Multi-instrumental Investigation of Affecting of Early Somatic Embryos of Spruce by Cadmium(II) and Lead(II) Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant material and cultivation conditions
2.3. Computer image analysis
2.4. Bright field photography of ESEs
2.5. Double staining
2.6. Intracellular esterases assay – Fluorimetric sensor
2.7. Electrochemical measurements
2.8. Nuclear magnetic resonance
2.9. Statistical analysis
3. Results and Discussion
3.1. Changes in morphology of somatic embryos under heavy metals stress
3.2. Determination of viability and growth of spruce embryos treated by heavy metals
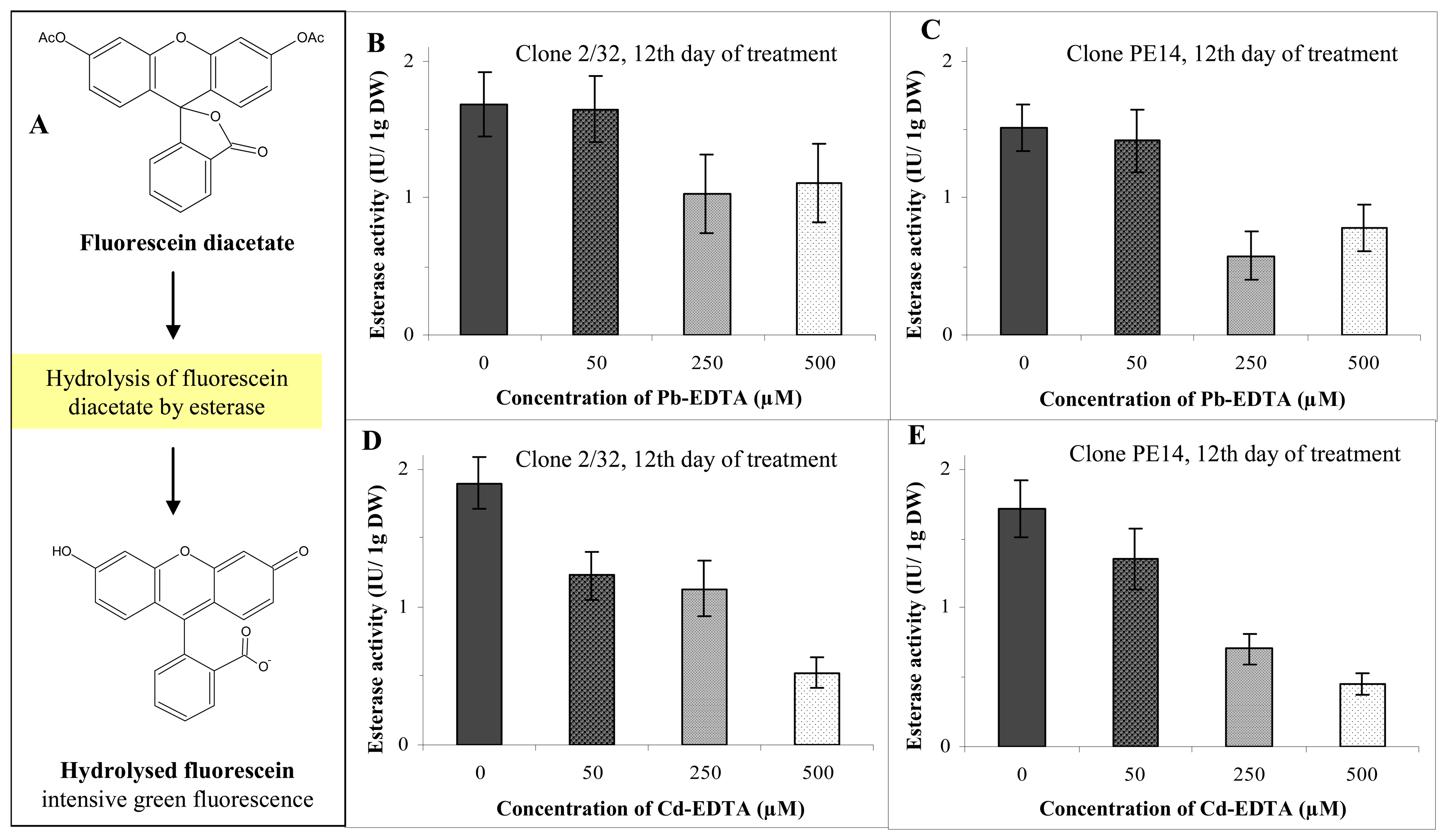
3.3. Determination of activity of intracellular esterases
3.4. Influence of heavy metals on the activity of intracellular esterases
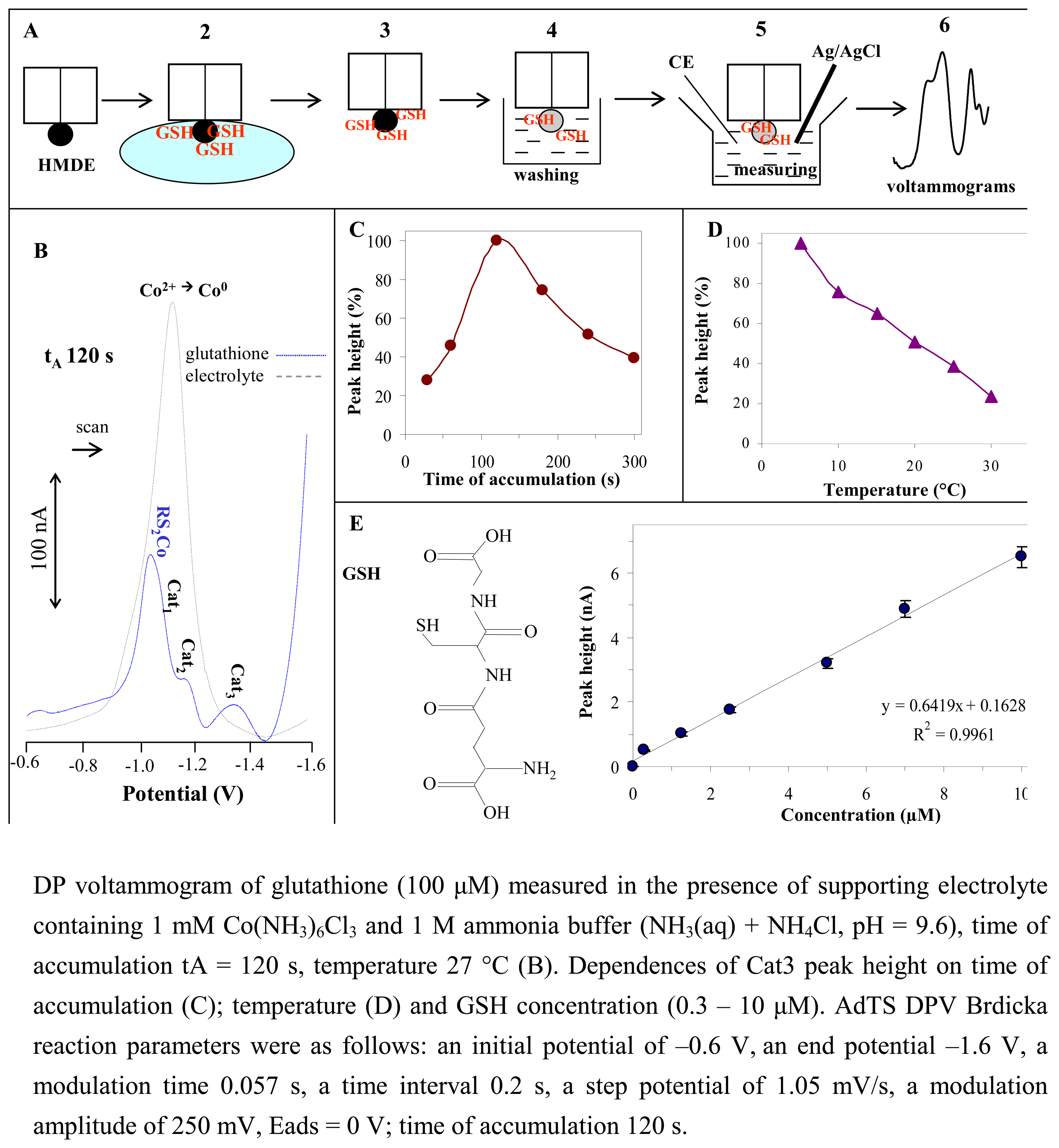
3.5. Electrochemical detection of glutathione – Use of the Brdicka reaction
3.6. Changes in content of glutathione in spruce embryos treated with heavy metals
4. Conclusions
Acknowledgments
References
- Cobbett, C.S. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Biol. 2000, 3, 211–216. [Google Scholar]
- Ha, S.B.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O'Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin synthase genes from arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1163. [Google Scholar]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins - the Principal Heavy-Metal Complexing Peptides of Higher-Plants. Science 1985, 230, 674–676. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar]
- Leopold, I.; Gunther, D.; Schmidt, J.; Neumann, D. Phytochelatins and heavy metal tolerance. Phytochemistry 1999, 50, 1323–1328. [Google Scholar]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 1985, 320, 674–676. [Google Scholar]
- Cobbett, C.S.; Goldsbrough, P.B. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar]
- Pacsial-Ong, E.J.; McCarley, R.L.; Wang, W.H.; Strongin, R.M. Electrochemical detection of glutathione using redox indicators. Anal. Chem. 2006, 78, 7577–7581. [Google Scholar]
- Davoine, C.; Douki, T.; Iacazio, G.; Montillet, J.L.; Triantaphylides, C. Conjugation of keto fatty acids to glutathione in plant tissues. Characterization and quantification by HPLC-tandem mass spectrometry. Anal. Chem. 2005, 77, 7366–7372. [Google Scholar]
- Jin, W.R.; Li, X.J.; Gao, N. Simultaneous determination of tryptophan and glutathione in individual rat hepatocytes by capillary zone electrophoresis with electrochemical detection at a carbon fiber bundle-Au/Hg dual electrode. Anal. Chem. 2003, 75, 3859–3864. [Google Scholar]
- Inoue, T.; Kirchhoff, J.R. Electrochemical detection of thiols with a coenzyme pyrroloquinoline quinone modified electrode. Anal. Chem. 2000, 72, 5755–5760. [Google Scholar]
- Potesil, D.; Zelena, J.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatog. A 2005, 1084, 134–144. [Google Scholar]
- Klejdus, B.; Zehnalek, J.; Adam, V.; Petrek, J.; Kizek, R.; Vacek, J.; Trnkova, L.; Roland, R.; Havel, L.; Kuban, V. Sub-picomolar HPLC/MS determination of glutathione in the maize (Zea mays L.) kernels exposed by cadmium. Anal. Chim. Acta 2004, 520, 117–124. [Google Scholar]
- Petrlova, J.; Mikelova, R.; Stejskal, K.; Kleckerova, A.; Zitka, O.; Petrek, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Kizek, R. Simultaneous determination of eight biologically active thiol compounds using gradient elution-liquid chromatography with Coul-Array detection. J. Sep. Sci. 2006, 29, 1166–1173. [Google Scholar]
- Vacek, J.; Petrek, J.; Kizek, R.; Havel, L.; Klejdus, B.; Trnkova, L.; Jelen, F. Electrochemical determination of lead and glutathione in a plant cell culture. Bioelectrochemistry 2004, 63, 347–351. [Google Scholar]
- Fojta, M.; Fojtova, M.; Havran, L.; Pivonkova, H.; Dorcak, V.; Sestakova, I. Electrochemical monitoring of phytochelatin accumulation in Nicotiana tabacum cells exposed to sub-cytotoxic and cytotoxic levels of cadmium. Anal. Chim. Acta 2006, 558, 171–178. [Google Scholar]
- Dorcak, V.; Sestakova, I. Electrochemical behavior of phytochelatins and related peptides at the hanging mercury drop electrode in the presence of cobalt(II) ions. Bioelectrochemistry 2006, 68, 14–21. [Google Scholar]
- Yosypchuk, B.; Sestakova, I.; Novotny, L. Voltammetric determination of phytochelatins using copper solid amalgam electrode. Talanta 2003, 59, 1253–1258. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Jelen, F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry 2004, 63, 19–24. [Google Scholar]
- Zitka, O.; Stejskal, K.; Kleckerova, A.; Adam, V.; Beklova, M.; Horna, A.; Supalkova, V.; Havel, L.; Kizek, R. Utilizing electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
- Huska, D.; Zitka, O.; Adam, V.; Beklova, M.; Krizkova, S.; Zeman, L.; Horna, A.; Havel, L.; Zehnalek, J.; Kizek, R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci. 2007, 52, 37–43. [Google Scholar]
- Zehnalek, J.; Adam, V.; Kizek, R. Influence of heavy metals on production of protecting compounds in agriculture plants. Listy Cukrov. 2004, 120, 222–224. [Google Scholar]
- Zehnalek, J.; Vacek, J.; Kizek, R. Application of higher plants in phytoremediation of heavy metals. Listy Cukrov. 2004, 120, 220–221. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7 in press. [Google Scholar]
- Mohr, H.; Schopfer, P. Plant Physiology; Springer; ? 1995; p. 629. [Google Scholar]
- Hader, D.P. Image analysis in biology; CRC Press: London, 1992. [Google Scholar]
- Ibaraki, Y.; Kenji, K. Application of image analysis to plant cell suspension cultures. Comput. Electron. Agric. 2001, 30, 193–203. [Google Scholar]
- Olofsdotter, M. Image processing: a non-destructive method for measuring growth in cell and tissue culture. Plant Cell Reports 1993, 12, 216–219. [Google Scholar]
- Gagna, C.E.; Winokur, D.; Larnbert, W.C. Cell biology, chemogenomics and chemoproteomics. Cell Biol. Int. 2004, 28, 755–764. [Google Scholar]
- Petrek, J.; Vitecek, J.; Vlasinova, H.; Kizek, R.; Kramer, K.J.; Adam, V.; Klejdus, B.; Havel, L. Application of computer imaging, stripping voltammetry and mass spectrometry to study the effect of lead (Pb-EDTA) on the growth and viability of early somatic embryos of Norway spruce (Picea abies/L./Karst.). Anal. Bioanal. Chem. 2005, 383, 576–586. [Google Scholar]
- Vitecek, J.; Adam, V.; Petrek, J.; Vacek, J.; Kizek, R.; Havel, L. Esterases as a marker for growth of BY-2 tobacco cells and early somatic embryos of the Norway spruce. Plant Cell Tissue Organ Cult. 2004, 79, 195–201. [Google Scholar]
- Vitecek, J.; Adam, V.; Petrek, J.; Babula, P.; Novotna, P.; Kizek, R.; Havel, L. Application of fluorimetric determination of esterases in plant material. Chem. Listy 2005, 99, 496–501. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Petrek, J.; Adam, V.; Havel, L.; Kramer, K.J.; Kizek, R. Application of fluorimetric analysis of plant esterases to study of programmed cell death and effects of cadmium(II) ions. Biol. Plant. 2007, 51, 551–555. [Google Scholar]
- Vitecek, J.; Wunschova, A.; Petrek, J.; Adam, V.; Kizek, R.; Havel, L. Cell death induced by sodium nitroprusside and hydrogen peroxide in tobacco BY 2 cell suspension. Biol. Plant. 2007, 51, 472–479. [Google Scholar]
- von Arnold, S.J. Improved efficiency of somatic embryogenesis in mature embryos of Picea abies (L.). Plant Phys. 1987, 128, 233–244. [Google Scholar]
- Havel, L.; Durzan, D.J. Apoptosis during diploid parthenogenesis and early somatic embryogenesis of Norway spruce. Int. J. Plant Sci. 1996, 157, 8–16. [Google Scholar]
- Vlasinova, H.; Mikulecky, M.; Havel, L. The mitotic activity of Norway spruce polyembryonic culture oscillates during the synodic lunar cycle. Biol. Plant. 2003, 47, 475–476. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Adam, V.; Havel, L.; Kramer, K.J.; Babula, P.; Kizek, R. A fluorimetric sensor for detection of one living cell. Sensors 2007, 7, 222–238. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Bartusek, K.; Dokoupil, Z.; Gescheidtova, E. Magnetic field mapping around metal implants using an asymmetric spin-echo MRI sequence. Meas. Sci. Technol. 2006, 17, 3293–3300. [Google Scholar]
- Bartusek, K.; Gescheidtova, E. Testing the quality of magnetic gradient fields for studying self-diffusion processes by magnetic resonance methods. Meas. Sci. Technol. 2006, 17, 2256–2262. [Google Scholar]
- Bartusek, K. Processing of MR images weighted by relaxation time T-2 to increase their contrast resolution. Meas. Sci. Technol. 2006, 17, 727–730. [Google Scholar]
- Bartusek, K.; Gescheidtova, E. MR measurement technique of rapidly switched gradient magnetic fields in MR tomography. Appl. Magn. Reson. 2005, 29, 675–686. [Google Scholar]
- Mojica, E.R.E.; Gomez, S.P.; Micor, J.R.L.; Deocaris, C.C. Lead detection using a pineapple bioelectrode. Philipp. Agric. Sci. 2006, 89, 134–140. [Google Scholar]
- Vassil, A.D.; Kapulnik, Y.; Raskin, I.; Salt, D.E. The Role of EDTA in Lead Transport and Accumulation by Indian Mustard. Plant Phys. 1998, 117, 447–453. [Google Scholar]
- Meagher, R.B. Phytoremediation of toxic elemental and organic pollutants. Curr. Opin. Plant Biol. 2000, 3, 153–162. [Google Scholar]
- Cruz, B.H.; Diaz-Cruz, J.M.; Arino, C.; Esteban, M. Complexation of heavy metals by phytochelatins: Voltammetric study of the binding of Cd2+ and Zn2+ ions by the phytochelatin (gamma-Glu-Cys)(3)Gly assisted by multivariate curve resolution. Environ. Sci. Technol. 2005, 39, 778–786. [Google Scholar]
- Cruz, B.H.; Diaz-Cruz, J.M.; Sestakova, I.; Velek, J.; Arino, C.; Esteban, M. Differential pulse voltammetric study of the complexation of Cd(II) by the phytochelatin (gamma-Glu-Cys)(2)Gly assisted by multivariate curve resolution. J. Electroanal. Chem. 2002, 520, 111–118. [Google Scholar]
- Sestakova, I.; Vodickova, H.; Mader, P. Voltammetric methods for speciation of plant metallothioneins. Electroanalysis 1998, 10, 764–770. [Google Scholar]
- Vodickova, H.; Pacakova, V.; Sestakova, I.; Mader, P. Analytical methods for determination of metallothioneins. Chem. Listy 2001, 95, 477–483. [Google Scholar]
- Dabrio, M.; Rodriguez, A.R.; Bordin, G.; Bebianno, M.J.; De Ley, M.; Sestakova, I.; Vasak, M.; Nordberg, M. Recent developments in quantification methods for metallothionein. J. Inorg. Biochem. 2002, 88, 123–134. [Google Scholar]
- Dragun, Z.; Raspor, B.; Erk, M.; Ivankovic, D.; Pavicic, J. The influence of the biometric parameters on metallothionein and metal level in the heat-treated cytosol of the whole soft tissue of transplanted mussels. Environ. Monit. Assess. 2006, 114, 49–64. [Google Scholar]
- Ivankovic, D.; Pavicic, J.; Erk, M.; Filipovic-Marijic, V.; Raspor, B. Evaluation of the Mytilus galloprovincialis Lam. digestive gland metallothionein as a biomarker in a long-term field study: Seasonal and spatial variability. Mar. Pollut. Bull. 2005, 50, 1303–1313. [Google Scholar]
- Raspor, B.; Dragun, Z.; Erk, M.; Ivankovic, D.; Pavicic, J. Is the digestive gland of Mytilus galloprovincialis a tissue of choice for estimating cadmium exposure by means of metallothioneins? Sci. Total Environ. 2004, 333, 99–108. [Google Scholar]
- Dragun, Z.; Erk, M.; Raspor, B.; Ivankovic, D.; Pavicic, J. Metal and metallothionein level in the heat-treated cytosol of gills of transplanted mussels Mytilus galloprovincialis Lmk. Environ. Int. 2004, 30, 1019–1025. [Google Scholar]
- Filipovic, V.; Raspor, B. Metallothionein and metal levels in cytosol of liver, kidney and brain in relation to growth parameters of Mullus surmuletus and Liza aurata from the Eastern Adriatic Sea. Water Res. 2003, 37, 3253–3262. [Google Scholar]
- Erk, M.; Ivankovic, D.; Raspor, B.; Pavicic, J. Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta 2002, 57, 1211–1218. [Google Scholar]
- Raspor, B.; Paic, M.; Erk, M. Analysis of metallothioneins by the modified Brdicka procedure. Talanta 2001, 55, 109–115. [Google Scholar]
- Erk, M.; Raspor, B. Interference of Pb leaching from the pH electrode on Cd-metallothionein complex. Anal. Chim. Acta 2001, 442, 165–170. [Google Scholar]
- Raspor, B. Elucidation of the mechanism of the Brdicka reaction. J. Electroanal. Chem. 2001, 503, 159–162. [Google Scholar]
- Erk, M.; Raspor, B. Advantages and disadvantages of voltammetric method in studying cadmium-metallothionein interactions. Cell. Mol. Biol. 2000, 46, 269–281. [Google Scholar]
- Raspor, B.; Pavicic, J. Electrochemical methods for quantification and characterization of metallothioneins induced in Mytilus galloprovincialis. Fresenius J. Anal. Chem. 1996, 354, 529–534. [Google Scholar]
- Selesovska-Fadrna, R.; Fojta, M.; Navratil, T.; Chylkova, J. Brdicka-type processes of cysteine and cysteine-containing peptides on silver amalgam electrodes. Anal. Chim. Acta 2007, 582, 344–352. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electorode for metallothionein electrochemical detection. Chem. Listy 2004, 98, 166–173. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Krizkova, S.; Adam, V.; Petrlova, J.; Zitka, O.; Stejskal, K.; Zehnalek, J.; Sures, B.; Trnkova, L.; Beklova, M.; Kizek, R. A suggestion of electrochemical biosensor for study of platinum(II)-DNA interactions. Electroanalysis 2007, 19, 331–338. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. Determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Kukacka, J.; Krizkova, S.; Zitka, O.; Prusa, R.; Adam, V.; Sures, B.; Beklova, M.; Kizek, R. Study of nucleic acids interactions with platinum based cytostatics using biosensor. FASEB J. 2007, 21, A262–A262. [Google Scholar]




© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental Investigation of Affecting of Early Somatic Embryos of Spruce by Cadmium(II) and Lead(II) Ions. Sensors 2007, 7, 743-759. https://doi.org/10.3390/s7050743
Supalkova V, Petrek J, Baloun J, Adam V, Bartusek K, Trnkova L, Beklova M, Diopan V, Havel L, Kizek R. Multi-instrumental Investigation of Affecting of Early Somatic Embryos of Spruce by Cadmium(II) and Lead(II) Ions. Sensors. 2007; 7(5):743-759. https://doi.org/10.3390/s7050743
Chicago/Turabian StyleSupalkova, Veronika, Jiri Petrek, Jiri Baloun, Vojtech Adam, Karel Bartusek, Libuse Trnkova, Miroslava Beklova, Vaclav Diopan, Ladislav Havel, and René Kizek. 2007. "Multi-instrumental Investigation of Affecting of Early Somatic Embryos of Spruce by Cadmium(II) and Lead(II) Ions" Sensors 7, no. 5: 743-759. https://doi.org/10.3390/s7050743
APA StyleSupalkova, V., Petrek, J., Baloun, J., Adam, V., Bartusek, K., Trnkova, L., Beklova, M., Diopan, V., Havel, L., & Kizek, R. (2007). Multi-instrumental Investigation of Affecting of Early Somatic Embryos of Spruce by Cadmium(II) and Lead(II) Ions. Sensors, 7(5), 743-759. https://doi.org/10.3390/s7050743



