Electroanalysis of NADH Using Conducting and Redox Active Polymer/Carbon Nanotubes Modified Electrodes-A Review
Abstract
:1. Introduction
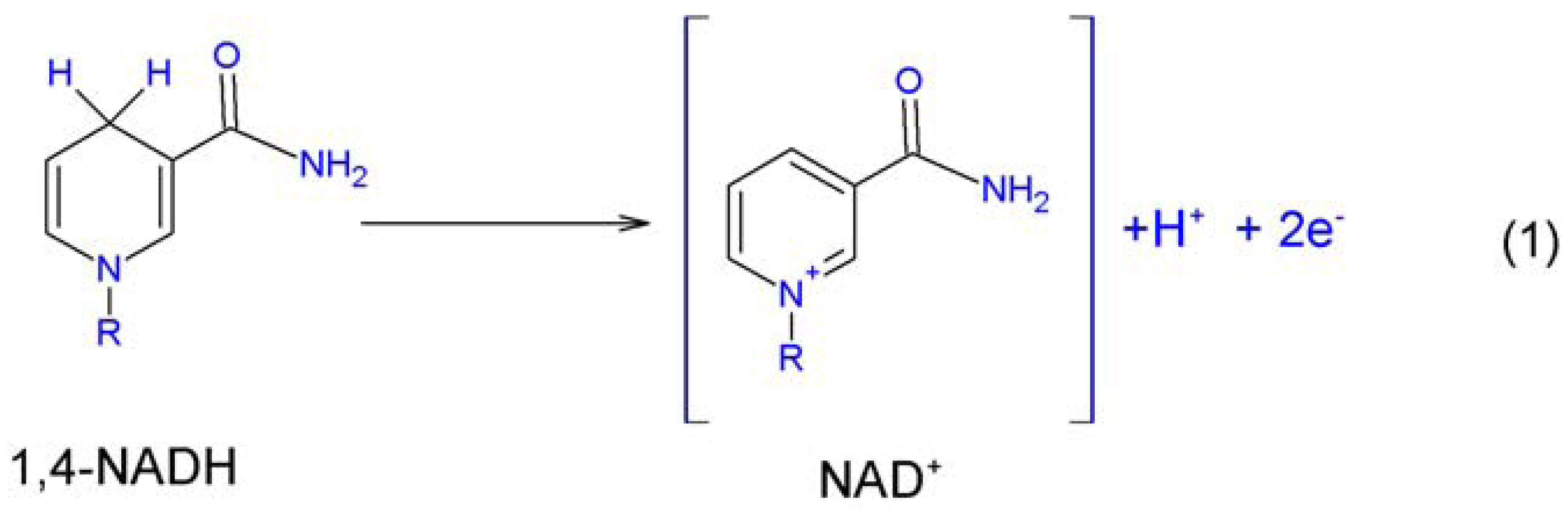
2. Electrocatalysis of NADH
2.1. Electrocatalytic oxidation of NADH at polyaniline (PANI)-co-polymer modified electrodes
2.2. Electrocatalysis of NADH at polymerized dye film modified electrodes
2.3 Photoelectrocatalytic Oxidation of NADH with Electropolymerized Toluidine Blue O
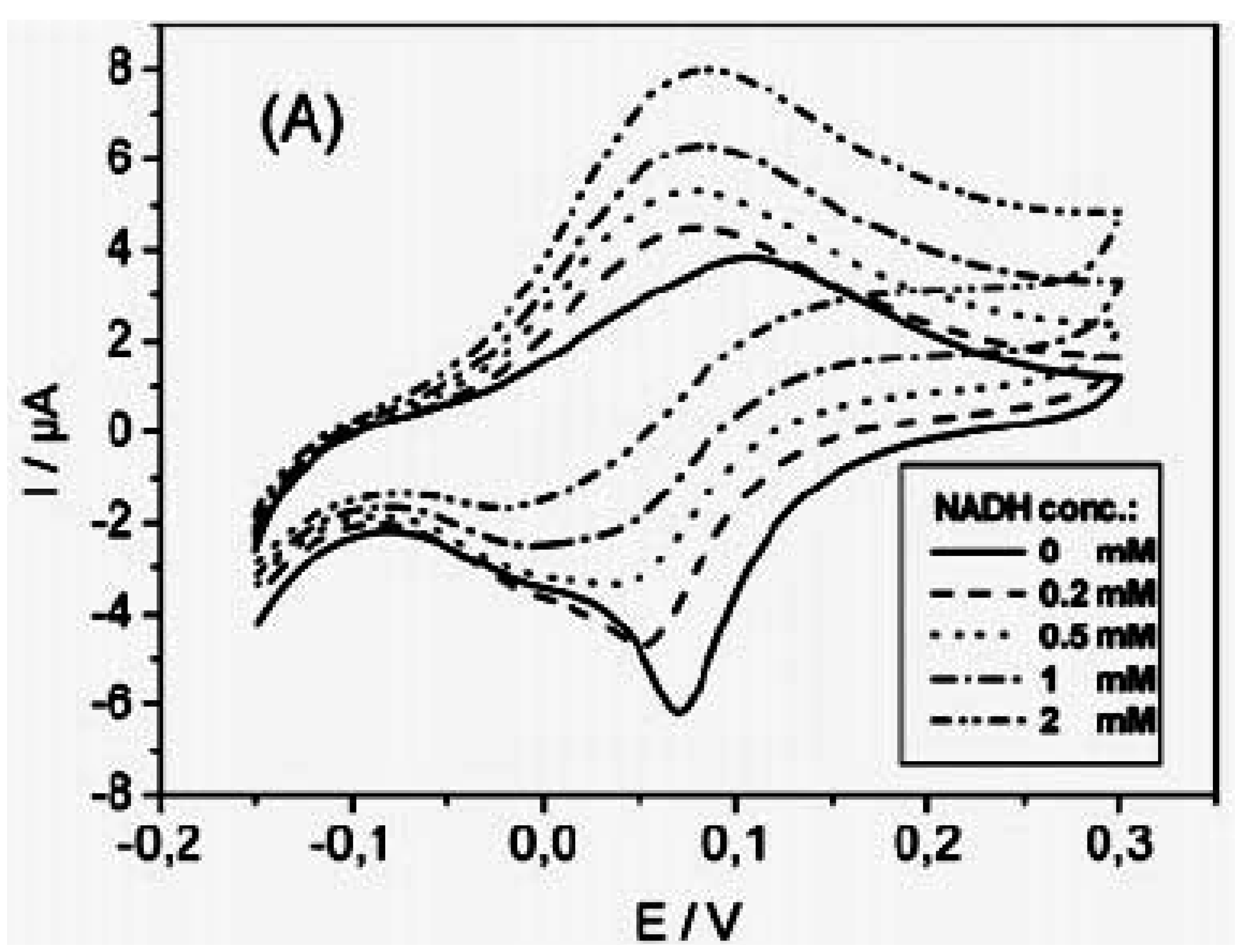
2.4. Electrocatalysis of NADH at polymer/carbon nanotubes composite modified electrodes
2.5 Other carbon nanotube based NADH transducers
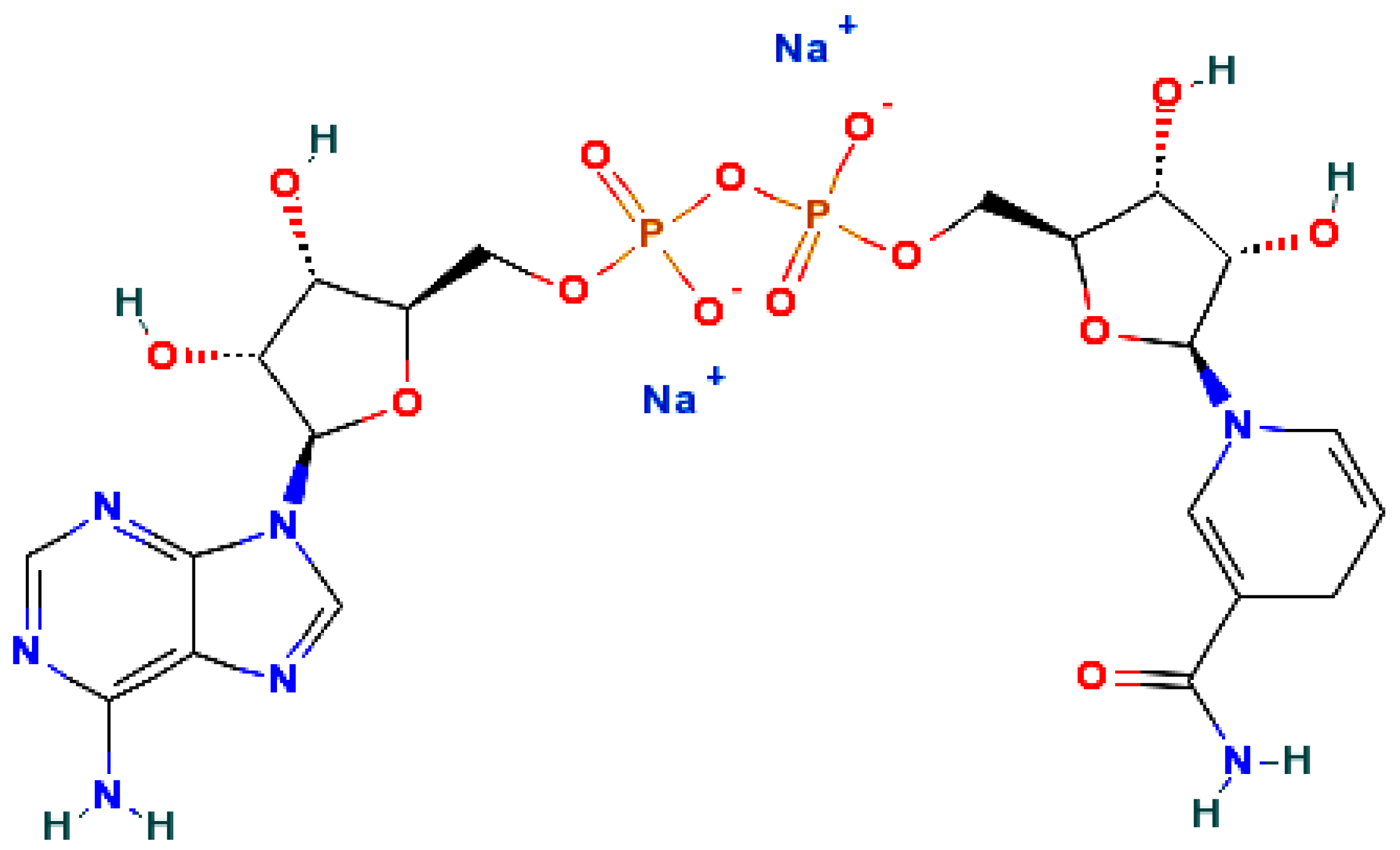
2.6 Biomolecules based NADH transducers
2.7 Mediators immobilized on inorganic materials surface for NADH electrocatalysis
3. Conclusions
Acknowledgments
References
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar]
- Pollak, N.; Dölle, C.; Ziegler, M. The power to reduce: pyridine nucleotides – small molecules with a multitude of functions. Biochem. J. 2007, 402, 205–18. [Google Scholar]
- Bergmer, H.U. (Ed.) Methods of Enzymatic Analysis, Third Edition; Weinheim; Verlag Chemie; Weinhein, Deerfield Beach, Florida, Basel; 1983; Vol. 3, p. 42.
- Turner, A.P.F.; Karube, I.; Wilson, G.S. (Eds.) Biosensors-Fundamentals and Applications; Oxford University Press, 1987; p. 21.
- Murray, R.W. Bard, A.J., Ed.; Electroanalytical Chemistry; Marcel Dekker: New York, 1984; Vol. 13, pp. 191–397. [Google Scholar]
- Murray, R.W. Chemically Modified Electrodes. Accts. Chem. Res. 1980, 13, 135–141. [Google Scholar]
- Redepenning, J.G. Chemically modified electrodes: a general overview. Trends in analytical chemistry 1987, 6, 18–22. [Google Scholar]
- Gorton, L.; Dominguez, E. Electrochemistry of NAD(P)+/NAD(P)H, encyclopedia of electrochemistry. In Bioelectrochemistry; Wilson, G.S., Ed.; Weinheim; Wiley-VCH, 2002; Vol. 9. [Google Scholar]
- Gorton, L.; Dominguez, E. Electrocatalytic oxidation of NAD(P)/H at mediator-modified electrodes. Reviews in Molecular Biotechnology 2002, 82, 371–392. [Google Scholar]
- Malinauskas, A.; Malinauskiene, J.; Ramanavicius, A. Conducting polymer-based nanostructurized materials: electrochemical aspects. Nanotechnology 2005, 16, R51–R62. [Google Scholar]
- Kossmehl, G.; Engelmann, G. Handbook of Oligo and Polythiophenes; Fichou, D., Ed.; Wiley-VCH: New York, 1999; Chapter 10. [Google Scholar]
- Lamy, C.; Leger, J.M.; Garnier, F. Handbook of Organic Conductive Molecules and Polymers; Nalwa, H.S., Ed.; John Wiley & Sons: New York, 1997; Vol. 3, Chapter 10. [Google Scholar]
- Cosnier, S. Biosensors based on electropolymerized films: new trends. Anal. Bioanal. Chem. 2003, 377, 507–520. [Google Scholar]
- Skotheim, T. (Ed.) Handbook of Conducting Polymers; Marcel Dekker: New York, 1986; Vols. 1 and 2.
- MacDiarmid, A.G. Synthetic Metals: A Novel Role for Organic Polymers (Nobel Lecture). Angew. Chem. 2001, 40, 2581–2590. [Google Scholar]
- Kumar, S.A.; Chen, S.M. Electrocatalytic reduction of oxygen and hydrogen peroxide at poly(p-aminobenzene sulfonic acid)-modified glassy carbon electrodes. Journal of Molecular Catalysis A: Chemical 2007, 278, 244–250. [Google Scholar]
- Kumar, S.A.; Tang, C.F.; Chen, S.M. Poly(4-amino-1-1′-azobenzene-3, 4′-disulfonic acid) coated electrode for selective detection of dopamine from its interferences. Talanta 2008, 74, 860–866. [Google Scholar]
- Kumar, S.A.; Chen, S.A. Electrochemical, microscopic, and EQCM studies of cathodic electrodeposition of ZnO/FAD and anodic polymerization of FAD films modified electrodes and their electrocatalytic properties. J Solid State Electrochem. 2007, 11, 993–1006. [Google Scholar]
- Malinauskas, A.; Garjonyt˙e, R.; Mažeikien˙e, R.; Jureviˇcīut˙e, I. Electrochemical response of ascorbic acid at conducting and electrogenerated polymer modified electrodes for electroanalytical applications: a review. Talanta 2004, 64, 121–129. [Google Scholar]
- Rahman, Md. A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Wiles, P.G.; Abrahamson, J. Carbon fibre layers on arc electrodes—I : Their properties and cool-down behavior. Carbon 1978, 16, 341–349. [Google Scholar]
- Abrahamson, J.; Wiles, P.G.; Rhoades, B.L. Structure of carbon fibres found on carbon arc anodes. Carbon 1999, 37, 1873–1874. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.; de Heer, W.A. Carbon Nanotubes--the Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1799. [Google Scholar]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar]
- Chen, R.S.; Huang, W.H.; Tong, H.; Wang, Z.L.; Cheng, J.K. Carbon fiber nanoelectrodes modified by single-walled carbon nanotubes. Anal. Chem. 2003, 75, 6341–6345. [Google Scholar]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes:edge-plane sites and tube ends are the reactive sites. Chem.Commun. 2005, 829–841. [Google Scholar]
- Luo, X.L.; Xu, J.J.; Wang, J.L.; Chen, H.Y. Electrochemically deposited nanocomposite of chitosan and carbon nanotubes for biosensor application. Chem. Commun. 2005, 2169–2171. [Google Scholar]
- Guo, M.L.; Chen, J.H.; Li, J.; Tao, B.; Yao, S.Z. Fabrication of polyaniline/carbon nanotube composite modified electrode and its electrocatalytic property to the reduction of nitrite. Anal. Chim. Acta 2005, 532, 71–77. [Google Scholar]
- Gao, M.; Huang, S.; Dai, L.; Wallace, G.; Gao, R.; Wang, Z. Aligned coaxial nanowires of carbon nanotubes sheathed with conducting polymers. Angew. Chem. 2000, 112, 3810–3813. [Google Scholar]
- Yao, X.; Wu, H.; Wang, J.; Qu, S.; Chen, G. Carbon Nanotube/Poly(methyl methacrylate) (CNT/PMMA) Composite Electrode Fabricated by In Situ Polymerization for Microchip Capillary Electrophoresis. Chem. Eur. J. 2007, 13, 846–853. [Google Scholar]
- Yogeswaran, U.; Thiagarajan, S.; Chen, S.M. Pinecone shape hydroxypropyl-β-cyclodextrin on a film of multi-walled carbon nanotubes coated with gold particles for the simultaneous determination of tyrosine, guanine, adenine and thymine. Carbon 2007, 45, 2783–2796. [Google Scholar]
- Yogeswaran, U.; Chen, S.-M. Electrocatalytic properties of electrodes which are functionalized with composite films of f-MWCNTs incorporated with poly(neutral red). J. Electrochem. Soc. 2007, 154, E178–E186. [Google Scholar]
- Gregory, G.; Wildgoose, G.G.; Banks, C.E.; Leventis, H.C.; Compton, R.G. Chemically Modified Carbon Nanotubes for Use in Electroanalysis. Microchim Acta 2006, 152, 187–214. [Google Scholar]
- Moiroux, J.; Elving, P.J. Effects of adsorption, electrode material, and operational variables on the oxidation of dihydronicotinamide adenine dinucleotide at carbon electrodes. Anal. Chem. 1978, 50, 1056–1062. [Google Scholar]
- Jaegfeldt, H. Adsorption and electrochemical oxidation behaviour of NADH at a clean platinum electrode. J. Electroanal. Chem. 1980, 110, 295–302. [Google Scholar]
- Bartalits, L.; Nagy, G.; Pungor, E. Determination of enzyme activity in biological fluids by means of electrochemical oxidation of NADH at a modified glassy carbon electrode. Anal. Lett. 1984, 17, 13–41. [Google Scholar]
- Bartlett, P.N.; Simon, E.; Toh, C.S. Modified electrodes for NADH oxidation and dehydrogenase-based biosensors. Bioelectrochemistry 2002, 56, 117–122. [Google Scholar]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar]
- Kanazawa, K.K.; Diaz, A.F.; Geiss, R.H.; Gill, W.D.; Kwak, J.F.; Logan, J.A.; Rabolt, J.F.; Street, G.B. Organic metals: polypyrrole, a stable synthetic metallic polymer. J. Chem. Soc., Chem. Commun. 1979, 19, 854–855. [Google Scholar]
- Persaud, K.C. Polymers for chemical sensing. Materials Today 2005, 8, 38–44. [Google Scholar]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc., Chem. Commun. 1977, 578–580. [Google Scholar]
- Shirakawa, H. The discovery of polyacetylene film: The dawning of an era of conducting polymers, Synth. Met. 2001, 125, 3–10. [Google Scholar]
- MacDiarmid, A.J. Synthetic metals: a novel role for organic polymers. Synth. Met. 2001, 125, 11–22. [Google Scholar]
- Heeger, A.J. Semiconducting and metallic polymers: the fourth generation of polymeric materials. Synth. Met. 2001, 125, 23–42. [Google Scholar]
- Skotheim, T. (Ed.) Handbook of Conducting Polymers; Marcel Dekker: New York, 1986; Vols. 1 and 2.
- Nalwa, H.S. Handbook of Organic Conductive Molecules and Polymers; Wiley: Chichester, 1997. [Google Scholar]
- Skotheim, T.A.; Elsenbaumer, R.L.; Reynolds, J.R. (Eds.) Handbook of Conducting Polymers, Second Edition; Marcel Dekker: New York, 1998.
- Lyons, M.E.G. Electroactive Polymer Electrochemistry; Plenum Press: New York, 1994. [Google Scholar]
- Ivaska, A. Analytical applications of conducting polymers. Electroanalysis 1991, 3, 247–254. [Google Scholar]
- Imisides, M.D.; John, R.; Riley, P.J.; Wallace, G.G. The use of lectropolymerization to produce new sensing surfaces: A review emphasizing electrode position of heteroaromatic compounds, Electroanalysis. 1991, 3, 879–889. [Google Scholar]
- Bidan, G. Electroconducting conjugated polymers: New sensitive matrices to build up chemical or electrochemical sensors. Sens. Actuators B 1992, 6, 45–56. [Google Scholar]
- Zotti, G. Electrochemical sensors based on polyconjugated conducting polymers. Synth. Met. 1992, 51, 373–382. [Google Scholar]
- Bartlett, P.N.; Birkin, P.R. The application of conducting polymers in biosensors. Synth. Met. 1993, 61, 15–21. [Google Scholar]
- Teasdale, P.R.; Wallace, G.G. Molecular recognition using conducting polymers: basis of an electrochemical sensing technology—Plenary lecture. Analyst 1993, 118, 329–334. [Google Scholar]
- Barlett, P.N.; Cooper, J.M. A review of the immobilization of enzymes in electropolymerized films. J. Electroanal. Chem. 1993, 362, 1–12. [Google Scholar]
- Josowicz, M. Applications of conducting polymers in potentiometric sensors. Analyst 1995, 120, 1019–1024. [Google Scholar]
- Emr, S.A.; Yacynych, A.M. Use of polymer films in amperometric biosensors. Electroanalysis 1995, 7, 913–923. [Google Scholar]
- Adeloju, S.B.; Wallace, G.G. Conducting polymers and the bioanalytical sciences: new tools for biomolecular communications. A review. Analyst 1996, 121, 699–703. [Google Scholar]
- Gcpel, W.; Schierbaum, K.D. Handbook of Organic Conductive Molecules and Polymers; Vol. 4, Conductive Polymers: Transport, Photophysics and Applications; Nalwa, H. S., Ed.; Wiley: Chichester, 1997; pp. 621–659. [Google Scholar]
- Fabre, B.; Simonet, J. Electroactive polymers containing crown ether or polyether ligands as cation-responsive materials. Coord. Chem. Rev. 1998, 178-180, 1211–1250. [Google Scholar]
- Giuseppi-Elie, A.; Wallace, G.G.; Matsue, T. Handbook of Conducting Polymers, Second Edition; Skotheim, T.A., Elsenbaumer, R.L., Reynolds, J.R., Eds.; Marcel Dekker: New York, 1998; pp. 963–991. [Google Scholar]
- Lewis, T.W.; Wallace, G.G.; Smyth, M.R. Electrofunctional polymers: their role in the development of new analytical systems. Analyst 1999, 124, 213–219. [Google Scholar]
- Wallace, G.G.; Smyth, M.; Zhao, H. Conducting electroactive polymer-based biosensors. Trends Anal. Chem. 1999, 18, 245–251. [Google Scholar]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar]
- Kane-Maguire, L.A.P.; Wallace, G.G. Communicating with the building blocks of life using organic electronic conductors. Synth. Met. 2001, 119, 39–42. [Google Scholar]
- Fabre, B. Handbook of Advanced Electronic and Photonic Materials and Devices, Vol. 8: Conducting Polymers; Nalwa, H.S., Ed.; Academic Press: San Diego, 2001; pp. 103–129. [Google Scholar]
- Sensors Update; Vol. 8, Leclerc, M.; Baltes, H.; Gcpel, W.; Hesse, J. (Eds.) Wiley-VCH: Weinheim, 2001; pp. 21–38.
- Ramanaviciene, A.; Ramanavicius, A. Application of Polypyrrole for the Creation of Immunosensors. Crit. Rev. Anal. Chem. 2002, 32, 245–252. [Google Scholar]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nature Materials 2003, 2, 19–24. [Google Scholar]
- Trojanowicz, M. Application of Conducting Polymers in Chemical Analysis. Microchim. Acta 2003, 143, 75–91. [Google Scholar]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors Based on Conducting Polymers. Electroanalysis 2003, 15, 366–374. [Google Scholar]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar]
- Ramanaviˇcius, A.; Ramanaviˇcien˙, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—polypyrrole. Electrochimica Acta 2006, 51, 6025–6037. [Google Scholar]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar]
- Diaz, A.F.; Logan, J.A. Electroactive polyaniline films. J. Electroanal. Chem. 1980, 111, 111–114. [Google Scholar]
- Ohsaka, T.; Ohnuki, Y.; Oyama, N.; Katagiri, K.; Kamisako, K. IR absorption spectroscopic identification of electroactive and electroinactive polyaniline films prepared by the electrochemical polymerization of aniline. J.Electroanal. Chem. 1984, 161, 399–405. [Google Scholar]
- Cui, S.Y.; Park, S.M. Electrochemistry of conductive polymers XXIII: polyaniline growth studied by electrochemical quartz crystal microbalance measurements. Synth. Met. 1999, 105, 91–98. [Google Scholar]
- Tian, S.; Baba, A.; Liu, J.; Wang, Z.; Knoll, W.; Park, M.K.; Advincula, R. Electroactivity of Polyaniline Multilayer Films in Neutral Solution and Their Electrocatalyzed oxidation of β-Nicotinamide Adenine dinucleotide. Adv. Funct. Mater. 2003, 13, 473–479. [Google Scholar]
- Toh, C.S.; Bartlett, P.N.; Mano, N.; Aussenac, F.; Kuhn, A.; Dufour, E.J. The effect of calcium ions on the electrocatalytic oxidation of NADH by poly(aniline)-poly(vinylsulfonate) and poly(aniline)-poly(styrenesulfonate) modified electrodes. Phys. Chem. Chem. Phys. 2003, 5, 588–593. [Google Scholar]
- Valentini, F.; Salis, A.; Curulli, A.; Palleschi, G. Chemical Reversibility and Stable Low-Potential NADH Detection with Nonconventional Conducting Polymer Nanotubule Modified Glassy Carbon Electrodes. Anal. Chem. 2004, 76, 3244–3248. [Google Scholar]
- Kumar, S.A.; Chen, S.M. Electrochemically polymerized composites of conducting poly(p-ABSA) and flavins (FAD, FMN, RF) films and their use as electrochemical sensors: A new potent electroanalysis of NADH and NAD+. Sensors and Actuators B 2007, 123, 964–977. [Google Scholar]
- Jin, G.; Zhang, Y.; Cheng, W. Poly(p-aminobenzene sulfonic acid)-modified glassy carbon electrode for simultaneous detection of dopamine and ascorbic acid. Sensors and Actuators B: Chemical 2005, 107, 528–534. [Google Scholar]
- Delbem, M.F.; Baader, W.J.; Serrano, S.H.P. Mechanism of 3,4-dihydroxybenzaldehyde electropolymerization at carbon paste electrodes - Catalytic detection of NADH. Quimica Nova 2002, 25, 741–747. [Google Scholar]
- Chen, S.M.; Lin, K.C. The electrocatalytic properties of biological molecules using polymerized luminol film-modified electrodes. J. Electroanal. Chem. 2002, 523, 93–105. [Google Scholar]
- Li, N.B.; Duan, J. P.; Chen, G. N. Electrochemical Polymerization of Azure Blue II and Its Electrocatalytic Activity toward NADH Oxidation. Chin. J. Chem. 2003, 21, 1191–1197. [Google Scholar]
- Gao, Q.; Cui, X.; Yang, F.; Ma, Y.; Yang, X. Preparation of poly(thionine) modified screen-printed carbon electrode and its application to determine NADH in flow injection analysis system. Biosensors and Bioelectronics 2003, 19, 277–282. [Google Scholar]
- Kumar, S.A.; Chen, S.M. Fabrication and characterization of Meldola's blue/zinc oxide hybrid electrodes for efficient detection of the reduced form of nicotinamide adenine dinucleotide at low potential. Analytica Chimica Acta 2007, 592, 36–44. [Google Scholar]
- Vasilescu, A.; Noguer, T.; Andreescu, S.; Calas-Blanchard, C.; Bala, C.; Marty, J.L. Strategies for developing NADH detectors based on Meldola Blue and screen-printed electrodes: a comparative study. Talanta 2003, 59, 751–765. [Google Scholar]
- Chen, Y.; Yuan, J.; Tian, C.; Wang, X. Flow-Injection analysis and voltammetric detection of NADH with a Poly-Toluidine Blue modified electrode. Analytical Sciences 2004, 20, 507–511. [Google Scholar]
- Gao, Q.; Wang, W.; Ma, Y.; Yang, X. Electrooxidative polymerization of phenothiazine derivatives on screen-printed carbon electrode and its application to determine NADH in flow injection analysis system. Talanta 2004, 62, 477–482. [Google Scholar]
- Sha, Y.; Gao, Q.; Qi, B.; Yang, X. Electropolymerization of Azure B on a Screen-Printed Carbon Electrode and its Application to the Determination of NADH in a Flow Injection Analysis System. Microchim. Acta 2004, 148, 335–341. [Google Scholar]
- Komura, T.; Niu, G.Y.; Yamaguchi, T.; Asano, M.; Matsuda, A. Coupled Electron-Proton Transport in Electropolymerized Methylene Blue and the Influences of Its Protonation Level on the Rate of Electron Exchange with β-Nicotinamide Adenine Dinucleotide. Electroanalysis 2004, 16, 1791–1800. [Google Scholar]
- Yuan, J.; Chen, Y.; Wang, X.; Tian, C. Electrocatalysis of β-nicotinamide adenine dinucleotide oxidation with electropolymerized films of toluidine blue. Fenxi Huaxue 2004, 32, 53–55. [Google Scholar]
- Nassef, H.M.; Radi, A.; O′Sullivan, C.K. Electrocatalytic sensing of NADH on a glassy carbon electrode modified with electrografted o-aminophenol film. Electrochem. Commun. 2006, 8, 1719–1725. [Google Scholar]
- Chen, S.M.; Liu, M.I.; Kumar, S.A. Electrochemical Preparation of Poly(acriflavine) Film-Modified Electrode and Its Electrolcatalytic Properties Towards NADH, Nitrite and Sulfur Oxoanions. Electroanalysis 2007, 19, 999–1007. [Google Scholar]
- Vasilescu, A.; Andreescu, S.; Bala, C.; Litescu, S.C.; Noguer, T.; Marty, J.L. Screen-printed electrodes with electropolymerized Meldola Blue as versatile detectors in biosensors. Biosensors and Bioelectronics 2003, 18, 781–790. [Google Scholar]
- Prieto-Simón, B.; Fàbregas, E. Comparative study of electron mediators used in the electrochemical oxidation of NADH, Biosensors and Bioelectronics. 2004, 19, 1131–1138. [Google Scholar]
- Lin, K.C.; Chen, S.M. Characterization of Hybrid Poly(acriflavine)/FAD Films and Their Electrocatalytic Properties with NAD+ and NADH. Journal of The Electrochemical Society 2006, 153, D91–D98. [Google Scholar]
- Dai, Z.H.; Liu, F.X.; Lu, G.F.; Bao, J.C. Electrocatalytic detection of NADH and ethanol at glassy carbon electrode modified with electropolymerized films from methylene green. J Solid State Electrochem 2008, 12, 175–180. [Google Scholar]
- Golabi, S.M.; Zare, H.R.; Hamzehloo, M. Electrochemistry and Electrocatalytic Activity of Pyrocatechol Violet (PCV) Film on a Glassy Carbon Electrode Towards the Oxidation of Reduced Nicotinamide Adenine Dinucleotide(NADH). Electroanalysis 2002, 14, 611–618. [Google Scholar]
- Dilgin, Y.; Gorton, L.; Nisli, G. Photoelectrocatalytic Oxidation of NADH with Electropolymerized Toluidine Blue O. Electroanalysis 2007, 19, 286–293. [Google Scholar]
- Liu, J.; Tian, S.; Knoll, W. Properties of Polyaniline/Carbon Nanotube Multilayer Films in Neutral Solution and Their Application for Stable Low-Potential Detection of Reduced β-Nicotinamide Adenine Dinucleotide. Langmuir 2005, 21, 5596–5599. [Google Scholar]
- Zeng, J.; Wei, W.; Wu, L.; Liu, X.; Liu, K.; Li, Y. Fabrication of poly(toluidine blue O)/carbon nanotube composite nanowires and its stable low-potential detection of NADH. J. Electroanal. Chem. 2006, 595, 152–160. [Google Scholar]
- Raj, C.R.; Chakraborty, S. Carbon nanotubes–polymer–redox mediator hybrid thin film for electrocatalytic sensing. Biosens. Bioelectron. 2006, 22, 700–706. [Google Scholar]
- Zeng, J.; Gao, X.; Wei, W.; Zhai, X.; Yin, J.; Wu, L.; Liu, X.; Liu, K.; Gong, S. Fabrication of carbon nanotubes/poly(1,2-diaminobenzene) nanoporous composite via multipulse chronoamperometric electropolymerization process and its electrocatalytic property toward oxidation of NADH. Sensors and Actuators B 2007, 120, 595–602. [Google Scholar]
- Tu, X.; Xie, Q.; Huang, Z.; Yang, Q.; Yao, S. Synthesis and Characterization of Novel Quinone-Amine Polymer/Carbon Nanotubes Composite for Sensitive Electrocatalytic Detection of NADH. Electroanalysis 2007, 19, 1815–1821. [Google Scholar]
- Agui, L.; Pena-Farfal, C.; Yanez-Sedeno, P.; Pingarron, J.M. Poly-(3-methylthiophene)/carbon nanotubes hybrid composite-modified electrodes. Electrochimica Acta 2007, 52, 7946–7952. [Google Scholar]
- Du, P.; Liu, S.; Wu, P.; Cai, C. Single-walled carbon nanotubes functionalized with poly(nile blue A) and their application to dehydrogenase-based biosensors. Electrochimica Acta 2007, 53, 1811–1823. [Google Scholar]
- Musameh, M.; Wang, J.; Merkoci, A.; Lin, Y. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochemistry Communications 2002, 4, 743–746. [Google Scholar]
- Lawrence, N.S.; Wang, J. Chemical adsorption of phenothiazine dyes onto carbon nanotubes: Toward the low potential detection of NADH, Electrochemistry Communications. 2006, 8, 71–76. [Google Scholar]
- Zhu, L.; Zhai, J.; Yang, R.; Tian, C.; Guo, L. Electrocatalytic oxidation of NADH with Meldola's blue functionalized carbon nanotubes electrodes, Biosensors and Bioelectronics. 2007, 22, 2768–2773. [Google Scholar]
- Radoi, A.; Compagnone, D.; Valcarcel, M.A.; Placidi, P.; Materazzi, S.; Moscone, D.; Palleschi, G. Detection of NADH via electrocatalytic oxidation at single-walled carbon nanotubes modified with Variamine blue. Electrochimica Acta 2008, 53, 2161–2169. [Google Scholar]
- Huang, M.; Jiang, H.; Zhai, J.; Liu, B.; Dong, S. A simple route to incorporate redox mediator into carbon nanotubes/Nafion composite film and its application to determine NADH at low potential. Talanta 2007, 74, 132–139. [Google Scholar]
- De-los-Santos-Alvarez, P.; Molina, P.G.; Lobo-Castanfon, M.J.; Miranda-Ordieres, A.J.; Tunfon-Blanco, P. Electrocatalytic Oxidation of NADH at Polyadenylic Acid Modified Graphite Electrodes. Electroanalysis 2002, 14, 1543–1549. [Google Scholar]
- De-los-Santos-Alvarez, N.; Lobo-Castanfon, M.J.; Miranda-Ordieres, A.J.; Tunfon-Blanco, P. Electrocatalytic Oxidation of NADH by Oxidized s-Adenosyl-LMethionine (SAMe): Application to NADH and SAMe Determinations. Electroanalysis 2004, 16, 881–887. [Google Scholar]
- De-los-Santos-Alvarez, N.; Lobo-Castanon, M.J.; Miranda-Ordieres, A.J.; Tunon-Blanco, P. Electrochemical and Catalytic Properties of the Adenine Coenzymes FAD and Coenzyme A on Pyrolytic Graphite Electrodes. Electroanalysis 2005, 17, 445–451. [Google Scholar]
- Golabi, S.M.; Irannejad, L. Preparation and Electrochemical Study of Fisetin Modified Glassy Carbon Electrode. Application to the Determination of NADH and Ascorbic Acid, Electroanalysis 2005, 17, 985–996. [Google Scholar]
- Vasantha, V.S.; Chen, S.M. Synergistic effect of a catechin-immobilized poly(3,4-ethylenedioxythiophene)-modified electrode on electrocatalysis of NADH in the presence of ascorbic acid and uric acid. Electrochimica Acta 2006, 52, 665–674. [Google Scholar]
- de S. Santos, A.; Gorton, L.; Kubota, L.T. Electrocatalytic NADH Oxidation Using an Electrode Based on Meldola Blue Immobilized on Silica Coated with Niobium Oxide. Electroanalysis 2002, 14, 805–812. [Google Scholar]
- de S. Santos, A.; Gorton, L.; Kubota, L.T. Nile blue adsorbed onto silica gel modified with niobium oxide for electrocatalytic oxidation of NADH. Electrochimica Acta 2002, 47, 3351–3360. [Google Scholar]
- Ce´sar Pereira, A.; de Santana Santos, A.; Kubota, L.T. o-Phenylenediamine adsorbed onto silica gel modified with niobium oxide for electrocatalytic NADH oxidation. Electrochimica Acta 2003, 48, 3541–3550. [Google Scholar]
- Munteanu, F.D.; Mano, N.; Kuhn, A.; Gorton, L. NADH electrooxidation using carbon paste electrodes modified with nitro-fluorenone derivatives immobilized on zirconium phosphate. Journal of Electroanalytical Chemistry 2004, 564, 167–178. [Google Scholar]
- Stergiou, D.V.; Prodromidis, M.I.; Veltsistas, P.G.; Evmiridis, N.P. Study of the Electrochemical Behavior of Disperse Blue 1-Modified Graphite Electrodes. Application to the Flow Determination of NADH. Electroanalysis 2004, 16, 949–954. [Google Scholar]
- Santiago, M.E.B.; Velez, M.M.; Borrero, S.; Diaz, A.; Casillas, C.A.; Hofmann, C.; Guadalupe, A.R.; Colon, J.L. NADH Electrooxidation Using Bis(1,10-phenanthroline-5,6-dione) (2,2′-bipyridine)ruthenium(II)-Exchanged Zirconium Phosphate Modified Carbon Paste Electrodes. Electroanalysis 2006, 18, 559–572. [Google Scholar]
- Salimi, A.; Hallaj, R.; Ghadermazi, M. Modification of carbon ceramic electrode prepared with sol–gel technique by a thin film of chlorogenic acid: application to amperometric detection of NADH. Talanta 2005, 65, 888–894. [Google Scholar]
- Kumar, S.A.; Chen, S.M. Electrocatalysis and Amperometric Detection of the Reduced Form of Nicotinamide Adenine Dinucleotide at Toluidine Blue/Zinc Oxide Coated Electrodes. Electroanalysis 2007, 19, 1952–1958. [Google Scholar]




| Polymer, copolymer or Poly(dye) films | Electroanalytical applications and characteristics | Ref. | |||
|---|---|---|---|---|---|
| PANI/SPANI multilayer (Layer by layer method) | Electrocatalysis of NADH in pH 7.1 phosphate buffer solution. | [80] | |||
| poly(aniline)-poly(vinylsulfonate) and poly(aniline)-poly(styrenesulfonate) | Catalytic current increases up to 12 and 25 times in the presences of Ca2+. | [81] | |||
| poly(1,2-diaminobenzene) conducting nanotubule coated GCE | Electrochemical oxidation of NADH at an applied potential of 450 mV, showed a sensitivity of 99 nA/mM, an operational stability for 2 days, a storage stability of 2 weeks at 4 °C, a linearity from 5 x 10-5 to 1 x 10-3M | [82] | |||
| poly(p-amino benzenesulfonic acid)/FAD film modified GCE | This electrode has a fast response to NADH and a good linear response observed in the range from 10 to 300 uM in pH 6.4 PBS. The detection limit is estimated to be luM (S/N = 3) | [83] | |||
| Poly (3,4-dihydroxybenzaldehyde) modified carbon paste electrodes | It has used for NADH catalytic detection at 0.23 V in the range 0.015 < [NADH] < 0.21 mmol L-1 | [85] | |||
| Poly(luminal) modified GCE | Electroanalysis of NADH oxidation in acidic and neutral aqueous solutions | [86] | |||
| Poly(azure blue II) film modified GCE | The electrocatalytic current increased linearly with NADH concentration from 1.0 x 10-5 to 8.0 x 10-3 mol/L in the presence of 4.0 x 10-2 mol/L Mg2+ cation. The detection limit (3sbi/S) was 5.0 x 10-6 mol/L | [87] | |||
| poly(thionine) modified screen-printed carbon electrode | Detection of NADH within dynamic range of 5-/100 uM. The resulting calibration plot had a slope of 1.14 uA/mM and correlation coefficient, 0.999. A detection limit was 3 uM (S/N=3). | [88] | |||
| Poly (Meldola) modified screen printed carbon electrode | Exhibited linear range between 8x/10-6 and /5x/10-4M NADH. Poly (MB) sensors allow detecting as low as 2uM NADH. | [90] | |||
| Poly-Toluidine Blue modified GCE | It has reduced the over a 450mV of the overpotential for NADH. The linear range was 5uM to 3.2mM. The detection limit was 0.1 uM. In this method L-ascorbic acid interfered with the determination of NADH in practical analysis | [91] | |||
| Poly(azure A) and Poly(toluidine blue 0) modified screen-printed carbon electrodes | Reduced overpotential of more than 500 mV, promising as an amperometric detector for the flow injection analysis of NADH, typically with a dynamic range of 0.5-100uM. | [92] | |||
| poly(azure B) modified screen-printed carbon electrode | Detection of NADH in the concentration range from 0.5 uM to 100 uM. A detection limit was 2.0xlO-7M (S/N=3). | [93] | |||
| Poly(methylene blue) modified GCE | This study examines NADH oxidation on monomeric dye and poly(methylene blue) modified electrode. Polymeric films showed enhanced electrocatalytic acitivity. | [94] | |||
| Poly-toduiline blue modified GC electrode | This electrode shift peak potential negatively to 450 mV and showed higher electrocatalytic current towards NADH. | [95] | |||
| Poly(acriflavine) modified GCE | Poly(acriflavine) found to be good mediator for electrochemical oxidation of NADH in pH 5 buffer solutions. The anodic current increased linearly with the additions of NADH concentration over the range from 80 to 720 uM. | [97] | |||
| Poly(meldola blue)/screen printed carbon electrode | Detection of NADH in the range from 8 - 500uM with detection limit of 2.5 uM and a sensitivity of 3713 uA/ mol in amperometric determinations at 0 V vs. Ag/AgCl | [98] | |||
| Poly(methylene green)modified GCE | Showed an excellent electrocatalytic activity toward NADH oxidation, reducing its overpotential by about 650 mV and exhibits a wide linear range of 5.6 - 420 μM NADH with the detection limit of 3.8 μM. | [101] | |||
| Electrodeposited film derivec from pyrocatechol violet on GCE | This electrode reduced overpotential of about 447 mV and the linear concentrations ranging from 2.5 |JM to 40 |JM. Limit of detection was 1.0 |JM | [102] | |||
| poly-TBO modified GCE with irradiation of 250W halogen lamp | A linear calibration graph for NADH was obtained in the range between 1.0* 10-5 and 1.0* 10-3 M and between 5.0*10-6 and 1.0x10-3 M for amperometric and photoamperometric studies at+100 mV. | [103] | |||
| Polymer/CNT composites | Electroanalytical applications and characteristics | Ref. | |||
|---|---|---|---|---|---|
| Polyaniline successfully assembled with poly(aminobenzenesulfonic acid)-modified single-walled carbon nanotubes (Layer by layer method) | This film shifts electrocataltyic activity of polyaniline to neutral pH. For a six-bilayer sample, the detection limit can go down to 1× 10-6 M as detected by the simple CV method, with a linear detection range for NADH at concentrations between 5 ×10-6 and 1×10-3 M. | [104] | |||
| poly(toluidine blue O)/multiwall carbon nanotube composite nanowires modified GCE | This electrode decreased the NADH oxidization overpotential by about 650 mV. A linear range from 2.0μM to 4.5 mM was observed with fast response (within 5 s) and a low detection limit of 0.5μM (based on S/N = 3). | [105] | |||
| MWCNTs/Nafion/oxidation product of serotonin modified GCE | This hybrid thin film modified electrode exhibits stable amperometric response and it linearly responds to NADH (0.5–400 μM). Detection limit as low as 0.1 μM at -0.05V with a sensitivity of 11.1 nA/μM in physiological pH. | [106] | |||
| poly(1,2-diaminobenzene)/ MWNTs/GCE | This nanoporous MCE-based electrode was applied to determination of NADH at low potential of 70mV, and a linear range from 2.0 μM to 4.0mM was observed with fast response (within 5 s) and a lower detection limit of 0.5 μM(based on S/N = 3). | [107] | |||
| Nanocomposite of quinone-amine polymer and multiwalled carbon nanotubes modified Au electrode | This film mediated the oxidation of NADH in pH 7.0 phosphate buffer, with an overpotential decrease by ca. 470 mV (vs. bare Au), a limit of detection of 6.4 nmol L-1 and good antiinterferent ability. The linear range was obtained from 0.04 to 300 mmol L-1 | [108] | |||
| Poly-(3-methylthiophene)— multi-walled carbon nanotubes hybrid composite electrode | Amperometric NADH detection at +300mV provided fast responses, a range of linearity between 5.0×10-7 and 2.0×10-5 mol l-1, and a detection limit of 1.7×10-7 mol l-1 | [109] | |||
| SWNTs with poly(nile blue A)/GCE | Electrocatalyze the oxidation of NADH at a very low potential (ca. -80mV versus SCE) and lead to a substantial decrease in the overpotential by more than 700mV | [110] | |||
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Kumar, S.A.; Chen, S.-M. Electroanalysis of NADH Using Conducting and Redox Active Polymer/Carbon Nanotubes Modified Electrodes-A Review. Sensors 2008, 8, 739-766. https://doi.org/10.3390/s8020739
Kumar SA, Chen S-M. Electroanalysis of NADH Using Conducting and Redox Active Polymer/Carbon Nanotubes Modified Electrodes-A Review. Sensors. 2008; 8(2):739-766. https://doi.org/10.3390/s8020739
Chicago/Turabian StyleKumar, S. Ashok, and Shen-Ming Chen. 2008. "Electroanalysis of NADH Using Conducting and Redox Active Polymer/Carbon Nanotubes Modified Electrodes-A Review" Sensors 8, no. 2: 739-766. https://doi.org/10.3390/s8020739
APA StyleKumar, S. A., & Chen, S.-M. (2008). Electroanalysis of NADH Using Conducting and Redox Active Polymer/Carbon Nanotubes Modified Electrodes-A Review. Sensors, 8(2), 739-766. https://doi.org/10.3390/s8020739





