Detection of Brominated By-Products Using a Sensor Array Based on Nanostructured Thin Films of Conducting Polymers
Abstract
:1. Introduction
2. Experimental
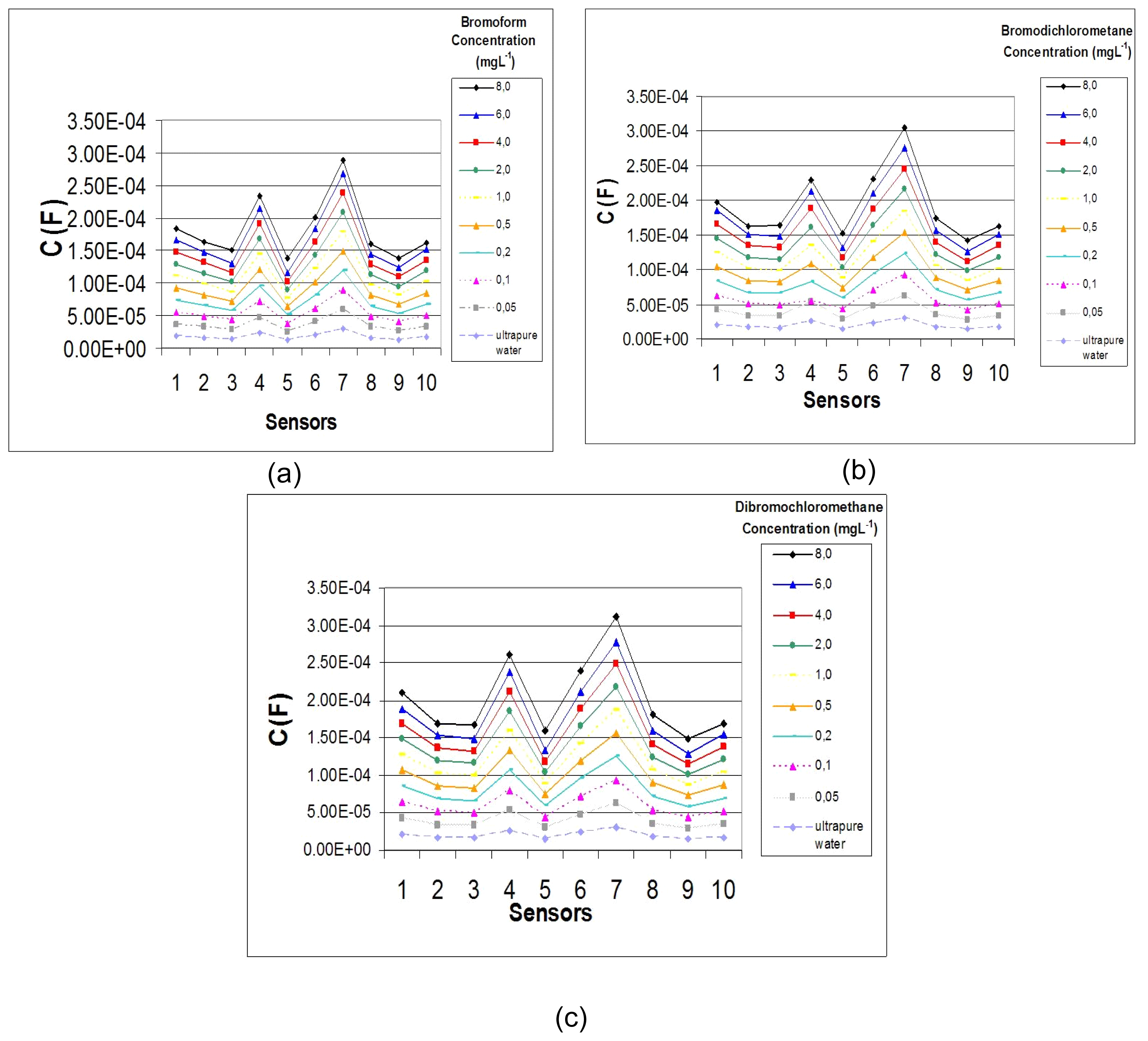
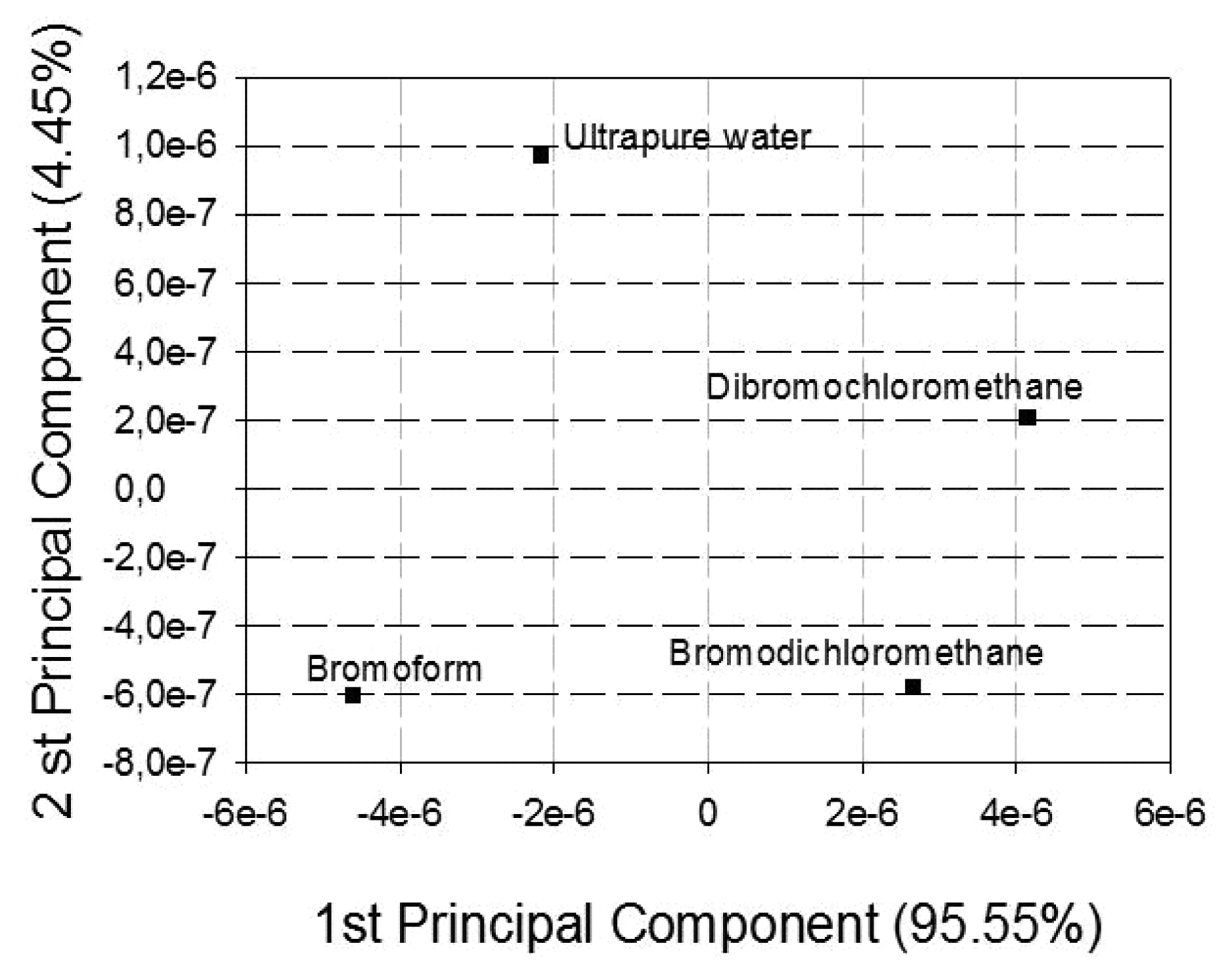
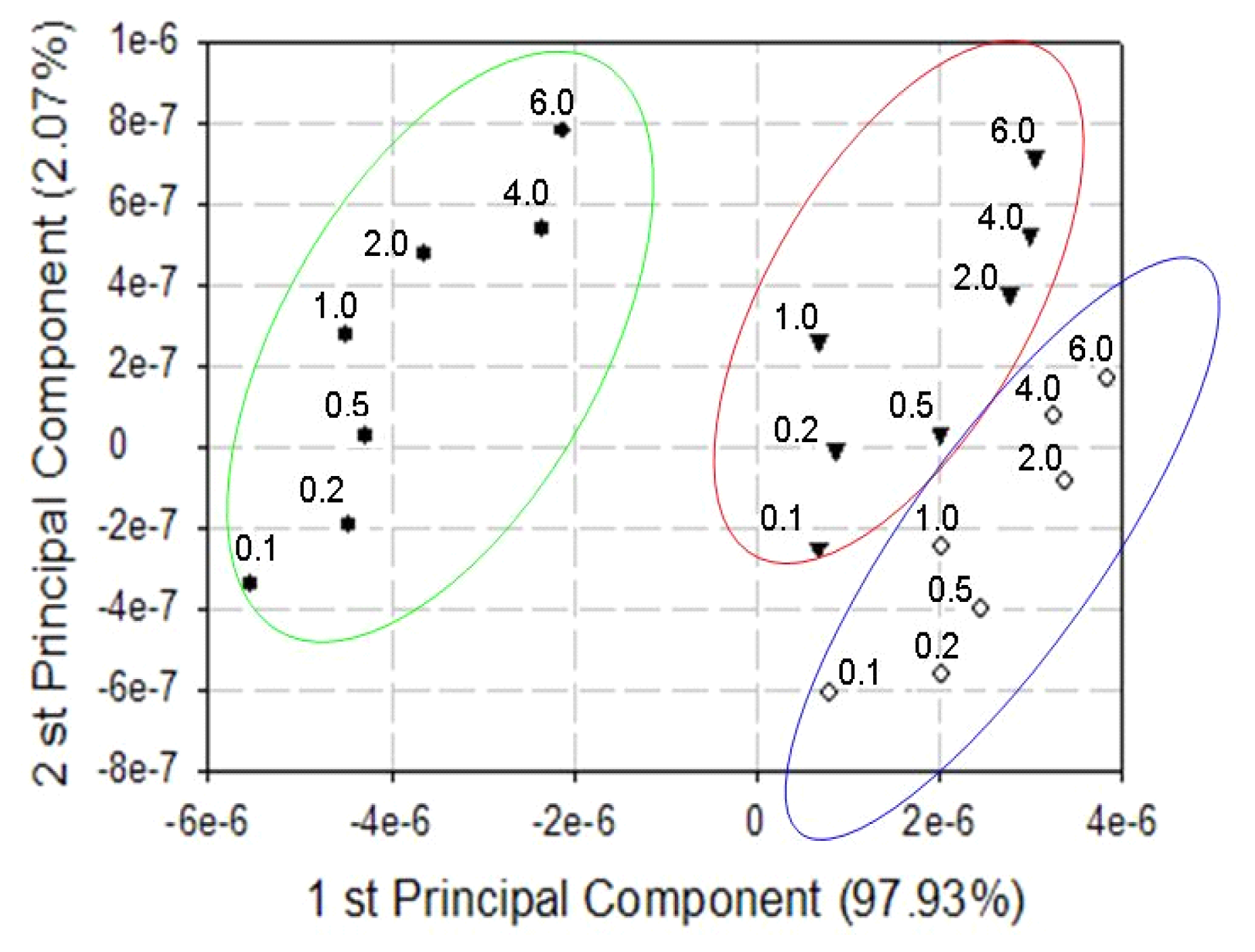
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Tominaga, M. Y.; Antonio, F. M. Exposição humana a trialometanos presentes em água tratada. Rev. Saúde Pública 1999, 33, 413–21. [Google Scholar]
- Melnick, R. L.; Dunnick, J. K.; Sandler, D. P.; Elwell, M. R.; Barrett, J. C. Trihalomethanes and other environmental-factors that contribute to colorectal-cancer. Environmental Health Perspectives 1994, 102, 586–588. [Google Scholar]
- Kasim, K.; Levallois, P.; Johnson, K. C.; Abdous, B.; Auger, P. Chlorination Disinfection By-products in Drinking Water and the Risk of Adult Leukemia in Canada. Am. J. Epidemiol. 2006, 163, 116–126. [Google Scholar]
- Pelizetti, E.; Maurino, V.; Minero, C.; Vinceti, M. Disinfection by-product in drinking water treatments. Mechanism of formation, analysis and research needs. Sci. Technol. 1994, 76, 701–707. [Google Scholar]
- Singer, P. C. Formation and characterization of disinfection by-products; In: Craun GF. Safety of water desinfection: balancing chemical and microbial risks; ILSI Press: Washington (DC), 1993; pp. 201–19. [Google Scholar]
- Gallard, H.; Von Gunten, U. Chlorination of natural organic matter: Kinetics of chlorination and THM formation. Water Res. 2002, 36, 65–74. [Google Scholar]
- Paraskeva, P.; Graham, N. J. D. Ozonation of municipal wastewater effluents. Water Environ. Res. 2002, 74, 569–581. [Google Scholar]
- (USEPA) United States Environmental Protection Agency. National primary drinking water standards. 2003. < http://www.epa.gov/safewater/standards.html> (cited April 20/2005).
- Budziak, D.; Carasek, E. Determination of Trihalomethanes in Drinking Water from Three Different Water Sources in Florianopolis–Brazil using Purge and Trap and Gas Chromatography. J. Braz. Chem. Soc. 2007, 18. [Google Scholar]
- Braga, F. M. G.; Araújo, J. C.; Sales, M. V.; Nascimento, F. R.; Padua, V.L. Diagnóstico da ocorrência de trialometanos (thms) na rede de abastecimento de água de Fortaleza – CE, Brasil. Projeto: “Potenciais fatores de risco à saúde decorrentes da presença de subprodutos de cloração na água utilizada para consumo humano”; PADETEC, da Universidade Federal do Ceará, 2002; p. 14p. [Google Scholar]
- Golfinopoulos, S. K.; Kostopoulou, M. N.; Lekkas, T. D. Volatile halogenated organics in the water supply system in Athens, Greece. Water Res. 1998, 32, 1811–1818. [Google Scholar]
- Minh, T.; Nicholas, D. O.; Birkett, J.; Kenneth, C. J.; Krewski, D.; Villeneuve, P. Chlorination Disinfection By-products and Pancreatic Cancer. Risk Environmental Health Perspectives 2005, 113, 418–424. [Google Scholar]
- Krewski, D.; Balbus, J.; Butler-Jones, D.; Haas, C.; Isaac-Renton, J.; Roberts, K. Managing health risks from drinking water: a report to the Walkerton Inquiry. J Toxicol. Environ. Health 2002, 65, 1635–1823. [Google Scholar]
- Fawell, J. Risk assessment case study-Chloroform and related substances. Food and Chemical Toxicology 2000, 38, 91–95. [Google Scholar]
- U.S. EPA (2005). U.S. EPA Drinking water criteria document for brominated trihalomethanes; Office of Science and Technology Office of Water U.S. Environmental, Protection, Agency: Washington, D.C., November 15 2005. [Google Scholar]
- Carvalho, E. R.; Consolin-Filho, N.; Firmino, A.; Oliveira, O. N., Jr.; Mattoso, L. H. C.; Martin-Neto, L. Sensorial System to Detect Chloroform in Water. Sensor Letters 2006, 4, 129–134. [Google Scholar]
- Riul, A., Jr.; Gallardo, S. A. M.; Mello, S. V.; Bone, S.; Taylor, D. M.; Mattoso, L. H. C. An electronic tongue using polypyrrole and polyaniline. Synthetic Metals 2003, 132, 109–116. [Google Scholar]
- Macdiarmid, A. G. Polyaniline and polypyrrole: where are we headed? Synthetic Metals 1997, 84, 27–34. [Google Scholar]
- Wessling, B. Dispersion as the key to processing conductive polymers. Handbook of conducting polymers; Marcel Dekker: New York, 1998. [Google Scholar]
- Janata, J. Principles of Chemical Sensors; Plenum Press: New York, 1989. [Google Scholar]
- Zhang, Q.; Jin, H.; Wang, X.; Jing, X. Morphology of conductive blend fibers of polyaniline and polyamide-11. Synthetic Metals 2001, 123, 481–485. [Google Scholar]
- Mattoso, L. H. C.; Manohar, S. K.; Macdiarmid, A. G.; Epstein, A. J. Studies on the chemical syntheses and on the characteristics of polyaniline derivatives. J. Polym. Sci., Part A, Polym. Chem. 1995, 33, 1227–1234. [Google Scholar]
- Carvalho, E. R.; Martin-Neto, L.; Milori, D. M. B. P.; Rocha, J. C.; Rosa, A. H. Interactions of chlorine with tropical aquatic fulvic acids and formation of intermediates observed by fluorescence spectroscopy. J. Braz. Chem. Soc. 2004, 15, 421–426. [Google Scholar]
- Aiken, G. R. Humic Substances in Soil, Sediment and Water; John Wiley & Sons: New York, 1985. [Google Scholar]
- Malcolm, R. L. Humic Substances in the Aquatic and Terrestrial Environment; Springer-Verlag: Berlin, 1989. [Google Scholar]
- Paterno, L. G.; Mattoso, L. H. C.; Oliveira, O. N., Jr. Filmes poliméricos ultrafinos produzidos pela técnica de automontagem: preparação, propriedades e aplicações. Quim. Nova 2001, 24, 228–235. [Google Scholar]
- Kern, W. Purifying Si and SiO/sub 2/ surfaces with hydrogen peroxide. Semicond. Int. 1984, 7, 94–99. [Google Scholar]
- Paterno, L. G.; Mattoso, L. H. C. Effect of pH on the preparation of self-assembled films of poly(o-ethoxyaniline) and sulfonated lignin. Polymer 2001, 42, 5239–5245. [Google Scholar]
- Crespilho, F. N.; Zucolotto, V.; Siqueira, J. R.; Constantino, C. J. L.; Nart, F. C.; Oliveira JR, O. N. Immobilization of humic acid in nanostructured layer-by-layer films for sensing applications. Env. Sci. and Technol. 2005, 39, 5385–5389. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Sys. 1987, 2, 37–52. [Google Scholar]
- Paterno, L. G.; Constantino, C. J. L.; Oliveira JR, O. N.; Mattoso, L. H. C. Self-assembled films of poly(o-ethoxyaniline) complexed with sulfonated lignin. Colloids and Surfaces B-Biointerfaces 2002, 23, 257–262. [Google Scholar]
- Venancio, E. C.; Consolin-Filho, N.; Constantino, C. J. L.; Martin-Neto, L.; Mattoso, L. H. C. Studies on the interaction between humic substances and conducting polymers for sensor application. J. Braz. Chem. Soc. 2005, 16, 24–30. [Google Scholar]
- Venancio, E. C.; Mattoso, L. H. C.; Herrmann, P. S. P. J.; MacDiarmid, A. G. Line Patterning of Graphite and the Fabrication of Cheap, Inexpensive, “Throw-away” Sensors. Sensors and Actuators B 2007, 1–26, Accepted Manuscript. [Google Scholar]
- Carvalho, E. R.; Correa, A. A.; Consolin Filho, N.; Oliveira, O. N., Jr.; Gomes, H. L.; Mattoso, L. H. C.; Martin-Neto, L. Detection of Chloroform with a Sensor Array Consisting of Electrochemically Deposited Polythiophenes Films: Processes Governing the Electrical Response. Sensor Letters 2007, 5, 1–6. [Google Scholar]
- Taylor, D. M.; MacDonald, A. G. AC admittance of the metal/ insulator/ electrolyte interface. J. Phys. D: Appl. Phys. 1987, 20, 1277–1283. [Google Scholar]
- Currie, L. A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities: IUPAC Recommendations 1995. Pure Appl. Chem. 1995, 67, 1669–1723. [Google Scholar]
- International Conference on Harmonisation (ICH). Validation of Analytical Procedures: Definitions and Terminology; Q2A (CPMP/ICH/381/95); 1995. [Google Scholar]
- International Conference on Harmonisation (ICH). Validation of Analytical Procedures: Methodology; Q2B (CPMP/ICH/281/95); 1995. [Google Scholar]
- Instituto Nacional de Metrologia, Normalização e Qualidade Industrial (INMETRO). Orientações sobre Validação de Métodos de Ensaios Químicos; DOQ-CGCRE-008; 2003. [Google Scholar]

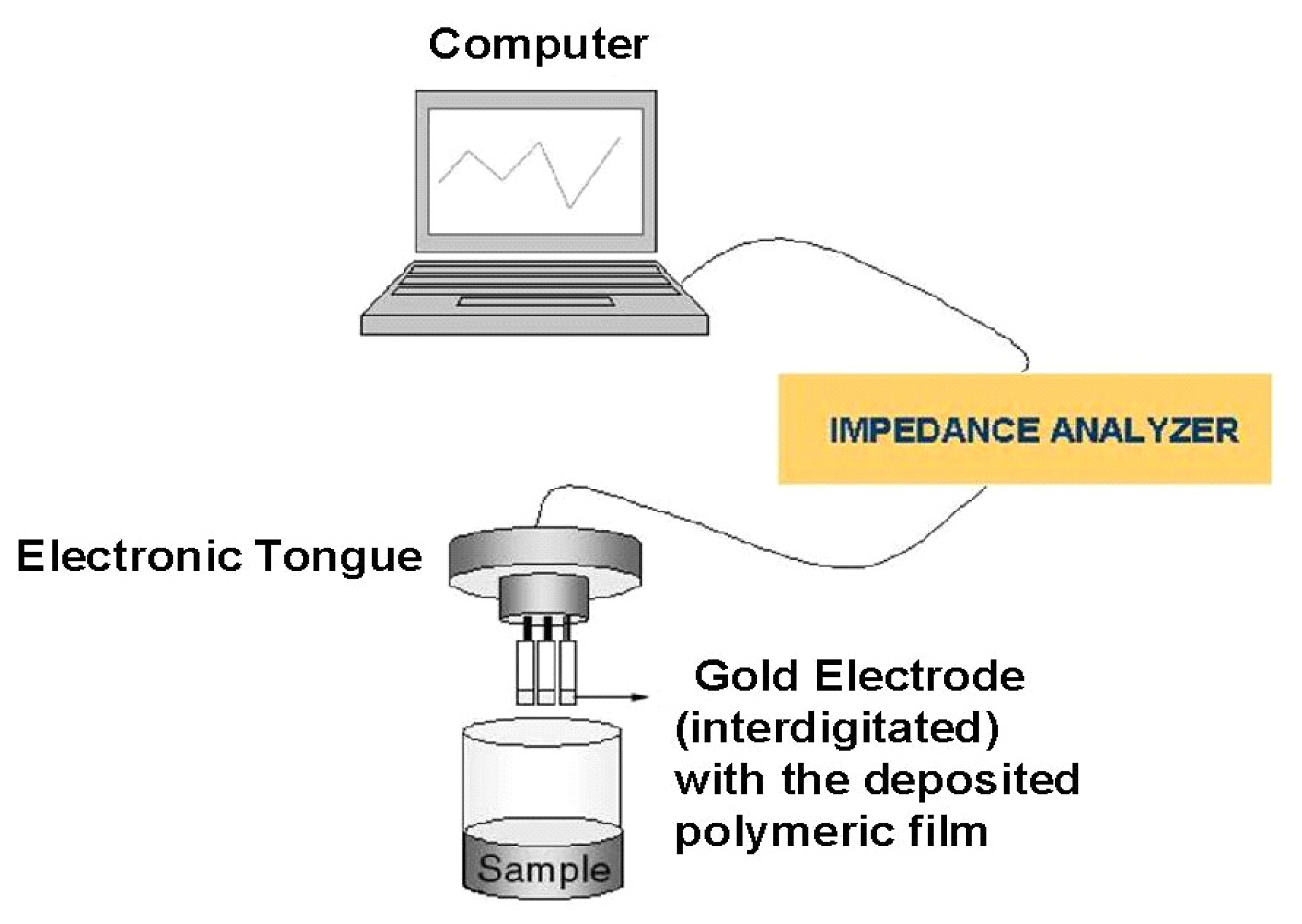
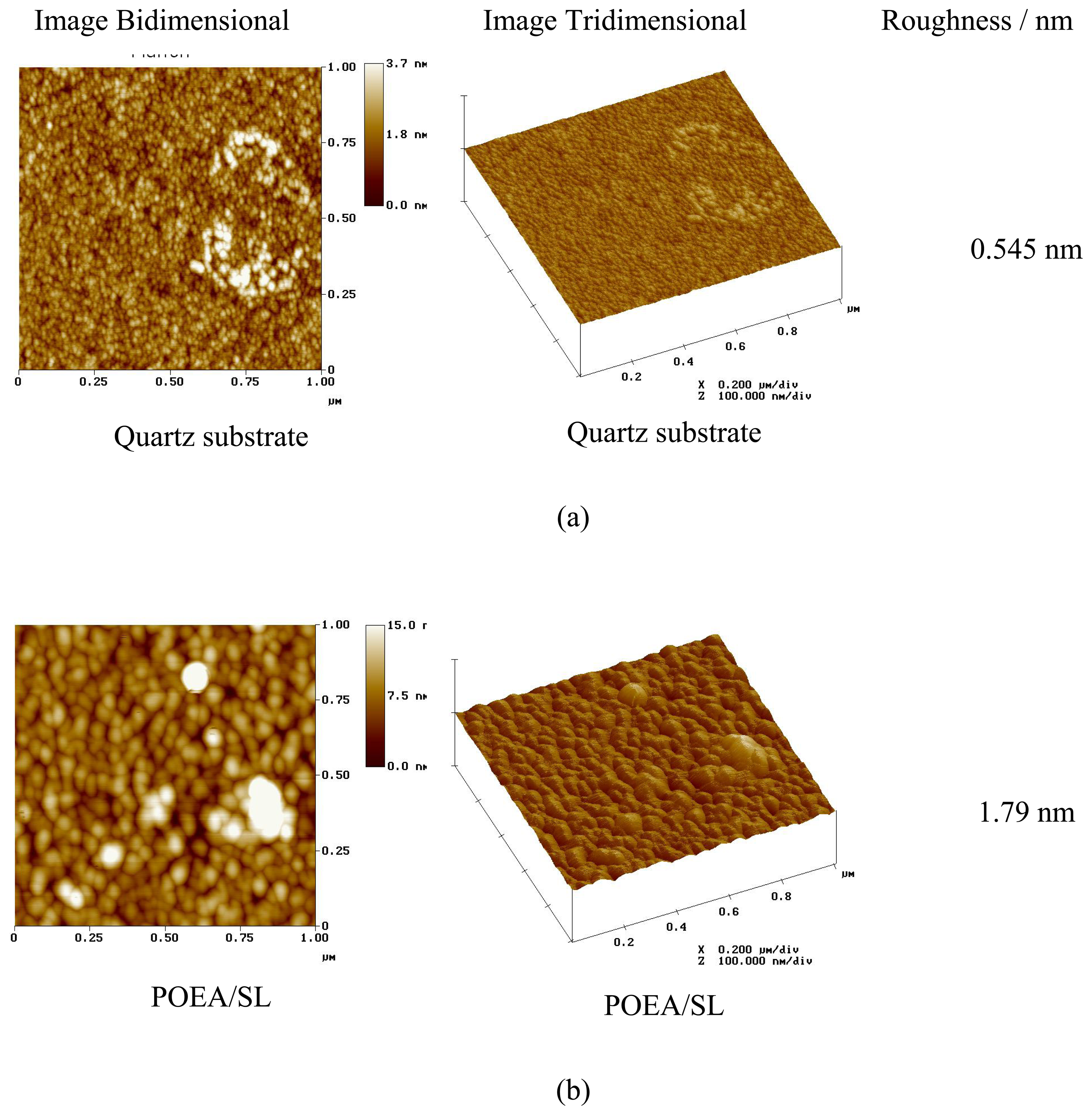

| Type of Material | Sensing Unit |
|---|---|
| without film | S1 |
| POEA | S2 |
| POEA/SL | S3 |
| POEA + SL | S4 |
| SL | S5 |
| AHS/POEA | S6 |
| POEA + AHS | S7 |
| PANI | S8 |
| AHS | S9 |
| PANI/SL | S10 |
| Sensors | DL (mg L-1) | |||
|---|---|---|---|---|
| CHBr3 | CHBrCl2 | CHBr2Cl | ||
| S1 | 0.074 ± 0.003 | 0.175 ± 0.004 | 0.115 ± 0.005 | |
| S2 | 0.082 ± 0.001 | 0.137 ± 0.005 | 0.145 ± 0.010 | |
| S3 | 0.165 ± 0.041 | 0.608 ± 0.050 | 0.257 ± 0.030 | |
| S4 | 0.033 ± 0.004 | 0.064 ± 0.000 | 0.070 ± 0.000 | |
| S5 | 0.071 ± 0.003 | 0.223 ± 0.004 | 0.253 ± 0.004 | |
| S6 | 0.084 ± 0.020 | 0.707 ± 0.020 | 0.203 ± 0.010 | |
| S7 | 0.020 ± 0.005 | 0.042 ± 0.002 | 0.091 ± 0.000 | |
| S8 | 0.043 ± 0.005 | 0.328 ± 0.100 | 0.205 ± 0.002 | |
| S9 | 0.104 ± 0.003 | 0.168 ± 0.001 | 0.309 ± 0.004 | |
| S10 | 0.050 ± 0.005 | 0.204 ± 0.030 | 0.137 ± 0.080 | |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Carvalho, E.R.; Consolin Filho, N.; Venancio, E.C.; O., O.N., Jr.; Mattoso, L.H.C.; Martin-Neto, L. Detection of Brominated By-Products Using a Sensor Array Based on Nanostructured Thin Films of Conducting Polymers. Sensors 2007, 7, 3258-3271. https://doi.org/10.3390/s7123258
Carvalho ER, Consolin Filho N, Venancio EC, O. ON Jr., Mattoso LHC, Martin-Neto L. Detection of Brominated By-Products Using a Sensor Array Based on Nanostructured Thin Films of Conducting Polymers. Sensors. 2007; 7(12):3258-3271. https://doi.org/10.3390/s7123258
Chicago/Turabian StyleCarvalho, Eduarda Regina, Nelson Consolin Filho, Everaldo Carlos Venancio, Osvaldo N. O., Jr., Luiz H. C. Mattoso, and Ladislau Martin-Neto. 2007. "Detection of Brominated By-Products Using a Sensor Array Based on Nanostructured Thin Films of Conducting Polymers" Sensors 7, no. 12: 3258-3271. https://doi.org/10.3390/s7123258
APA StyleCarvalho, E. R., Consolin Filho, N., Venancio, E. C., O., O. N., Jr., Mattoso, L. H. C., & Martin-Neto, L. (2007). Detection of Brominated By-Products Using a Sensor Array Based on Nanostructured Thin Films of Conducting Polymers. Sensors, 7(12), 3258-3271. https://doi.org/10.3390/s7123258




