca Bak. | X | X | X | ||||||
| Tangerine | Citrus reticulata Blanco (Citrus nobilis Andreus) | X | X | |||||||
| Tea tree | Melaleuca alternifolia Cheel etc. | X | ||||||||
| Thyme | Thymus vulgaris L. | X | X | X | ||||||
| Turpentine rectified | Pinus palustris Miller; Pinus pinaster Aiton | X | X | X | X | X |

Table 2.
Essential Oils Used in the Treatment of Catarrhal Diseases of Children.
| Aetherolum | Common oil name | Administration | Age of children in years | |||
|---|---|---|---|---|---|---|
| route | 0 – 1 | >1 – 4 | >4 – 10 | >10 – 16 | ||
| Eucalypti | Eucalyptus | Inhalation b | 1 – 2 dr | 4 – 6 dr | 4 – 6 dr | 4 – 6 dr |
| Foeniculi | Fennel | Oral | -------- | -------- | 0,05 – 0,2 ml | 0,1 – 0,6 ml |
| Menthae arvensis | Japanese peppermint | Inhalation c | -------- | 1 – 3 dr | 2 – 4 dr | 3 – 6 dr |
| Menthae piperitae | Peppermint | Inhalation c | 1 dr | 1 – 2 dr | 2 – 3 dr | 3 – 4 dr |
| Piceae | Norway spruce fir, Siberian pine needle | Inhalation a | 2 dr/l | 2 – 4 dr/l | 3 – 4 dr/l | 3 – 4 dr/l |
| Pini | Dwarf or Scots pine needle, Austrian or French turpentine | Inhalation c | 1 – 2 dr | 2 – 3 dr | 3 – 4 dr/l | 3 – 4 dr/l |
| Terebinthinae rect. | Rectified turpentine | Inhalation a | -------- | -------- | 3 – 4 dr/l | 3 – 4 dr/l |
Notes: a) Inhalation with hot water, b) pure oil is given on a pillow, c) a) or b). Abbreviations used: dr = drops
Most essential oils consist of many, in part over 100 individual compounds, which are responsible as individuals or in their natural composition for beneficial and adverse effects of the respective entire oil. In this paper some effort was done to select safe compounds from essential oils with simultaneously antimicrobial activity against children pathogens and to compare the selected compounds with medicinal antibiotics used for the therapy of children diseases.
Information about treatment of children infections with pure, individual compounds from essential oils is not much available from literature. A very early report described the rectal administration of eugenol 3, the main constituent of clove oil (Syzygium aromaticum), in the trheatment of systemic infections of several children. Eugenol decreased body temperature and reduced fever, however, this was not sufficient to prevent death among all treated patients [6].
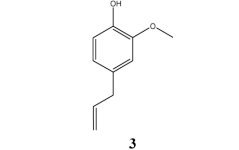
Further, oral, topical and inhalative administration of camphor 2, the main constituent of rosemary oil (Rosmarinum officinale), is recommended in supportive therapy of respiratory tract infection [7].
Because of the limited availability of pharmacological data obtained with living organisms, another selection strategy was necessary as a substitute in the selection of promising antimicrobials. In pharmacology the so-called ‘Therapeutic-Index’ (TI) is a measure for the effectiveness of pharmacological active drugs [8]. It is deduced from laboratory test results and the animal toxicity of compounds, respectively. The use of the TIs presupposes a collection of microbiological inhibitory and toxicological data. Such a collection is already available as a computer database [9], which was used for calculating TIs for children pathogenic microorganisms. The so-selected compounds were controlled for side effects as they are documented in materials safety data sheets (MSDS) or in ‘Toxicology Reports’ [10]. The effectiveness on the basis of TI-calculations of the selected compounds was then compared to antibiotics used in the therapy of children infections [11,16].
2. Material and Methods
The volume of the database used for the data analysis exceeds 153.000 data records [9]. It contains information about 6800 compounds and over 2500 species of bacteria, fungi and yeast, which is scheduled from almost 3000 microbiology and over 4500 toxicology references (Figure 1).
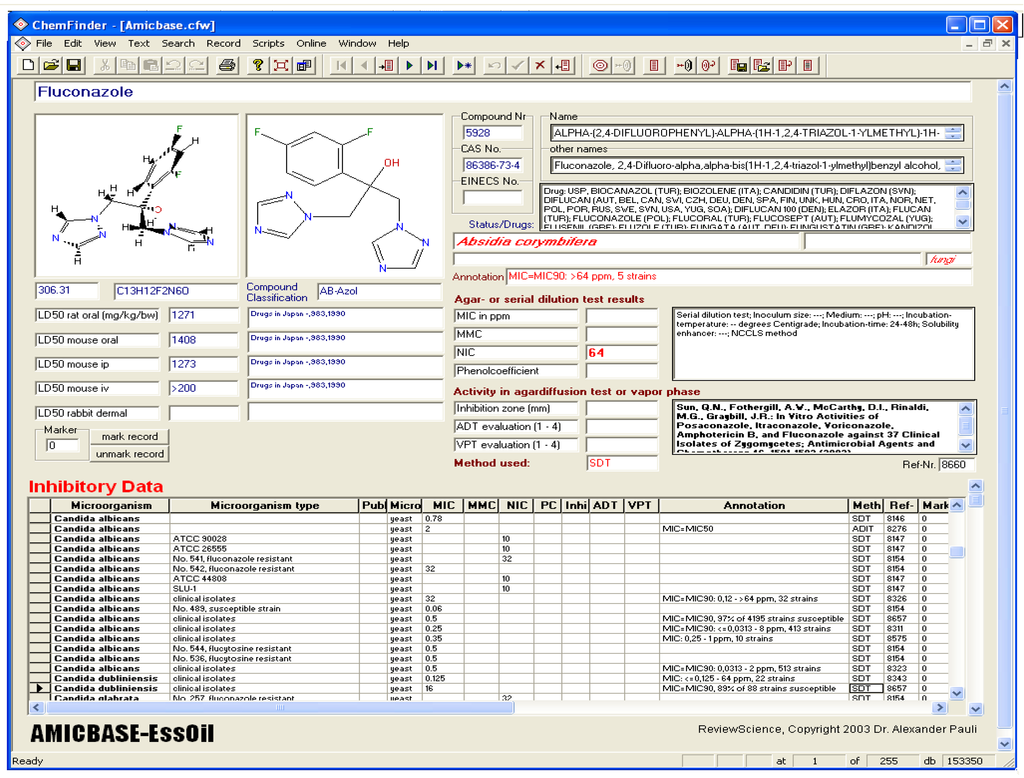
Figure 1.
Example of one data record of the antimicrobial database.
Among constituents of higher plants, terpenoids (20.000 data records) and aliphatic compounds (16.000 data records) are the largest groups in the database. The antibiotics database comprises 84.000 data records, of which 53.000 data records relate to officinal drugs being available in Japan, Europe and USA.
The calculation of ‘Therapeutic Indices’ (TI) is done usually by combining ED50 and LD50: the effective dose 50 (ED50) is the amount causing 50% of a desired effect, and the lethal dose 50 (LD50) is the amount causing 50% death of test animals [8].
TI calculation used for pharmaceutical products and substances: 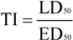 .
.
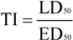 .
.In the case of antimicrobials the concentration preventing microbial growth (minimal inhibitory concentration, MIC in ppm) is of interest and was used instead of ED50.
Among toxicity data towards animals different types exist depending on the route of administration. Comprehensive toxicological data material exists following oral administration. In the case of skin affections the dermal toxicity of a compound is of interest. Therefore, oral ‘Therapeutic Indices’ (oral TI) and dermal ‘Therapeutic Indices’ (dermal TI) are given both.
TI calculation used for antimicrobials:
The 1530 compounds presented in the database are characterized by their oral toxicity and 523 compounds by their dermal toxicity. Defined inhibitory concentrations (MIC) are necessary to calculate ‘Therapeutic Indices’ (TIs). About 40.000 data records of the entire database yield MIC-data of compounds from higher plants. The number of TIs calculated from combined antimicrobial and toxicological data exceeds 30.000 without antibiotics.
Typical children’s diseases, infection sites and causative microorganisms were compiled together (Table 3) from the ‘Merck Manual of Diagnosis and Therapy’ [11].

Table 3.
Diseases caused by Important Children’s Pathogens.
| Causative Microorganism | Children disease | Localization |
|---|---|---|
| Streptococcus pneumoniae | Pneumonia | Respiratory tract |
| Meningitis | Nasopharynx | |
| Rhinitis, Sinusitis | Nose, nasal cavity | |
| Otitis media | Ear | |
| Conjunctivitis, Orbital cellulitis | Eye | |
| Bacteremia, Sepsis | Bloodstream | |
| Staphylococcus aureus | Rhinitis, Sinusitis | Nose, nasal cavity |
| Conjunctivitis, Orbital cellulitis | Eye | |
| Infectious gastroenteritis | Gastrointestinal tract | |
| Sepsis | Bloodstream | |
| Impetigo, Staphylococcal skin infections | Skin | |
| Staphylococcal scalded skin syndrome | Skin | |
| Escherichia coli | Pneumonia | Respiratory tract |
| Meningitis | Nasopharynx | |
| Urinary tract infections | Urinary tract | |
| Infectious gastroenteritis | Gastrointestinal tract | |
| Salmonella spec. | Bacteremia, Sepsis | Bloodstream |
| Infectious gastroenteritis | Gastrointestinal tract | |
| Mycobacterium tuberculosis, M. bovis, M. africanum | Pulmonary tuberculosis Lymphadenopathy | Respiratory tract Lymph nodes |
| Microsporum audouinii, M. canis, M. gypseum, Trichophyton tonsurans, T. violaceum | Tinea capitis | Skin, hair |
| Candida albicans | Pneumonia | Bloodstream, respiratory tract |
| Nappy (diaper) rush | Skin | |
| Oral candidiasis | Mouth | |
| Bordetella pertussis | Pertussis | Respiratory tract |
| Haemophilus influenzae | Epiglottis | Respiratory tract |
| Clostridium tetani | Tetanus | Skin, wounds |
The most hazardous infectious for children - according to the statistics of the World Health Organization dating from the year 2001 [12] - are shown in Table 4. Among children’s diseases caused by bacteria in particular respiratory infections followed by diarrhea, pertussis, syphilis, meningitis and tetanus are most frequent causes of death.

Table 4.
WHO Statistic on Causes of Death among Children in the Year 2001.
| No. cases of death in age group 0 – 4 years | No. cases of death in age group 5 – 14 years | |||||||
| Male | Female | Both | Male | Female | Both | |||
| Lower respiratory infect. | 1070739 | 963096 | 2033836 | Measles | 86435 | 87434 | 173868 | |
| Diarrhoeal diseases | 685979 | 667149 | 1353128 | Other infectious diseases | 62495 | 53268 | 115763 | |
| Malaria | 454970 | 502027 | 956997 | Lower respiratory infect. | 57849 | 43601 | 101450 | |
| Other infectious diseases | 308522 | 310123 | 618645 | Diarrhoeal diseases | 54967 | 39016 | 93983 | |
| Measles | 276372 | 277156 | 553527 | Malaria | 54388 | 29391 | 83779 | |
| HIV/AIDS | 178049 | 174069 | 352118 | Tuberculosis | 17257 | 17584 | 34842 | |
| Pertussis | 142430 | 142111 | 284541 | HIV/AIDS | 15102 | 13904 | 29006 | |
| Tetanus | 100657 | 100784 | 201441 | Tetanus | 12840 | 12746 | 25587 | |
| Syphilis | 78084 | 64292 | 142376 | Meningitis | 12623 | 10605 | 23229 | |
| Meningitis | 42999 | 36473 | 79471 | Trypanosomiasis | 6183 | 11125 | 17309 | |
| Tuberculosis | 27979 | 21351 | 49330 | Leishmaniasis | 7135 | 9663 | 16797 | |
| Upper respiratory infect. | 16179 | 18672 | 34851 | Dengue | 1904 | 1171 | 3075 | |
| Dengue | 2730 | 6393 | 9123 | Hepatitis B | 1180 | 1500 | 2680 | |
| Leishmaniasis | 4833 | 4040 | 8872 | Upper respiratory infect. | 1087 | 835 | 1922 | |
| Japanese encephalitis | 2133 | 4490 | 6623 | Trichuriasis | 878 | 907 | 1785 | |
| Diphtheria | 2470 | 1976 | 4446 | Ascariasis | 869 | 739 | 1608 | |
| Trypanosomiasis | 2237 | 1245 | 3482 | Japanese encephalitis | 1130 | 357 | 1487 | |
| Hepatitis B | 1668 | 1782 | 3450 | Hepatitis C | 563 | 736 | 1299 | |
| Otitis media | 1420 | 997 | 2417 | Otitis media | 622 | 222 | 843 | |
| Ascariasis | 1059 | 1094 | 2153 | Schistosomiasis | 159 | 659 | 818 | |
| Hepatitis C | 646 | 831 | 1476 | Diphtheria | 518 | 212 | 730 | |
| Other intestinal infections | 517 | 540 | 1057 | Pertussis | 254 | 252 | 506 | |
| Leprosy | 206 | 169 | 375 | Other intestinal infections | 111 | 115 | 226 | |
| Other STDs | 184 | 140 | 324 | Leprosy | 68 | 52 | 120 | |
| Trichuriasis | 227 | 95 | 323 | Poliomyelitis | 45 | 52 | 97 | |
| Schistosomiasis | 110 | 21 | 131 | Chlamydia | 74 | 0 | 74 | |
| Poliomyelitis | 46 | 34 | 80 | Syphilis | 10 | 15 | 25 | |
| Gonorrhoea | 0 | 33 | 33 | Other STDs | 3 | 6 | 9 | |
| Trachoma | 31 | 0 | 31 | Hookworm disease | 9 | 0 | 9 | |
| Lymphatic filariasis | 31 | 0 | 31 | Chagas disease | 4 | 1 | 5 | |
| Hookworm disease | 5 | 3 | 7 | Gonorrhoea | 0 | 1 | 1 | |
| Onchocerciasis | 6 | 0 | 6 | Trachoma | 0 | 1 | 1 | |
| Chagas disease | 4 | 1 | 5 | Lymphatic filariasis | 0 | 0 | 0 | |
| Chlamydia | 0 | 0 | 0 | Onchocerciasis | 0 | 0 | 0 | |
Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15, Table 16, Table 17 summarize data about the effectiveness of antimicrobials from higher plants and antibiotic drugs against children pathogens. The selected compounds possess ‘Therapeutic Indices’ equal and higher than 100 units. Thus, the toxic dose towards animals is in minimum over 100 - fold higher than the inhibitory dose towards microorganism.
The results of natural antimicrobials are compared to results of antibiotic drugs obtained with resistant and non-resistant microbial strains. Because of the data variety additional information was included, which is the reported minimum and maximum MIC, the minimum and maximum TI, the number of tested strains. Of antibiotic drugs the MIC-breakpoints have frequently been determined [13]. This allows to distinguished between strains that are regarded as normal or as resistant. The minimum MIC of resistant strains is given as MIC-res for each antibiotic drug. When the quality data range for antibiotic drugs is given only [14], the double amount of the highest concentration was taken as MIC-res. TI-res is the ‘Therapeutic Index’ calculated from MIC-res for resistant strains, respectively. Side effects are characterized and annotated in the status field, which indicates whether a compound is used as drug (‘Drug’) or is listed by the Unites States Food and Drug Administration as compound that may occur in food (‘Food’) [15].
3. Results
The results of effective compounds against causative microorganisms of children’s diseases are scheduled according to Table 3.
3.1. Streptococcus pneumoniae
Streptococci are human parasites, which colonize skin and mucous membranes and can be isolated from alimentary, respiratory and genital tracts. Among Pneumococci several types exist, of which capsulated strains are regarded as pathogen. Antibiotic resistances of Pneumococci isolated from children in Germany are as follows: penicillin G 8,6%, cefotaxime 3,1%, erythromycin 27,4%, tetracycline 10,7% [16]. In addition, resistances of Pneumococci towards trimethoprim, sulfonamides and chloramphenicol are reported [17]. Table 5 shows the calculations on pharmaceutical drugs used for the treatment of Pneumococcus infection and the calculations of most successful compounds from higher plants in comparison. The data is sorted first by TI-res and if not available by minimum TIs.
When Pneumococci strains without resistance mechanisms were tested, the approved antibiotics dominate the list of oral TIs (Table 5), of which penicillin G is preferably be used in the therapy of Pneumococcus infections in children. The TIs of compounds from higher plants including essential oils are inferior to such therapeutically used drugs.
Pneumococci resistant to penicillin G are characterized by MIC-res >=1 ppm, however, even at this concentration the TI-calculation for penicillin G is much better than for compounds from higher plants. Tetracycline, cotrimoxazol and chloramphenicol are not much different at their lowest level of effectiveness (TI-res) from meta-menthene-thymol 4, meta-menthene-phenol 5, alpha-bisabolol 6, a component from chamomile (Matricaria recutita) essential oil, or lupulone 7, a component present in hop extract (Humulus lupulus). However, only a small number of strains of Pneumococci have been tested with these compounds, which impairs the drawing of general conclusions.

Table 5.
Effective Inhibitors of Streptococcus pneumoniae, oral TI max.>= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC-range in ppm | TI max. - TI min. | MIC-res | TI-res | Status |
|---|---|---|---|---|---|---|---|---|
| Cefotaxime | Mou-o | >20000 | >100 | 0,004 – 4 | >5000000 - 5000 | >2 | <10000 | Drug |
| Erythromycin | Mou-o | 2580 | >100 | 0,0008 – 64 | 3225000 - 80 | >0,5 | <5160 | Drug |
| Penicillin G | Mou-o | >5000 | >100 | 0,008 –16 | 625000 – 312 | >1 | <5000 | Drug |
| Linoleic acid | Mou-o | >50000 | 2 | 13 | >3846 | --- | --- | Food* |
| n-Dodecanol | Rat-o | >12800 | 2 | 13 | >984 | --- | --- | Food* |
| m-Menthene-phenol | Mou-o | 2900 | 1 | 3 | 966 | --- | --- | --- |
| Lauric acid | Rat-o | 12000 | 2 | 13 | 923 | --- | --- | Food* |
| Lauric aldehyde | Rat-o | 23000 | 2 | 25 | 920 | --- | --- | Food* |
| Lupulone | Mou-o | 1500 | 2 | 3,3 | 454 | --- | --- | Food |
| Sulfamethoxazole | Mou-o | 2300 | 5 | 1 – 8 | 2300 - 340 | --- | --- | Drug |
| Tetracycline | Mou-o | 678 | >100 | 0,12 – 4 | 5650 - 170 | >2 | <340 | Drug |
| Cotrimoxazol | Mou-o | 3740 | >100 | 0,12 – 32 | 31000 - 233 | >32 | <233 | Drug |
| alpha-Bisabolol | Rat-o | 14850 | >4 | 32 - 64 | 464 - 232 | --- | --- | --- |
| Myristic acid | Rat-o | >10000 | 2 | 50 | >200 | --- | --- | Food |
| Chloramphenicol | Mou-o | 1500 | >100 | 1 – 32 | 1500 - 46 | >8 | <184 | Drug |
| m-Menthene-thymol | Mou-o | 1925 | 1 | 12 | 160 | --- | --- | ---- |
| Lauramine HCl | Mou-o | 1160 | 2 | 10 – 12,5 | 116 - 93 | --- | --- | Food* |
| Palmitic acid | Rat-o | >10000 | 3 | 7 – 125 | >1428 - >80 | --- | --- | Food |
| Trimethoprim | Mou-o | 2764 | >100 | <0,06 – 128 | >2750 - 80 | --- | --- | Drug |
| Oleic acid | Rat-o | 74000 | 4 | 4 – 1000 | 1850 - 74 | --- | --- | Food* |
| Undecylenic acid | Mou-o | 8150 | 2 | 50 – 1000 | 163 - 8 | --- | --- | Drug |
| Stearic acid | Rat-o | 4600 | 3 | 7 – 1000 | 657 - 4,6 | --- | --- | Food |
Notes: * may cause digestive tract irritation. Abbreviations used: -o = orally administered, Mou = mouse
The in vitro activity of fatty acids like oleic, linoleic and linolenic acid is inactivated by addition of blood serum [18]. Such compounds might have no practical significance in systemic treatment of infectious diseases, and therefore, they are not discussed in the following tables. The low toxicity of oleic acid and related long-chain aliphatic compounds indicate a possible use as topical antiseptics.
Treating respiratory tract infections by inhalation of volatile compounds lies near by hand. Very active compounds against Streptococcus pneumoniae in the vapor phase are shown in Table 6.

Table 6.
Strong Inhibitors of Streptococcus pneumoniae in the Vapor Phase.
| Trivial name | Microorganism | Activity evaluation | Status |
|---|---|---|---|
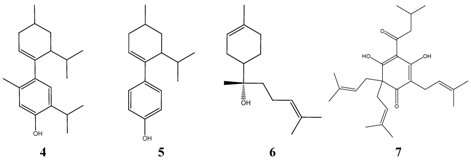
| Thymol 8 | Streptococcus pneumoniae type II | ++++ | Food * |
| Thymol | Streptococcus pneumoniae type III | ++++ | Food * |
| Thymol | Streptococcus pneumoniae type VI | ++++ | Food * |
| Carvacrol 9 | Streptococcus pneumoniae type III | ++++ | Food *? |
| Cinnamic aldehyde 10 | Streptococcus pneumoniae type VI | ++++ | Food * |
| Limonene 11 | Streptococcus pneumoniae | ++++ | Food * |
| Citronellol 12 | Streptococcus pneumoniae | ++++ | Food * |
| Citronellal 13 | Streptococcus pneumoniae | ++++ | Food * |
| Geraniol 14 | Streptococcus pneumoniae | ++++ | Food * |
Notes: * may cause respiratory tract irritation, ++++: strong inhibitory activity
Almost all compounds listed in Table 6. may cause irritation of the respiratory system, and therefore, their use as therapeutics via inhalation is questionable, although thymol 8 superimposes the germicidal activity of phenol, which has a long history as disinfecting agent, about 5 to 30 times towards gram-positive bacteria. The antimicrobial properties of alpha-bisabolol are unexamined in the vapor phase. No toxic signs were observed in animal experiments following inhalation (7h exposure in a highly enriched and/or saturated atmosphere at 20 °C, Worksafe Australia). In toxicology studies alpha-bisabolol 6 was found to be safe [19]. Alpha-bisabolol strongly inhibits Streptococcus pneumoniae in the serial dilution tests (MIC = 32 - 64 ppm). This may explain why inhalation of vapors of chamomile flowers is recommended in German traditional medicine.
3.2. Staphylococcus aureus
Staphylococci are found mainly on human skin, skin glands and mucous membranes and sometimes in the mouth, blood, mammary glands, intestinal, genitourinary and upper respiratory tracts. Pathogenic strains are known to form toxins. Antibiotic resistance is reported towards ß-lactam antibiotics (oxacillin, ampicillin), aminoglycosides (gentamycin), macrolides (erythromycin), quinolones (ciprofloxacin), clindamycin, chloramphenicol, tetracycline, trimethoprim [17] and the reserve antibiotic vancomycin in 1999 [20].
In the antibiotic therapy first choice drugs are flucloxacillin (methicillin susceptible strains, MSSA), vancomycin and teicoplanin (methicillin resistant strains, MRSA) and vancomycin + flucloxacillin or vancomycin + gentamycin (glycopeptide intermediate resistant strains, GISA). Recommendations as second choice antibiotics are: cefaclor, cefuroximaxetil, and loracarbef (MSSA), quinopristin + dalfopristin, linezolid and further alternatives: vancomycin + rifampicin, cefazolin + vancomycin + netilmycin, imipenem + vancomycin + netilmycin, fusidic acid + rifampicin, Cotrimoxazol + fusic acid or rifampicin, minocycline, fosomycin + cefotaxime, and cotrimatzol + nitrofurantoin (MRSA), and quinopristin + dalfopristin, ampicillin + sulbactam, and linezolid (GISA). No differences were made in the choice of antibiotic treatment between grownups and children [16].

Table 7.
Effective Inhibitors of Staphylococcus aureus, oral TI max. > 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. – TI min. | MIC-res | TI-res | Status |
|---|---|---|---|---|---|---|---|---|
| Netilmicin | Rat-o | Su: >10000 | >100 | 0,125 – 32 | >80000 – 312 | >1 | >10000 | Drug |
| Cefazolin | Mou-o | Na: >11000 | >100 | 0,01 - >256 | >110000 - <43 | 0,25 – 1 | **<5500 | Drug |
| Flucloxacillin | Mou-o | Na: 7600 | >100 | 0,125 – 32 | 60800 – 238 | ***0,125 – 1 | 3800 | Drug |
| Minocycline | Mou-o | 3100 | >100 | <0,015 - 12,5 | >206666 – 248 | 0,06 - 0,5 | **<3100 | Drug |
| Fusidic acid | Mou-o | 1500 | >100 | 0,032 – 16 | 46875 - 94 | >0,5 | <3000 | Drug |
| Cefotaxime | Mou-o | Na: >20000 | >100 | 0,12 - >60 | >166666 - <333 | 0,5 - 4 | **<2500 | Drug |
| Cefaclor | Mou-o | >20000 | >100 | 0,25 – 128 | >80000 - 156 | 1 – 4 | **<2500 | Drug |
| Vancomycin | Mou-o | 5000 | >100 | 0,125 – 8 | 40000 – 625 | >2 | <2500 | Drug |
| Bakuchiol | Mou-o | 2560 | 1 | 1,4 | 1828 | --- | --- | --- |
| Oleic acid | Rat-o | 74000 | 3 | 15 – 90 | 4933 - 822 | --- | --- | Food |
| Mupirocin | Mou-o | 5000 | >100 | 0,05 – 512 | 100000 – 10 | >8 | 625 | Drug |
| Gentamicin | Mou-o | >10000 | >100 | 0,035 - >256 | >285714 - <39 | >16 | <624 | Drug |
| Cefuroxime | Mou-o | >10000 | >100 | 0,13 - >60 | >76923 - <167 | >32 | <624 | Drug |
| Rifampicin | Mou-o | 500 | >100 | 0,002 - 3,1 | 250000 - 161 | >1 | <500 | Drug |
| Loracarbef | Dog-o | >2000 | >100 | 0,5 – 16 | >4000 - 125 | 0,5 – 2 | **<500 | Drug |
| Fosfomycin | Mou-o | Ca: >3500 | >100 | 0,5 - >128 | >7000 - <27 | 0,5 - 4 | **<440 | Drug |
| n-Tridecanol | Rat-o | 17200 | 3 | 20 – 50 | 860 – 344 | --- | --- | Food* |
| Imipenem | Mou-o | >5000 | >100 | 0,006 – >256 | >833333 – <20 | >16 | <312 | Drug |
| Teicoplanin | Mou-o | 1000 | >100 | 0,125 - 6,3 | 8000 - 160 | >4 | <250 | Drug |
| Linoleic acid | Mou-o | >50000 | 10 | 4 - >200 | >12500 - <250 | --- | --- | Food* |
| Lupulone | Mou-o | 1500 | 7 | 2 - 6,25 | 750 – 240 | --- | --- | Food |
| Cotrimoxazol | Mou-o | 3740 | >100 | 0,015 - 2,86 | 249333 – 1300 | >16 | <234 | Drug |
| Lauricidin | Rat-o | ****53400 | 11 | 10 – 250 | 5340 – 213 | --- | --- | Food |
| 3-Heptylacrolein | Rat-o | 5000 | 3 | 25 | 200 | --- | --- | Food |
| Shikonin | Mou-o | >1000 | 2 | 4 – 5 | >250 – 200 | --- | --- | --- |
| Swartziadione | Mou-o | *****>300 | 1 | 1,56 | >192 | --- | --- | --- |
| alpha-Bisabolol | Mou-o | 11350 | 9 | 32 – 100 | 354 - 113 | --- | --- | --- |
| m-Menthene-phenol | Mou-o | 2900 | 1 | 28 | 103 | --- | --- | --- |
| Sorbic acid | Rat-o | 7360 | 50 | 50 – 100 | 147 - 73 | --- | --- | Food |
| Butylparaben | Mou-o | 13200 | 20 | 60 – 270 | 220 - 49 | --- | --- | Food |
| Ampicillin-sulbactam | Mou-o | >6000 | >100 | <=0,06 – 128 | >100000 - 47 | --- | --- | Drug |
| o-Methoxy-cinnamaldehyde | Rat-o | >5000 | 3 | 50 – 200 | >100 - 25 | --- | --- | Food |
| Dimethyl fumarate | Rat-o | 2240 | 2 | 10 – 100 | 224 - 22 | --- | --- | --- |
| Diethyl fumarate | Rat-o | 1780 | 2 | 10 – 100 | 178 - 18 | --- | --- | --- |
| Lauric aldehyde | Rat-o | 23000 | 8 | 50 - >2000 | 460 - <11,5 | --- | --- | Food* |
| ortho-Phenylphenol | Rat-o | 2000 | 10 | 15,6 – 200 | 128 - 10 | --- | --- | Food |
| Nitrofurantoin | Mou-o | 360 | >100 | 0,75 – 32 | 480 - 11 | >32 | <11 | Drug |
| n-Dodecanol | Rat-o | >12800 | 15 | 12,5 - >2000 | >1024 - <6,4 | --- | --- | Food* |
| Linolenic acid | Mou-o | >3200 | 8 | 5 – 500 | 640 – 6,4 | --- | --- | Food* |
| Undecylen aldehyde | Rat-o | >5000 | 5 | 50 – 1000 | >100 - 5 | --- | --- | Food |
| Lauric acid | Rat-o | 12000 | 22 | 22 – 2500 | 545 - 4,8 | --- | --- | Food* |
| Falcarindiol | Mou-o | ca. 100 | 3 | 1 – 25 | 100 – 4 | --- | --- | --- |
| Hinokitiol | Mou-o | 760 | 51 | 0,2 – 200 | 3800 -3,8 | --- | --- | --- |
| Capric alcohol | Mou-o | 6500 | 16 | 40 – 2000 | 162 - 3,2 | --- | --- | Food |
| n-Tetradecanol | Rat-o | 32,5 ml/kg | 4 | 40 - >10000 | 812 - <3,2 | --- | --- | Food |
| Farnesol | Mou-o | 7400 | 16 | 25 – 2000 | 123 - 3,7 | --- | --- | Food |
| Isoborneol | Rat-o | 5200 | 4 | 50 – 2000 | 104 - 2,6 | --- | --- | Food |
| Hyacinthin | Mou-o | 3890 | 6 | 32 - >2000 | 121 – 2 | --- | --- | Food |
| Nerolidol | Mou-o | 15000 | 25 | 25 - >10000 | 600 - <1,5 | --- | --- | Food |
| Eugenol | Mou-o | 3000 | 49 | 25 – 2100 | 120 - 1,4 | --- | --- | Food |
| n-Undecyl alcohol | Rat-o | 3000 | 13 | 25 - >2000 | 120 - <1,3 | --- | --- | Food |
| Citral | Mou-o | 6000 | 49 | 12,5 – 5000 | 480 - 1,2 | --- | --- | Food |
| Diacetyl | Rat-o | 1580 | 11 | <9,75 – 1000 | >162 - 0,16 | --- | --- | Food |
| Linezolid | --- | --- | >100 | 0,5 – 8 | --- | >4 | --- | Drug |
| Quinupristin-dalfopristin | --- | --- | >100 | 0,06 – 32 | --- | >2 | --- | Drug |
Notes: * may cause digestive tract irritation, ** calculated with double MIC max. of quality data range [14], *** range of 87 clinical isolates [21] assumed as quality data range, **** 53,4 ml/kg calculated with density = 1, ***** LDL = lethal dose lowest. Abbreviations used: Na = Na-salt, Ca = Ca-salt, Su = sulfate-salt,
The list of effective inhibitors of Staphylococcus aureus is dominated again by therapeutics in use (Table 7). According to TI calculations, bakuchiol 15, a natural long-chain phenol occurring in Malaya tea (Psoralea corylifolia) was the most successful compound from higher plants and it superimposes antibiotics towards which Staphylococci strains had developed a high level of resistance, e.g. mupirocin, fosfomycin or nitrofurantion. Bakuchiol was further examined on its activity against dental plaque forming microorganisms and the authors concluded that the compound is of value as food additive or as additive in mouth washes [22]. Promising in vitro results were again obtained with lupulone 7. Swartziadione 16, a constituent of the African tree Swartzia madagascariensis, was patented for treatment or inhibition of microbial infections. Shikonin 17, another patented constituent of higher plants [23], occurs in the extract of Groomwell root (Arnebia euchroma), which is used traditionally in China as topical wound healing therapeutic.
Further compounds having in part good results in the TI calculation are citral 18 from e.g. lemongrass (Cymbopogon citratus), hinokitiol 19 from the heartwood of Thuja trees, which possesses an extraordinary tropolone-ring substructure, and eugenol 3. The wide range of activity of these compounds points to the existence of microbial resistance mechanisms and to a natural variability towards antimicrobials, which can not be explained alone with variations of the testing methods used in the examinations of the respective compounds.
Highly calculated compounds from essential oils are the sesquiterpenes nerolidol 20 and farnesol 21, which are both methyl-substituted long-chain C-12 alcohols, and further primary alcohols having a carbon chain of 12 to 14 atoms and the monocyclic sesquiterpene alpha-bisabolol 6.
Because S. aureus is also prominent for skin and wound infections, a second list on the basis of dermal ‘Therapeutic indices’ was calculated (Table 8).

Table 8.
Effective Inhibitors of Staphylococcus aureus, dermal TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Triclosan | Rabbit-dermal | 9300 | 10 | 0,01 - 0,03 | 930000 - 310000 | Drug*,** |
| Haloprogine | Rabbit-dermal | 1625 | 6 | 1,56 - 3,12 | 1041 - 520 | Drug*,** |
| Hexetidine | Rabbit-dermal | 1,86 ml/kg | 1 | 5 | 372 | Drug |
| Linolenic acid | Rabbit-dermal | >20000 | 2 | 5 – 100 | >4000 - 200 | Food* |
| Hexachlorophene | Rabbit-dermal | >600 | 10 | 0,3 - 3 | >2000 - 200 | Drug* |
| Hinokitiol | Rabbit-dermal | >2000 | 14 | 0,2 - 12,5 | >10000 - 160 | --- |
| 3-Heptylacrolein | Rabbit-dermal | 3400 | 2 | 25 | 136 | Food* |
| Oxolinic acid | Rabbit-dermal | >2000 | 9 | 0,4 - 25 | >5000 - 80 | Drug |
| Hydroquinone | Mammal-dermal | 5970 | 11 | 10 – 90 | 597 - 66 | Drug*,** |
| o-Methoxy-cinnamaldehyde | Rabbit-dermal | >5000 | 3 | 50 – 200 | >100 - 25 | Food*,** |
| Dimethyl fumarate | Rabbit-dermal | 1250 | 2 | 10 – 100 | 125 - 12,5 | ---* |
| beta-Naphthol | Rabbit-dermal | >10000 | 2 | 100 - 1000 | >100 - 10 | ---*,** |
| Isobornyl acetate | Rabbit-dermal | >20000 | 6 | 200 - >2000 | >100 - 10 | Food |
| n-Tridecanol | Rabbit-dermal | 5600 | 3 | 20 - >800 | 280 - 7 | Food* |
| Diacetyl | Rabbit-dermal | >5000 | 11 | 9,75- 1000 | >512 - 5 | Food* |
| Hyacinthin | Rabbit-dermal | >5000 | 6 | 32 - >1000 | >156 - 5 | Food* |
| Isoborneol | Rabbit-dermal | >5000 | 5 | 50 - >1000 | >100 - 5 | Food* |
| Undecylen aldehyde | Rabbit-dermal | >5000 | 5 | 50 – 1000 | >100 - 5 | Food* |
| alpha-Amylcinnamyl alcohol | Rabbit-dermal | >5000 | 6 | 50 - >2000 | >100 - 2,5 | Food |
| n-Undecyl alcohol | Rabbit-dermal | 4,76 ml/kg | 13 | 25 - >2000 | 190 - 2,4 | Food* |
| n-Dodecanol | Rabbit-dermal | >10 ml/kg | 16 | 12,5 - >10000 | >800 - 1 | Food* |
| n-Tetradecanol | Rabbit-dermal | 7,13 ml/kg | 25 | 40 - >10000 | 173 - <0,7 | Food* |
| Nerolidol | Rabbit-dermal | >5000 | 8 | 25 - >10000 | >200 - 0,5 | Food* |
Notes:* may cause skin irritation ** mutagen
The most effective inhibitors of Staphylococcus aureus are again approved drugs used to treat skin infections. Almost all compounds listed in Table 8 are classified as skin irritants. The dermal toxicity of alpha-bisabolol 6 has not been determined in numbers, however, it is regarded as safe and allergic reaction is not reported although humans are exposed to it to a large extend, because it is widely used in cosmetics. Interestingly, (+)-epi-alpha-bisabolol from Peperomia galioides turned out to be as active wound healing agent, when a series of commercial available terpenoids was studied [24]. A good result in the dermal TI calculation was obtained with linolenic acid 22. It is the main constituent of the fatty oil of ‘evening primrose’ (Oenanthera biennis), which is used internally for the adjuvant treatment of neurodermitis in Germany.
Other active compounds are long-chain alcohols (C11 – C14), nerolidol 20, alpha-amylcinnamyl alcohol 23, isoborneol 24 and its acetate ester, unsaturated aldehydes (3-heptylacrolein 25, undecylen aldehyde 26) and hinokitiol 19.
3.3. Escherichia coli
Escherichia coli belongs to the normal intestinal microflora of humans. Pathogenic strains are characterized by the ability to form toxins. Diarrhea is caused by enteropathogenic Escherichia coli strains. Antibiotic resistance is reported towards ß-lactam antibiotics (ampicillin), aminoglycosides, quinolones, chloramphenicol, tetracycline (doxycycline), and trimethoprim [17]. Diarrhea is treated with orally administered colistin in children and infants [16].

Table 9.
Effective Inhibitors of Escherichia coli, oral TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Colistin | Mou-o | LD >2000 | 3 | 0,5 – 10 | >4000 –200 | Drug |
| trans-Nerolidol | Mou-o | ***15000 | 1 | ****125 | 120 | Food |
| para-Thymol | Mou-o | 6280 | 2 | 60 – 96 | 104 - 65 | --- |
| Diethyl fumarate | Rat-o | 1780 | 2 | 10 – 100 | 178 – 18 | --- |
| Hinokitiol | Rat-o | >500 | 12 | 1,56 – 120 | >320 – 6 | --- |
| Sorbic acid | Rat-o | 7360 | 11 | 50 - <2000 | 147 - <3,7 | Food*, ** |
| Butylparaben | Mou-o | 13200 | 6 | 120 – 4000 | 110 – 3,3 | Food |
| Vanillin | Mou-o | 3925 | 5 | 25 – 2000 | 157 – 2 | Food |
| Eugenol | Mou-o | 3000 | 23 | <25 – 2100 | >120 – 1,4 | Food* |
| Linoleic acid | Mou-o | >50000 | 2 | 90 - >100000 | >555 - <0,2 | Food* |
| Citral | Mou-o | 6000 | 16 | 12,5 – 6660 | 480 – 0 | Food** |
| Nerolidol | Mou-o | 15000 | 4 | 400 – 9500 | 37,5 – 1,6 | Food |
| Thymol | Mou-o | 640 | 31 | 100 – 1000 | 6,4 – 0,6 | Food** |
Notes: * may cause digestive tract irritation ** mutagen *** calc. with nerolidol toxicity, **** minimal bactericidal conc.
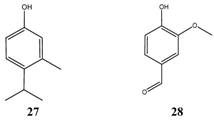
Only a few compounds are effective inhibitors of Escherichia coli (Table 9). The most interesting one, a synthetic isomer of thymol 8 with the name para-thymol 27, is about 10 times less toxic than thymol (TI max. = 6,4) itself. Not much data is available for para-thymol, e.g. spectrum of antimicrobial activity, side effects. The trans-isomer of nerolidol had strong inhibitory properties, but further testing is necessary, because stereochemically undefined nerolidol 20 was much less active. The results of hinikitiol 19 vary and were influenced by different test conditions. Variation of data is given also with citral 18, vanillin 28 and eugenol 3.
3.4. Salmonella species
Salmonella species have been isolated from humans and almost all animals throughout the world and they constitute another genus being prominent for causing diarrhea in humans all kind of age. Humans present the only known reservoir for Salmonella typhi and paratyphi serotypes. Resistances of
Salmonella species towards antibiotics are reported from tetracycline, trimethoprim, quinolones and chloramphenicol [17]. In diarrhea treatment caused by Salmonella infections the drug of first choice is amoxicillin. Alternatively, quinolones, ceftriaxone and cotrimoxazol can be used [16].

Table 10.
Effective Inhibitors of Salmonella sp., oral TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Ceftriaxone | Mou-o | >10000 | 90 | 0,031 – 0,25 | 322000 - 40000 | Drug |
| Amoxicillin | Mou-o | >25000 | >100 | 0,01 - >100 | >2500000 - <250 | Drug |
| Isoborneol | Rat-o | 5200 | 1 | 50 | 104 | Food |
| Ciprofloxaxin | Mou-o | 5000 | >100 | 0,008 – >64 | 625000 - <78 | Drug |
| Cotrimoxazol | Mou-o | 3740 | >100 | <0,015 - >100 | 250000 - <37 | Drug |
| Butylparaben | Mou-o | 13200 | 6 | 125 – 1000 | 105 – 13 | Food |
| p-Benzylphenol | Mou-o | >20000 | 2 | *160 – 2170 | >125 – 9 | --- |
| Allyl isothiocyanate | Mou-o | 308 | 5 | 1 – 47 | 308 – 6,5 | Food |
Notes: * bactericidal after15 min. exposure
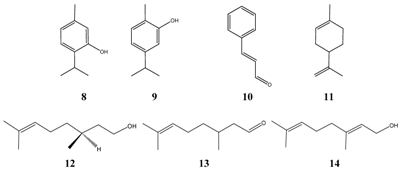
Similar to Escherichia coli, not many compounds are selected (Table 10). Allyl isothiocyanate 29 or with its more common name ‘oil of mustard’ (German “Senföl”) is a very irritant compound with pungent taste. Its inhibitory data as well as the data of the preservative butylparaben 30 is not conclusive due to the small number of tested Salmonella strains, respectively. Nevertheless, butylparaben was shown previously as effective compound against E. coli. Only one data record exists with isoborneol 24, which occurs together with borneol as main constituents of the essential oil of annual wormwood (Artemisia annua), a plant growing in India.
3.5. Mycobacterium tuberculosis
Most species of Mycobacteria are living in soil or water in contrast to microorganisms belonging to the Mycobacterium tuberculosis complex (M. bovis, M. microti, and M. africanum, M. tuberculosis), which are found in diseased tissues of humans and warm-blooded animals suffering on tuberculosis. Resistances are found in particular towards all antibiotic drugs (ethambutol, rifampicin, streptomycin, isoniazid, and pyrazinamide), which represents a global problem [17]. The calculation of TIs resulted in a new ranking favoring compounds from higher plants (Table 11).

Table 11.
Effective Inhibitors of Mycobacterium tuberculosis, oral TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | MIC-res | TI-res | Status |
|---|---|---|---|---|---|---|---|---|
| cis-Phytol | Rat-o | **>5000 | 1 | 2 | >2500 | --- | --- | --- |
| Ethambutol | Mou-o | 8700 | >10 | 0,95 – 7,5 | 9157 – 1160 | ***10 | 870 | Drug |
| Sclareol | Rat-o | >5000 | 1 | 6 | >833 | --- | --- | --- |
| Farnesol | Mou-o | 7400 | 1 | 8 | 825 | --- | --- | Food |
| Propylparaben | Mou-o | 6332 | 1 | 8 | 791 | --- | --- | Food |
| Phenyl salicylate | Rat-o | 3000 | 1 | 4 | 750 | --- | --- | Drug |
| trans-Phytol | Rat-o | **>5000 | 4 | 2 – 8 | >2500 – 625 | --- | --- | --- |
| Streptomycin | Rat-o | 9000 | >30 | 0,1 – 100 | 5000 - 5 | ***16 | 560 | Drug |
| Rifampicin | Mou-o | 500 | >100 | 0,01 – 16 | 50000 – 31 | ***2 | 250 | Drug |
| beta-Naphthol | Rat-o | 1960 | 1 | 8 | 245 | --- | --- | Drug |
| alpha-Naphtol | Rat-o | 1870 | 1 | 8 | 233 | --- | --- | --- |
| Methylparaben | Mou-o | >8000 | 1 | 40 | >200 | --- | --- | Food |
| Pyrazinamide | Mou-o | >3000 | >20 | 4 - 4000 | >750 – 0,7 | ***16 | 187 | Drug |
| asym.-m-Xylenol | Rat-o | 3200 | 1 | 40 | 160 | --- | --- | --- |
| Retinoic acid | Rat-o | 1960 | 3 | 13 | 150 | --- | --- | Food |
| Isoniazid | Mou-o | 133 | >50 | 0,01 – 100 | 13300 – 1 | ***1 | 133 | Drug |
| 4-Hexylresorcinol | Mou-o | 1040 | 2 | * 1 - 8 | 1040 - 130 | --- | --- | Drug |
| Bakuchiol | Mou-o | 2560 | 1 | 10 - 20 | 256 – 128 | --- | --- | --- |
| Retinol | Mou-o | 4000 | 3 | 40 - 50 | 100 - 80 | --- | --- | Food |
| Lupulone | Mou-o | 1500 | 13 | 10 – 110 | 150 – 14 | --- | --- | --- |
| Thymol | Rat-o | 980 | 7 | 8 – 100 | 122 – 10 | --- | --- | Food |
Notes: * bactericidal conc., ** calc. both with toxicity of pythol, *** estimated MIC for resistant strains
The results indicate that several constituents from higher plants seem to be superior to antibiotic drugs being used for therapy of tuberculosis, however, only a small number of strains have been tested so far. Beside sclareol 31 from Clary Sage (Salvia sclarea) the hop (Humulus lupulus) constituent lupulone 7 was selected among most effective inhibitors. The latter was once tested in tuberculosis infected mice, which had received intramuscularly injections of 60 mg/kg body weight over a 4 weeks period, but the renal toxicity of lupulone prevented its development as pharmaceutical drug [25].
Other compounds of Table 11 can be classified in three groups: long-chain aliphatic compounds, aromatic acid esters and phenols.
The selected compounds of group 1, cis- 32 and trans-phytol 33, farnesol 21, retinoic acid 34 and retinol 35 possess structural similarities, which are in particular a long side chain consisting of 3 to 4 isoprene-units plus a terminal polar group. Similarly, fatty acids were strong inhibitors in vitro (data not shown). However, the effectiveness in vivo of all selected lipophilic compounds seems to be questionable due to their possible adsorption to lipophilic lung tissue and inactivation.
Surprisingly, preservatives like methyl- 36 and propylparaben 37 are very successful in the TI-calculations. A further ester of aromatic acids, phenyl salicylate 38, is well known as anti-infective drug for a long time.
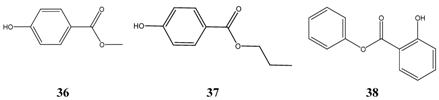
The third group consists of phenols (asym.-m-xylenol 39, 4-hexylresorcinol 40, alpha- 41 and beta-naphtol 42, bakuchiol 15) having a similar range of lipophilicity, of which bakuchiol is the most fat-soluble representative.
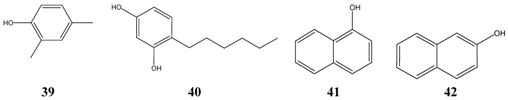
In comparison with the water solubility of antitubercular drugs, all investigated compounds are less soluble in water, which might prevent their use as lung therapeutics.
3.6. Dermatophytes
Skin and hair infections of children (Tinea capitis) are caused by dermatophytes belonging to the genera Trichophyton and Microsporum. They occur either in soil or possess host preference (humans or animals), e.g. Microsporum canis infections are often result of contact between susceptible children and stray kittens [17]. The calculations made with Microsporum species, compounds from higher plants and orally administered antifungal drugs are shown in Table 12.
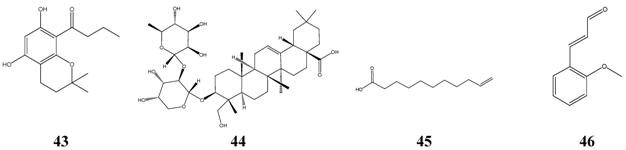
Interestingly, a series of 1’-oxo-substituted phloroglucinols 43 turned out to be as successful and non-toxic inhibitors of Microsporum species. Good results were obtained again with bakuchiol 15 and alpha-hederin 44, which originates from hederasaponine C, the main saponine of Common Ivy (Hedera helix). Alpha-hederin was found to be non-toxic following oral administration, but it was rather toxic when given intravenously. Undecylenic acid 45, a naturally occurring compound, has a long history as antifungal drug. When compared with the lowest level of activity of antibiotics, the effectiveness of alpha-bisabolol 6 was similar to griseofulvin, nystatin, fluconazole, itraconazole and ketoconazole. Other compounds seem be to of minor importance, e.g. o-methoxycinnamaldehyde 46, due to their mutagenic or tumorigenic properties.

Table 12.
Effective Inhibitors of Microsporum Species, oral TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Terbinafine | Mou-o | 4000 | >40 | 0,002 - 1 | 2000000 – 4000 | Drug |
| Oenanthic acid | Mou-o | 6400 | 2 | 2 | 3200 | Food |
| Amphotericin B | Mou-o | >8000 | >100 | 0,125 - 8 | >64000 - 1000 | Drug |
| Decanoic acid | Rat-o | >10000 | 2 | 12,5 | >800 | Food* |
| o-Methoxycinnamaldehyde | Mou-o | 4430 | 2 | 3,13 - 6,25 | 1415 - 708 | Food*** |
| Pelargonic acid | Rat-o | 3200 | 2 | 2 – 5 | 1600 – 640 | Food* |
| 1-(2,4,6-Trihydroxy- 3-isobutyl-phenyl)-hexan-1-one | Mou-o | >1000 | 1 | 1,6 | >625 | --- |
| Bakuchiol | Mou-o | 2560 | 5 | 0,5 - 5 | 27777 - 512 | --- |
| 1-(3-Butyryl-2,4,6-trihydroxy-phenyl)-butan-1-one | Mou-o | >1000 | 1 | 3 | >333 | --- |
| 4-(1,1-Dimethylethyl)phenol | Rat-o | 3250 | 2 | 10 | 325 | ---** |
| alpha-Hederin | Mou-o | >4000 | 1 | 12,5 | >320 | --- |
| Thiabendazole | Mou-o | 2400 | 10 | 0,2 - 7,8 | 12000 – 307 | Drug |
| alpha-Bisabolol **** | Rat-o | 14850 | 1 | 50 | 297 | Food |
| Griseofulvin | Mou-o | >5000 | >100 | 0,25 - 25 | >20000 – 200 | Drug |
| 4-Methyl-1-(2,4,6-trihydroxy-3-isobutyl-phenyl)-pentan-1-one | Mou-o | 1000 | 1 | 6 | 166 | --- |
| Cyclohexyl-(2,4,6-trihydroxy-3-isobutyl-phenyl)-methanone | Mou-o | >1000 | 1 | 6 | >166 | --- |
| 1-(3-Allyl-2,4,6-trihydroxy-phenyl)-propan-1-one | Mou-o | >1000 | 1 | 6 | >166 | --- |
| 1-(3-Allyl-2,4,6-trihydroxy-phenyl)-hexan-1-one | Mou-o | >1000 | 1 | 6 | >166 | --- |
| 1-(3-Allyl-2,4,6-trihydroxy-phenyl)-4-methyl-pentan-1-one | Mou-o | >1000 | 1 | 6 | >166 | --- |
| 1-(3-Benzyl-2,4,6-trihydroxy-phenyl)-4-methyl-pentan-1-one | Mou-o | 1000 | 1 | 6 | 166 | --- |
| 1-(5,7-Dihydroxy-2,2-dimethyl-chroman-8-yl)-butan-1-one | Mou-o | >1000 | 1 | 6 | >166 | --- |
| Nystatin | Mou-o | 8000 | 10 | <0,1 - 50 | 80000 - 160 | Drug |
| Lauric acid | Rat-o | 12000 | 100 | 100 | 120 | Food* |
| Undecylenic acid | Mou-o | 8150 | 6 | 25 - 100 | 326 - 81 | Drug* |
| Fluconazole | Mou-o | 1408 | >100 | 0,06 - >64 | 23467 - 33 | Drug |
| Itraconazole | Mou-o | >320 | >100 | 0,03 - 10 | >10666 - 32 | Drug |
| Citral | Mou-o | 6000 | 3 | 20 - 190 | 288 - 31 | Food*** |
| Ketoconazole | Mou-o | 618 | >100 | 0,01 - 50 | 61800 - 12 | Drug |
| Miconazole | Mou-iv | *****90 | >100 | 0,01 - 16 | 9000 – 6 | Drug |
Notes: * may cause digestive tract irritation, ** tumorigen, *** mutagen, **** active against ‘dermatophytes’, ***** intravenous LD50
When the activity of essential oil and related compounds was examined on their effectiveness against Trichophyton species three sesquiterpene alcohols and two C-11 alcohols were selected (Table 13).

Table 13.
Effective Inhibitors of Trichophyton Species, oral TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Undecylenic alcohol | Rat-o | >5000 | 1 | 10 | 500 | Food* |
| Farnesol | Rat-o | 6000 | 1 | 12,5 | 480 | Food |
| Nerolidol | Rat-o | >5000 | 1 | 12,5 | 400 | Food |
| 4-(1,1-Dimethylethyl)phenol | Rat-o | 3250 ul/kg | 5 | 10 | 325 | ---*** |
| n-Undecyl alcohol | Rat-o | 3000 | 1 | 12,5 | 240 | Food* |
| alpha-Bisabolol | Rat-o | 14850 | 6 | 50 – 100 | 297 – 149 | Food |
| Piperonal | Rat-o | 2700 | 1 | 25 | 108 | Food*, ** |
| cis-Jasmone | Rat-o | 5000 | 1 | 50 | 100 | Food* |
| Cinnamic aldehyde | Rat-o | 2220 | 18 | 5 – 400 | 444 – 1 | Food*, ** |
Notes: * may cause skin irritation, ** mutagen, *** tumorigen
The structures of further effective inhibitors (4-(1,1-dimethylethyl)phenol 47, piperonal 48, cis-jasmone 49) are shown next, which are all reported to have undesired side effects.
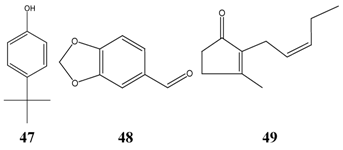
To get information about the most successful compounds by the way of dermal application, the TIs of compounds against Microsporum species were calculated. As result, only a few compounds were selected as effective inhibitors and none of the investigated natural compounds were free of side effects. The highest calculations were obtained with the synthetic antifungal drugs haloprogine and triclosan (Table 14).

Table 14.
Effective Inhibitors of Microsporum Species, dermal TI max. >= 100.
| Trivial name | Animal test | LD50-dermal mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Haloprogine | Rabbit-dermal | 1625 | 10 | 0,001 - 0,4 | 1625000 - 4062 | Drug |
| Triclosan | Rabbit-dermal | 9300 | 5 | 1 – 10 | 9300 - 930 | Drug |
| o-Methoxycinnamaldehyde | Rabbit-dermal | >5000 | 2 | 3,13 – 6,25 | >1597 – 625 | Food* |
| 4-(1,1-Dimethylethyl)phenol | Rabbit-dermal | 2520 | 1 | 10 | 252 | ---** |
| Citral | Rabbit-dermal | 2250 | 3 | 20,8 – 190 | 108 - 12 | Food* |
| Hexachlorophene | Rat-dermal | >600 | 7 | 1 – 100 | >600 - 6 | Drug |
Notes: *mutagen, ** tumorigen
It would be desirable to compare dermal TIs of natural compounds with drugs used to treat topical fungal infection. However, the dermal toxicity of most of topical antifungal drugs is not published [10]. A list of TIs calculated with oral toxicity data is shown below (Table 15).

Table 15.
Effectiveness of Topical Antifungals towards Microsporum Species.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Tolciclate | Mou-o | 4000 | >20 | 0,001 - 0,13 | 1000000 - 64516 | Drug |
| Sulconazole | Mou-o | nitrate: 2475 | 2 | <0,04 – 0,08 | >61875 – 30937 | Drug |
| Siccanin | Mou-o | >6000 | 2 | 0,8 - 1,6 | >7500 - 3750 | Drug |
| Oxiconazole | Mou-o | nitrate: 2630 | 2 | 0,3 - 1 | 8766 - 2630 | Drug |
| Tioconazole | Mou-o | 1870 | 10 | 0,2 - 1,56 | 9350 - 1200 | Drug |
| Fenticonazole | Mou-o | >3000 | 1 | 2,5 | >1200 | Drug |
| Amphotericin B | Mou-o | >8000 | >100 | 0,125 - 8 | >64000 - 1000 | Drug |
| Amorolfin | Mou-o | 400 | 50 | 0,001 - 0,2 | 400000 - 800 | Drug |
| Bifonazole | Mou-o | 2629 | >100 | 0,025 - 5 | 105000 - 525 | Drug |
| Triclosan | Mou-o | 4530 | 4 | 0 - 10 | 4530 – 453 | Drug |
| Flutrimazole | Mou-o | >1000 | 14 | 0,025 - 2,5 | >40000 – 400 | Drug |
| Econazole | Mou-o | nitrate: 463 | 10 | 0,03 - 12,5 | 15433 – 370 | Drug |
| Clotrimazol | Mou-o | 923 | >100 | 0,01 - 2,69 | 92300 – 369 | Drug |
| Ciclopirox | Mou-o | 1740 | >40 | 0,98 - 10 | 1775 – 174 | Drug |
| Nystatin | Mou-o | 8000 | 10 | <0,1 - 50 | 80000 - 160 | Drug |
| Natamycin | Mou-o | 1500 | 1 | 12,5 | 120 | Drug |
| Haloprogine | Mou-o | LD >3000 | 7 | 31,2 | >2000000 - 96 | Drug |
| Miconazole | Mou-o | 519 | >100 | 0,01 - 16 | 51900 - 16 | Drug |
| Ketoconazole | Mou-o | 618 | >100 | 0,01 - 50 | 61800 - 12 | Drug |
| Tolnaftate | Mou-o | 10000 | >60 | 0,0015 - 1000 | 6666666 - 10 | Drug |
| Protiofate | Mou-o | 423 | 8 | 0,78 - 50 | 542 - 8 | Drug |
3.7. Candida albicans
Yeasts live as normal microorganisms in and on the human body. Many species are without any clinical significance, however, some of them develop pathological changes in debilitated persons, e.g. following administration of anticancer drugs or immunsuppressive agents such as corticosteroids and by overuse of broad-spectrum antibiotics [17]. Candida albicans causes systemic and topical infections in children, and therefore, respective TIs are calculated for oral (Table 16) and topical (Table 17) administration routes.

Table 16.
Effective Inhibitors of Candida albicans, oral TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Lapachol | Mou-o | 487 | 1 | 0,03 | 16233 | --- |
| Dodecanal | Rat-o | 23000 | 1 | 25 | 920 | Food* |
| Linoleic acid | Mou-o | >50000 | 1 | 125 | >400 | Food* |
| Swartziadione | Mou-o | LDL0 >300 | 1 | 0,78 | >384 | --- |
| Cerulenin | Mou-o | 547 | 1 | <1,5 | >364 | --- |
| Amphotericin B | Mou-o | >8000 | >100 | 0,02 - 25 | >400000 - 320 | Drug |
| Ketoconazole | Mou-o | 618 | >100 | 0,039 - 2 | 19776 - 309 | Drug |
| Nystatin | Mou-o | 8000 | >100 | 0,78 - 33 | 10256 - 242 | Drug |
| Oenanthic acid | Mou-o | 6400 | 1 | 32,5 | 197 | Food |
| Fluconazole | Mou-o | 1408 | >100 | <=0,0313 - 8 | >45000 - 176 | Drug |
| Flucytosine | Mou-o | >15000 | >100 | 0,005 - >128 | >3000000 - <117 | Drug |
| 4-(1,1-Dimethylethyl)phenol | Rat-o | 3250 | 1 | 31,25 | 104 | ---** |
| Butylparaben | Mou-o | 13200 | 6 | 75 - 130 | 176 - 101 | Food |
| o-Methoxycinnamaldehyde | Rat-o | >5000 | 3 | 50 | >100 | Food*** |
| Decylenic alcohol | Rat-o | >10000 | 1 | 100 | >100 | --- |
| Octanoic acid | Rat-o | 10080 | 2 | 18 – 145 | 560 – 69 | Food* |
| alpha-Bisabolol | Mou-o | 11350 | 4 | 100 – 500 | 113 - 22 | --- |
| Miconazole | Mou-iv | 90 | 82 | 0,02 - >25 | 4500 - <3,6 | Drug |
| Citral | Mou-o | 6000 | 16 | 10 – 10000 | 600 – 0 | Food |
| n-Dodecanol | Rat-o | >12800 | 2 | 25 – 100000 | >512 - 0 | Food* |
| Undecylenic acid | Mou-o | 8150 | 1 | 70 – 1000 | 116 - 8 | Drug* |
| Sorbic acid | Mou-o | 3200 | 1 | 25 – 10000 | 128 - 0 | Food |
Notes: * may cause digestive tract irritation, ** tumorigen, *** mutagen
The most successful compound against Candida albicans was lapachol 50 according to the oral TI calculations. Lapachol is a green-yellow substance found in the inner bark of Brazilian Taheboo tree (Tabebuia avellanedae), which is traditionally used in South America to treat Candida and bacterial infections. Lapachol inhibited growth of Candida albicans at a very low concentration, but the germicidal concentration was found to be much higher (32 ppm). Beside long-chains aliphatics having a terminal polar group and 8 to 12 carbon atoms, swartziadione 16 was selected, a structurally related compound to lapachol, and in addition, cerulenin 51 from microbial origin (e.g. Cephalosporium caerulens). Cerulenin was found to inhibit growth of yeast-type fungi by inhibiting the biosynthesis of fatty acids [26]. All before mentioned compounds superimpose the minimum TIs of orally administered antibiotics usually taken to treat Candida infections. Among terpenoids only alpha-bisabolol resulted in promising a TI, while acute toxicity data of other interesting compounds have not been determined.
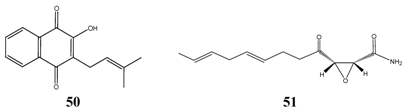
When calculating dermal TIs of anti-candidal drugs no results were calculable with standard therapeutics amphothericin B, nystatin, clotrimazole and miconazol due to missing acute dermal toxicity data [10]. The residual calculations of the most effective compounds are shown in Table 17.

Table 17.
Effective Inhibitors of Candida albicans, dermal TI max. >= 100.
| Trivial name | Animal test | LD50-oral mg/kg bw | n-strains | MIC range in ppm | TI max. - TI min. | Status |
|---|---|---|---|---|---|---|
| Capsaicin | Mou-dermal | >512 | 1 | <1,5 | >340 | Food**, *** |
| Triclosan | Rabbit-dermal | 9300 | 12 | 10 - 33 | 930 - 282 | Drug |
| Haloprogine | Rabbit-dermal | 1625 | >100 | 0,05 - 6,25 | 32500 - 260 | Drug |
| Sclareol | Rabbit-dermal | >5000 | 1 | 32 | >156 | --- |
| Undecanal | Rabbit-dermal | >5000 | 1 | 50 | >100 | Food* |
| o-Methoxycinnamaldehyde | Rabbit-dermal | >5000 | 2 | 50 | >100 | Food** |
| Cloconazole | Rabbit-dermal | HCl: >310 | >100 | 0,12 - 8 | >160 - 39 | Drug |
| Caprylic acid | Rabbit-dermal | >5000 | 4 | 18 - 1000 | >277 - 5 | Food* |
| Camphor | Rabbit-dermal | >5000 | 5 | 50 - 2000 | >100 - 2,5 | Food* |
| Citral | Rabbit-dermal | 2250 | 17 | 10 - 10000 | 225 - 0 | Food*, ** |
Notes: * may cause skin irritation, **mutagen, *** tumorigen
Only a few effective compounds were found, of which the most successful compound is sclareol 31 from Clary Sage (Salvia sclarea). This compound was patented to treat human fungal infections and its recommended to be used internally at daily doses of 1 to 1000 mg/kg [27]. Other compounds may have undesired side effects, such as capsaicin 52, the main compound in Chilly (Capsicum frutescens) or Red Pepper (Capsicum annuum).
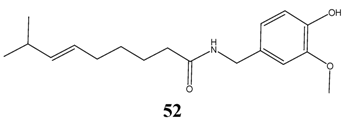
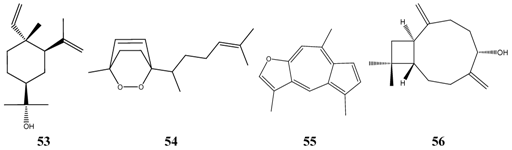
Alpha-bisabolol (MIC = 100 ppm) 6 is widely used in topical cosmetic preparations and it is regarded as safe on human skin, although its numeric dermal LD50 value has not been published [19]. A few other compounds show also promising activity against Candida albicans, however, their acute toxicity is unknown. These compounds are commercially available elemol (MIC = 70 ppm) 53, while others (3,6-epoxydioxy-bisabola-1,10-diene: MIC: 6,25 ppm 54, linderazulene: 12,5 ppm 55 and caryophyllodienol: 12,5 ppm 56) are isolated from plants only in a few cases.
3.8. Other Important Microorganisms Causing Children’s Infections
About causative microorganism of other important diseases typical for children, such like pertussis, epiglottis, tetanus and diphtheria, not much information is obtained about effective inhibitors in TI calculations.
Bordetella pertussis (responsible for pertussis) was found to be susceptible towards aliphatic acids. No effective compound was found in the cases of Haemophilus influenzae (epiglottis) and Clostridium tetani (tetanus). Alpha-bisabolol (oral TI > 200) 6 and to a lesser extend camphor (oral TI = 90) 2 were effective against the diphtheria causing microorganism Corynebacterium diphtheriae.
4. Discussion
The effectiveness of antibiotics used to treat children’s diseases is superior to that of compounds produced by higher plants according to the before mentioned criteria and data analysis.
The antimicrobial plant constituents differ in their spectrum of activity from antibiotic drugs and show only poor effectiveness against gram-negative pathogens, such like Escherichia coli, Salmonella species, Bordetella pertussis and Haemophilus influenzae. Several effective inhibitors of low mammalian toxicity (TI min. >100) were selected in the analysis with gram-positive bacteria: Streptococcus pneumoniae (m-menthene-phenol 5, alpha-bisabolol 6, lupulone 7), Staphylococcus aureus (m-menthene-phenol 5, alpha-bisabolol 6, lupulone 7, bakuchiol 15, swartziadione 16, shikonin 17, hinokitiol 19) and Mycobacterium tuberculosis (bakuchiol 15, farnesol 21, sclareol 31, cis- 32 and trans-phytol 33, vitamin A acid 34, methyl- 36 and propylparaben 37, phenylsalicylate 38, asym.-m-xylenol 39, hexyresorcinol 40, alpha- 41 and beta-naphthol 42). As effective inhibitors of dermatophytic fungi were found: alpha-bisabolol 6, bakuchiol 15, nerolidol 20, farnesol 21, a series of phenylbutanone derivatives 43, alpha-hederin 44, o-methoxycinnamaldehyde 46, 4-(1,1-dimethylethyl)phenol 47, piperonal 48, and cis-jasmone 49. As the most successful compound against Candida albicans turned out to be: swartziadione 15, sclareol 31, lapachol 50, capsaicin 52. In addition, alpha-bisabolol 6 was effective against Corynebacterium diphtheriae.
Among the typical components of essential oils especially sesquiterpene and diterpene alcohols were selected (alpha-bisabolol 6, nerolidol 20, farnesol 21, cis- 32 and trans-phytol 33, sclareol 31). Monoterpenes, such as thymol 8 or carvacrol 9, and phenylpropanes, such as eugenol 3, being prominent for their antimicrobial activity failed in this analysis due to their comparatively high mammalian toxicity.
Not many of the isolated natural inhibitors of children’s pathogens have been tested against a greater number of microbial strains of one species as it the case with standard therapeutics. Thus, the ranges of antimicrobial activity are generally unknown, which are important for the judgement about the usefulness of such compounds as therapeutic agent. Another important topic aspect is the use of non-standardized test methods in microbiology, which also causes variation of data.
The calculation of oral TIs aimed to analyze the effectiveness of compounds by oral application. Other than in laboratory in vitro experiments an orally administered compound undergoes metabolic changes inside the body of living organism. For instance, the metabolites of trans-anethole 57, the main constituent of the essential oil of anise (Pimpinella anisum), were studied in two human volunteers [28]. Following ingestion of 1 mg methoxy 14C-labelled trans-anethole approx. 21% of the administered dose was demethylated within 8 hours as it could be determined by the amount of exhaled 14CO2. Within 24 hours approx. 65% of the totally administered radioactivity was found in the urine, while about 10% of the radioactivity was not recovered (Figure 2).
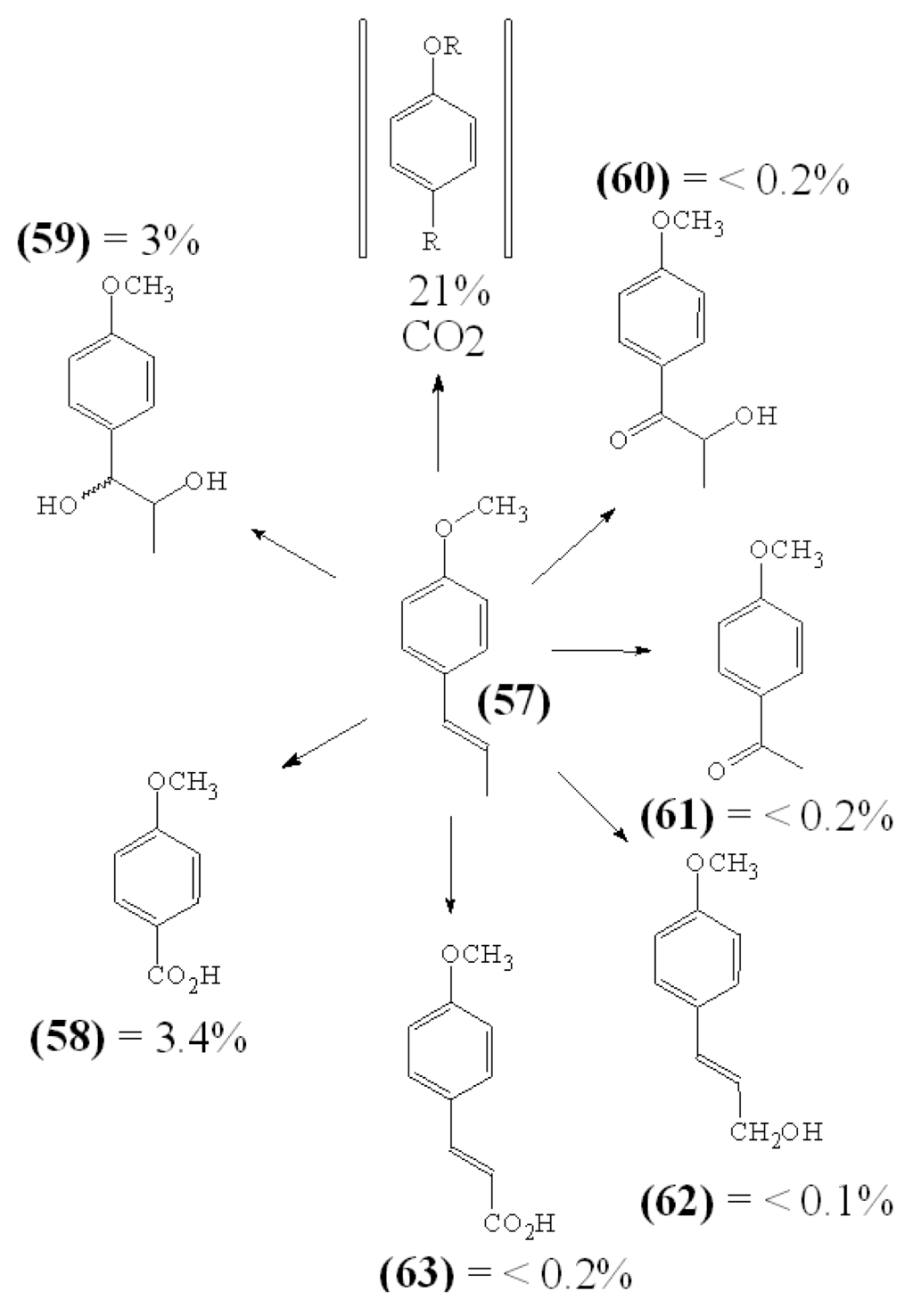
Figure 2.
Metabolization of Orally Administered trans-Anethole in Humans.
Among urinary metabolites main conversion products were O-demethylated compounds and compounds of known structure 57 to 62. This rapid biotransformation of a trans-anethole may help to interpret the findings obtained in a comparative in vivo - in vitro study with mycobacteria and trans-anethole [29]. Anethole was most effective in tuberculosis protection of guinea pigs in vivo, but failed to have considerable activity in vitro (Table 18).

Table 18.
Comparison of the in vitro Inhibition of Mycobacterium tuberculosis by Essential Oils and some of their Components with the in vivo Disease Protection by such Materials of Guinea Pigs Infected with Tubercle bacilli.
| Test materials | in vivo | in vitro |
|---|---|---|
| Anethole | ++++ | ++ |
| Lemon oil | +++ | + |
| Nutmeg oil | ++ | + |
| Terpineol | ++ | ++++ |
| d-Limonene | + | +++ |
| l-Limonene | + | +++ |
| Geraniol | + | ++ |
| Eugenol | + | ++++ |
| d-Pinene | + | +++ |
| l-Pinene | - | ++ |
Notes: ++++ very active, +++ moderate active, ++ active, poor effect, - no effect
By means of these results, it is obvious that the effects of test compounds can not be explained with in vitro testing alone, although this method-strategy was successful many times in antibiotic research.
If a metabolizing system, however, is absent or plays a minor role, the active compound is available without major losses. Treatment of localized infections of outer and inner surfaces of the body with antimicrobials from higher plants is imaginable, e.g. topical application onto skin, inhalation (respiratory tract) and ingestion (gastrointestinal tract).
The compounds selected on the basis of dermal toxicity data are may be useful in the therapy or support of therapy of localized skin infections. Especially, several alcohols occurring in essential oils were effective against hair and skin pathogens, which are n-undecanol, n-undecenol, alpha-bisabolol 6, nerolidol 20 and farnesol 21.
Inhalation of volatile antimicrobials causes direct contact to undesired microorganisms present on mucous membranes of the respiratory tract. Similar to entire essential oils, a treatment of respiratory tract diseases with selected compounds from essential oils is imaginable.
In the gastrointestinal tract structural changes of essential oil compounds are known and influence in part markedly the chemical constitution. For instance, reports exist on rapid rearrangement of linalool to geraniol 14 through influence of gastric juice in the stomach [30], or the formation 3,4-dihydroxy-propylbenzene from methyl isoeugenol (3,4-dimethoxy-1-propenylbenzene) by action of the caecal microbial flora [31].
Appearance of essential oil compounds in the bloodstream following administration to skin and percutaneous resorption [32] or following inhalation [33] was demonstrated in humans and animals, respectively, which implies further pharmacological effects.
Among compounds found in essential oils, alpha-bisabolol 6 from Chamomile turned out to be safe following inhalation and simultaneously it is inhibitory to several microbial species pathogenic for children.
5. Conclusion
A comparison of the effectiveness of natural compounds with such of antibiotics on the basis of oral ‘Therapeutic Indices’ calculations revealed that natural compounds are inferior to antibiotics in general. The natural compounds are mild oral antimicrobials and they are unfit to treat severe infections of children.
Only in the case of tuberculosis several natural compounds of low mammalian toxicity and strong in vitro activity exist, however, these findings need further verification in living organisms.
Despite the limited value of essential oil compounds as oral antibiotics, the dermal ‘Therapeutic Index’ calculations may lead to findings, which are helpful in the therapy or in the supportive therapy of skin infections in children. Inhalation of isolated compounds from essential oil may be also useful in the support of therapy of respiratory tract infections.
This data analysis shows that the natural resources of antimicrobials are not fully explored. The effectiveness of most of the natural compounds as antimicrobials cannot be fully evaluated due to many missing toxicity data. The existing data material on volatile sesquiterpenes indicates their low mammalian toxicity and relatively strong antimicrobial activity, and therefore, this group of compounds turned out to be the most interesting one.
References
- Schilcher, H. Pharmakologie und Toxikologie ätherischer Öle. Therapiewoche 1986, 36, 1100–1112. [Google Scholar]
- Österreichisches Arzneibuch (1990) + supplements 1-6 (1990-1999), European Pharmacopoeia 3 (1997) + supplements (1998 – 2000), Pharmacopée Française X + supplements 1-13 (1983 – 1996), Deutsches Arzneibuch (2000) and Deutscher Arzneimittel-Codex (1997-2000), Farmacopea Ufficiale della Repubblica Italiana IX + supplements I-IV (1985 – 1996), The Japanese Pharmacopoeia XIII (1996) + supplements I + II (1999), Pharmacopoeia Helvetica 8 (1997) + supplements (1998-1999), Britisch Pharmacopoeia (1999) and US National Formulary 19 (2000) + supplements (2000).
- Craig, J.O. Poisoning by the Volatile Oils in Childhood. Archives of Disease in Childhood 1953, 28, 475–483. [Google Scholar]
- Schilcher, H. Sind pflanzliche Arzneimittel bzw. ist die “NATURMEDIZIN” eine Gefahr für den Anwender. Ärztezeitschrift für Naturheilverfahren 2002, 43, 253–254. [Google Scholar]
- Dorsch, W.; Loew, D.; Meyer-Buchtela, E.; Schilcher, H. Kinderdosierungen von Phytopharmaka. Teil I. Empfehlungen zur Anwendung und Dosierungen von Phytopharmaka, monographierte Arzneidrogen und ihren Zubereitungen in der Pädiatrie, 2nd ed; Kooperation Phytopharmaka GbR: Bonn, 1998; pp. 9–185, ISBN 3-929964-14-7. [Google Scholar]
- Giacosa, P. Studj sulla azione fistologica di alcune sostanze aromatiche messa in rapporto collo loro struttura atomica. Ann. Chim. Medicofarm. Farm. Ser. 1886, 3, 273–293. [Google Scholar]
- Schilcher, H. Phytotherapie in der Kinderheilkunde – Handbuch für Ärzte und Apotheker, 3rd ed; Wissenschaftliche Verlagsgesellschaft mbH Stuttgart: Stuttgart, 1999; ISBN 3-8047-1644-X. [Google Scholar]
- Kuschinski, G.; Lüllmann, H.; Peters, T. Kurzes Lehrbuch der Pharmakologie und Toxikologie, 10th ed; Georg Thieme Verlag: Stuttgart – New York, 1984; ISBN 3-13-368510-4. [Google Scholar]
- Pauli, A. AMICBASE-EssOil: Database on Natural Antimicrobials and Officinal Antiinfectives. ReviewScience. 2003. Zirndorf. Available online: http://www.reviewscience.com.
- Woodard, D.; Stuart, R. Toxicology Reports; Vermont Safety Information Resources, Inc.: Vermont, 2003. Available online: http://www.hazard.com/msds.
- Merck Sharp and Dohme Research Laboratories <Rahway, N.Y.>, Merck Manual of Diagnosis and Therapy, 16th ed; Urban & Schwarzenberg: München – Wien – Baltimore, 1993; ISBN 3-541-01855-0.
- World Healh Organization, World Health Report: Global Burden of Disease Estimates; Geneva, 2002.
- Kahlmeter, G.; Olsson-Liljequist, B. Species related MIC-breakpoints; Landstinget Kronoberg: Kronoberg, 2003. Available online: http://www.ltkronoberg.se.
- World Health Organization: Department of Communicable Disease Surveillance and Response, Whonet 5: Microbiology Laboratory, Database Software; World Health Organization (Geneva) and WHO Collaborating Centre for Surveillance of Antimicrobial Resistance: Microbiology Department, Brigham and Women's Hospital, Boston, 1999.
- United States Food and Drug Administration, Code of Federal Regulations. Title 21. Food and Drugs; Rockville, 2003.
- Daschner, F. Antibiotika am Krankenbett, 11th ed; Springer Verlag: Berlin – Heidelberg – New York – Barcelona – Hongkong – London – Mailand – Paris – Singapur – Tokio., 2002. [Google Scholar]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Yolken, R.H. (Eds.) Manual of Clinical Microbiology; ASM Press: Washington, D.C., 1995; ISBN 1-55581-086-1.
- Lamar, R.V. Some Further Observations Upon the Action of Certain Soaps on the Pneumococcus and its Experimental Infection. Journal of Experimental Medicine (New York) 1911, 14, 256–264. [Google Scholar]
- Anonymous. Final report on the Safety Assessment of Bisabolol. International Journal of Toxicology 1999, 18 (Supplement 3), 33–40. [Google Scholar]
- Sieradzki, K.; Roberts, R.B.; Haber, S.W.; Tomasz, A. The Development of Vancomycin Resistance in a Patient with Methicillin-Resistant Staphylococcus aureus Infection. The New England Journal of Medicine 1999, 340, 517–523. [Google Scholar]
- Endtz, H.P.; Mouton, J.W.; Den Hollander, J.G.; Van den Braak, N.; Verbrugh, H.A. Comparative in vitro Activities of Trovafloxacin (CP-99,219) against 445 Gram-positive Isolates from Patients with Endocarditis and those with other Bloodstream Infections. Antimicrobial Agents and Chemotherapy 1997, 41, 1146–1149. [Google Scholar]
- Katsura, H.; Tsukiyama, R.-I.; Suzuki, A.; Kobayashi, M. In Vitro Antimicrobial Activities of Bakuchiol against Oral Microorganisms. Antimicrobial Agents and Chemotherapy 2001, 45, 3009–3013. [Google Scholar]
- Lin, Y. Compositions for Treatment of Antibiotic-Resistant Gram-Positive Bacterial Infections and Methods for Using and Preparing the Same. United States Patent 6025400, 2000. [Google Scholar]
- Villegas, L.F.; Marcalo, A.; Martin, J.; Fernandez, I.D.; Maldonado, H.; Vaisberg, A.J.; Hammond, G.B. (+)-epi-Alpha-bisabolol [correction of bisbolol] is the Wound-Healing Principle of Peperomia galioides: Investigation of the in Vivo Wound-Healing Activity of Related Terpenoids. Journal of Natural Products 2001, 64, 1357–1359, 2002, 65, 248 (erratum). [Google Scholar]
- Chin, Y.; Anderson, H.H.; Alderton, G.; Lewis, J.C. Antituberculous Activity and Toxicity of Lupulon for the Mouse. Proceedings of the Society for Experimental Biology and Medicine 1949, 70, 158–162. [Google Scholar]
- Royer, G.P.; Townsend, C.A. Cerulenin Compounds for Fatty Acid Synthesis Inhibition. United. States Patent 5539132, 1996. [Google Scholar]
- Nozoe, S.; Takahashi, A.; Masuda, J.; Segawa, T. Antifungal Agent. Japanese Patent 10298070, 1998. [Google Scholar]
- Sangster, S.S.; Caldwell, J.; Hutt, A.J.; Anthony, A.; Smith, R.L. The Metabolic Disposition of [Methoxy-14C]-Labelled trans-Anethole, Estragole and p-Propylanisole in Human Volunteers. Xenobiotica 1987, 17, 1223–1232. [Google Scholar]
- Imura, K. On the Influence of the Ethereal Oils Upon the Culture of Tubercle Bacilli and Upon Development of Experimental Tuberculosis in Animals. Journal of the Shanghai Science Institute (Section 4) 1935, 1, 235–270. [Google Scholar]
- Franchomme, P. Analytical and Pharmacological Approach of Aforementioned Species; Lecture. In 4th International Scientific Aromatherapy Symposium, Grasse, March 2 – 4; 2001. [Google Scholar]
- Solheim, E.; Scheline, R.R. Metabolism of Alkenebenzenes in the Rat. II. Eugenol and Isoeugenol Methyl Ethers. Xenobiotica 1976, 6, 137–150. [Google Scholar]
- Schalter, R. Perkutane Resorption ätherischer Öle mit und ohne Zusatz von Nicotinsäuerestern. Dissertation, München, 1985. [Google Scholar]
- Buchbauer, G.; Jirovetz, L.; Jäger, W.; Plank, C.; Dietrich, H. Fragrance Compounds and Essential Oils with Sedative Effects Upon Inhalation. Journal of Pharmaceutical Sciences 1993, 82, 660–664. [Google Scholar]
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).