An influence of polyHEMA gate layer on properties of ChemFETs
Abstract
:Introduction
Experimental
Chemicals
- -
- 2-hydroxyethyl methacrylate (HEMA), purum - Fluka, Buchs
- -
- 3-trimethoxysilylpropyl methacrylate, purum - Fluka, Buchs
- -
- ethylene glycol dimethacrylate (EGDMA), purum - Fluka, Buchs
- -
- polyvinylpyrrolidone K-15 (PVP K-15), pract. - Fluka, Buchs
- -
- polyvinylpyrrolidone K-90 (PVP K-90), BioChemika - Fluka, Buchs
- -
- 2,2’-dimethoxy-2-phenylacetophenone (DMPAP, photoinitiator), 99% - Aldrich
- -
- toluene, p.a. - POCh, Gliwice
- -
- 2-butanone (ethyl methyl ketone), pure – CHEMPUR, Piekary Slaskie
- -
- ethanol, 95%
- -
- hydrogen peroxide solution, 3%
- -
- nonactine, Selectophore® - Fluka, Buchs
- -
- potassium tetrakis(4-chlorophenyl)borate (KTpClPB), Selectophore® - Fluka, Buchs
- -
- Siloprene K 1000, Selectophore® - Fluka, Buchs
- -
- Siloprene Crosslinking Agent K-11, Selectophore® - Fluka, Buchs
- -
- tetrahydrofuran (THF) - Riedel de Haën or Fluka
- -
- ammonium chloride, p.a. – POCh, Gliwice
- -
- sodium chloride, p.a. – Merck, Darmstadt or POCh, Gliwice
- -
- sodium dihydrogenphosphate, p.a. – POCh, Gliwice
- -
- sodium hydroxide, p.a., pre-weighed concentrate samples – POCh, Gliwice
- -
- hydrochloric acid, p.a., pre-weighed concentrate samples – POCh, Gliwice
- -
- nitrogen, technically pure (99,9%) - Praxair, Warsaw
Equipment for Fabrication of PolyHEMA Hydrogel Coatings
Silanization
- Round-bottomed flanged reactor of 1000 ml capacity, with five-necked cover, flange diameter 100 mm, reflux condenser, thermometer, Teflon sleeves for ground joints – Bibby Quickfit, Great Britain.
- Electric heating mantles matching the reactor.
- Autotransformer for power supply control of the heating mantle.
Photopolymerization
- -
- Centrifuge with controlled spin-rate for uniform distribution of liquid HEMA mixture onto 3”-wafers – Laboratory of Biochemical Sensors of the Institute of Biocybernetics and Biomedical Engineering of Polish Academy of Sciences (IBBE).
- -
- UV lamp – EPROM eraser, model 910 EE, MOMIK Electronics, Warsaw –- with additional accessories for fastening of mounted sensors and blowing through the casing with nitrogen, fabricated at the IBBE.
- -
- Photolithography station, consisting of illuminator with light-guide, power supply unit, wafer support with mask aligner and optical bench.
Characterization of the Partial Technological Processes
Hydration
Silanization
- -
- toluene: 450 ml,
- -
- 3-(trimethoxysilyl)propyl methacrylate: 50 ml,
- -
- deionized water: 2 ml,
Deposition of Hydrogel Layer

Optimization of Technology of PolyHEMA Layer Deposition for Coating of Wafers
Method 1 – PolyHEMA with PVP K-15 as a Filler
| Additives to HEMA | Weight percent related to HEMA |
|---|---|
| PVP K-15 | 10% |
| DMPAP | 4% |
| EGDMA | 0.5% |
Method 2 - PolyHEMA with PVP K-15 as a Filler, with Additional Preparation of Silicon Nitride Surface and Increasing of the Thickness of Already Deposited PolyHEMA Layers
Increasing of Thickness of PolyHEMA Layer Deposited According to the Method 2
Method 3 - PolyHEMA with PVP K-90 as a Filler, with Addition of KCl Solution
| Additives to HEMA | Weight percent related to HEMA |
|---|---|
| PVP K-90 | 10% |
| DMPAP | 4% |
| EGDMA | 0.5% |
| 0.1 M aqueous KCl solution | 40% |
| Test No. | Spin rate | Spin Time | Coating thickness in µm | ISFET structures | |
| mean | at wafer edge | ||||
| 1 | 500 min-1 | 30 s | 60 – 100 | up to 160 | yes |
| 2 | 500 min-1 | 5 min | 40 – 60 | up to 120 | no |
| 3 | 1000 min-1 | 5 min | 20 – 30 | up to 50 | no |
| 4 | 1500 min-1 | 5 min | 15 – 25 | up to 40 | no |
| 5 | 2000 min-1 | 5 min | 5 – 15 | up to 20 | no |
| 6 | 1000 min-1 | 7 min | 15 – 20 | 35* | yes |
Characterization of ISFETs with Deposited PolyHEMA Layer
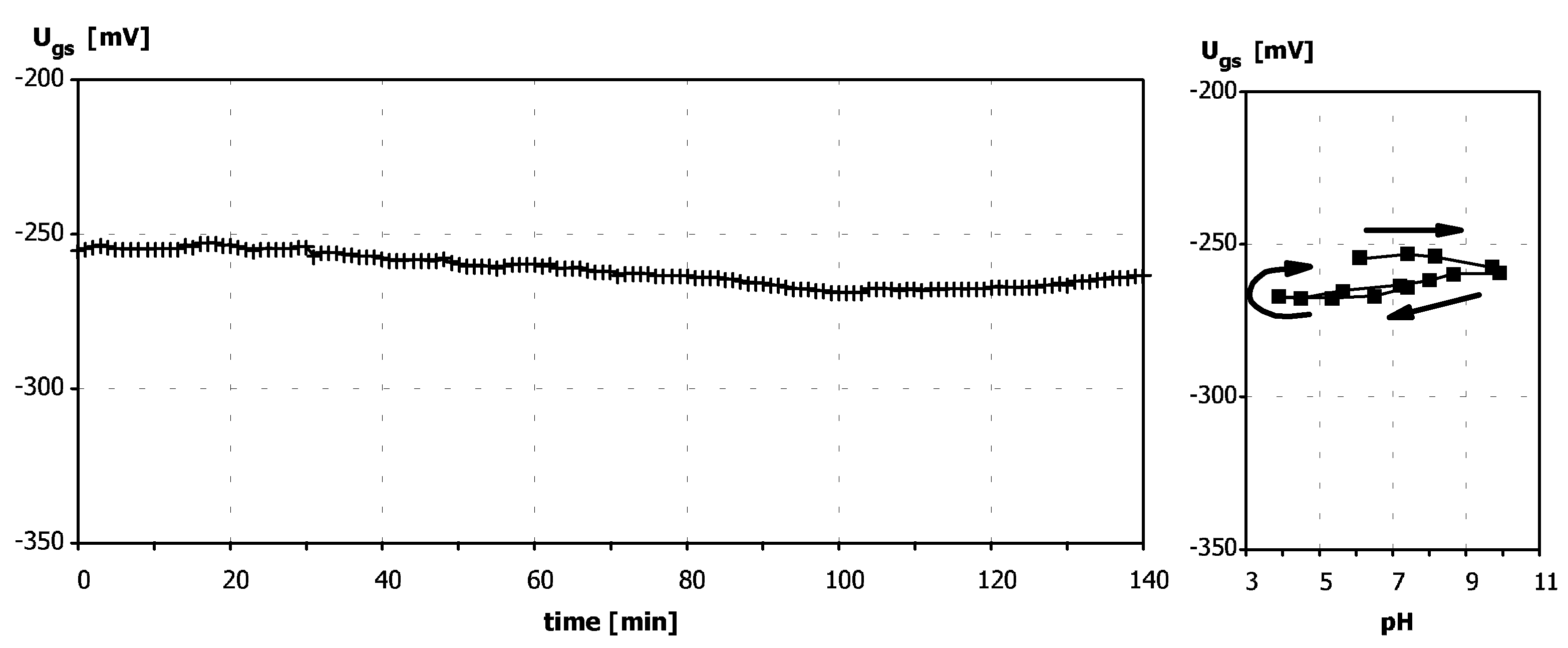
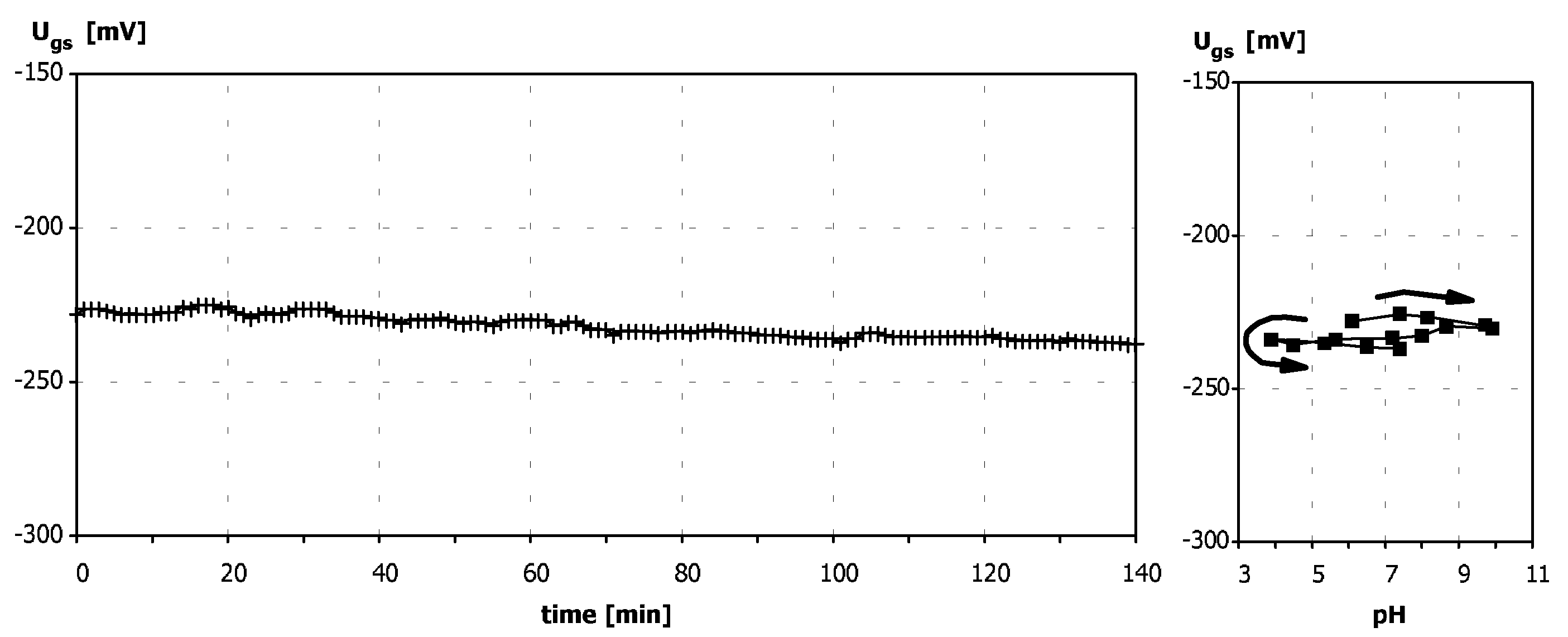
Sensitivity to pH
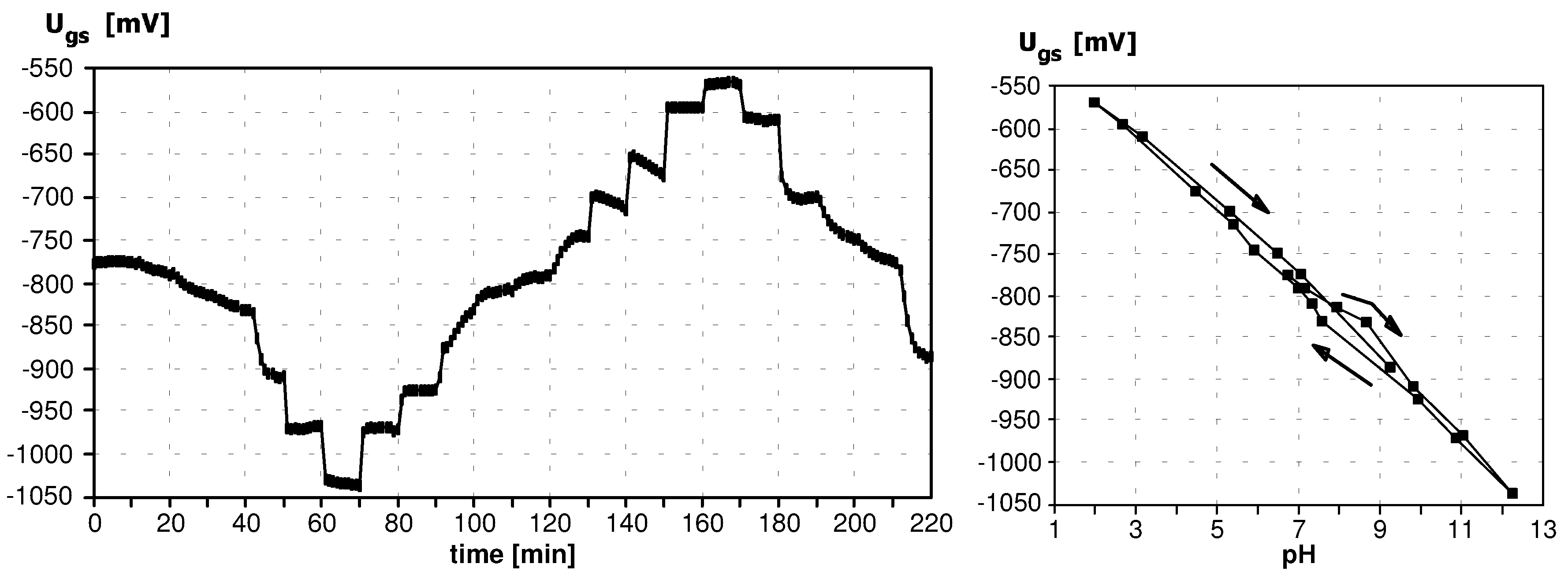
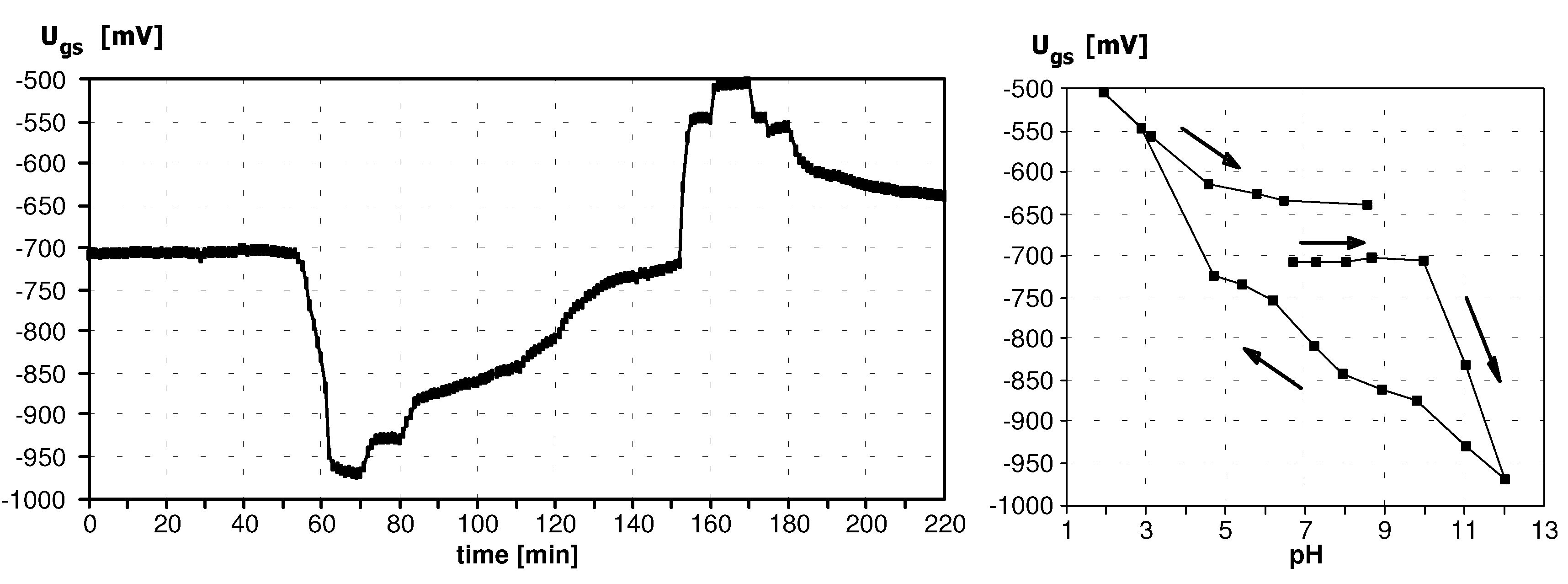
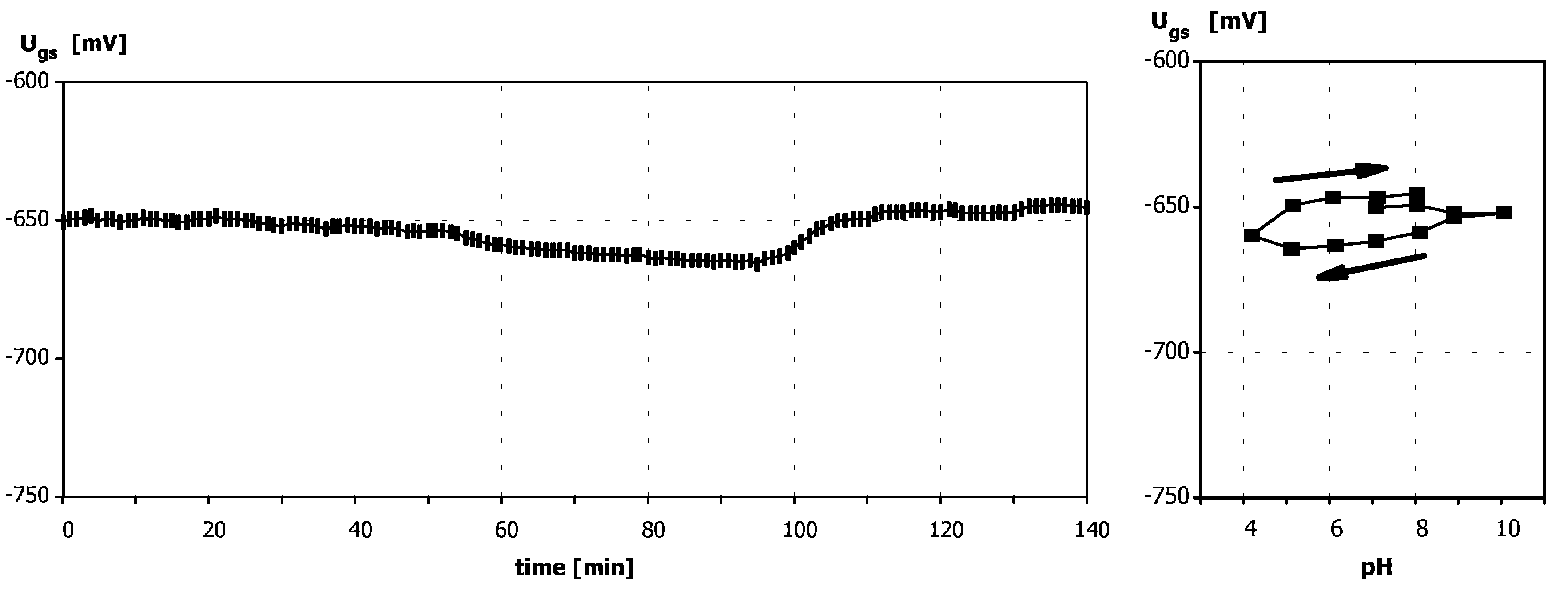
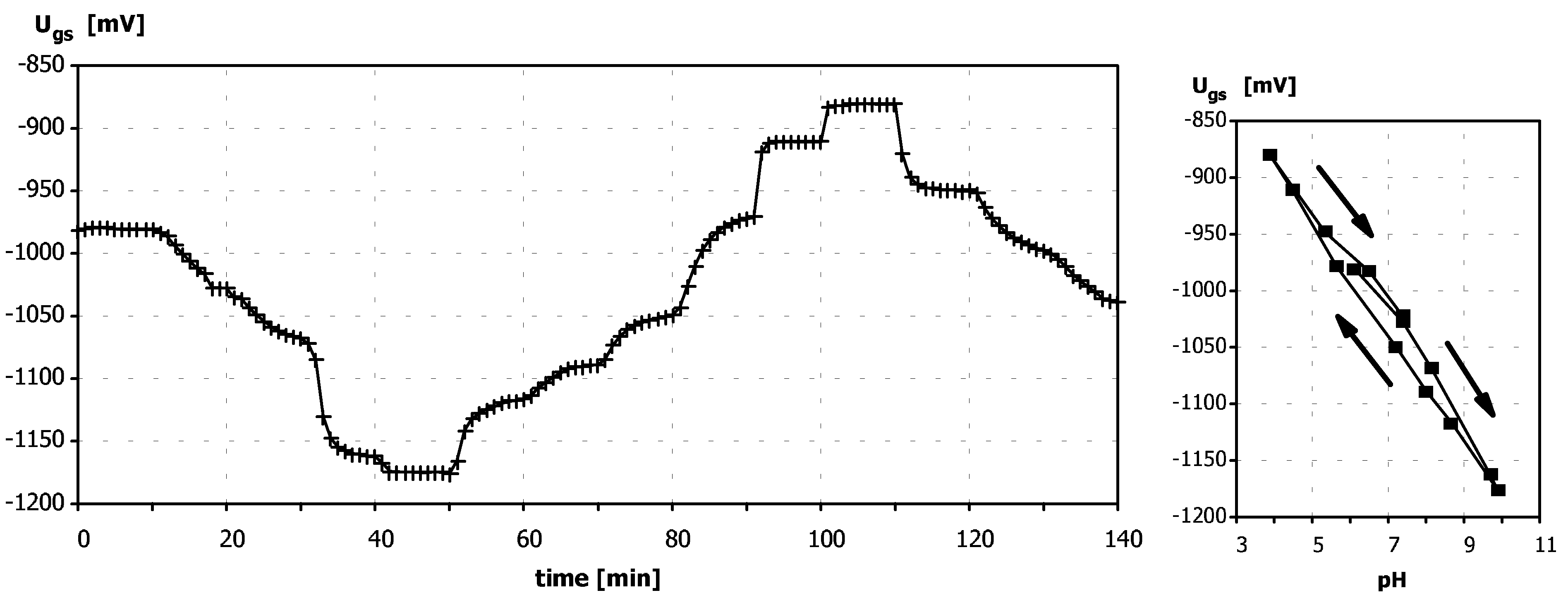
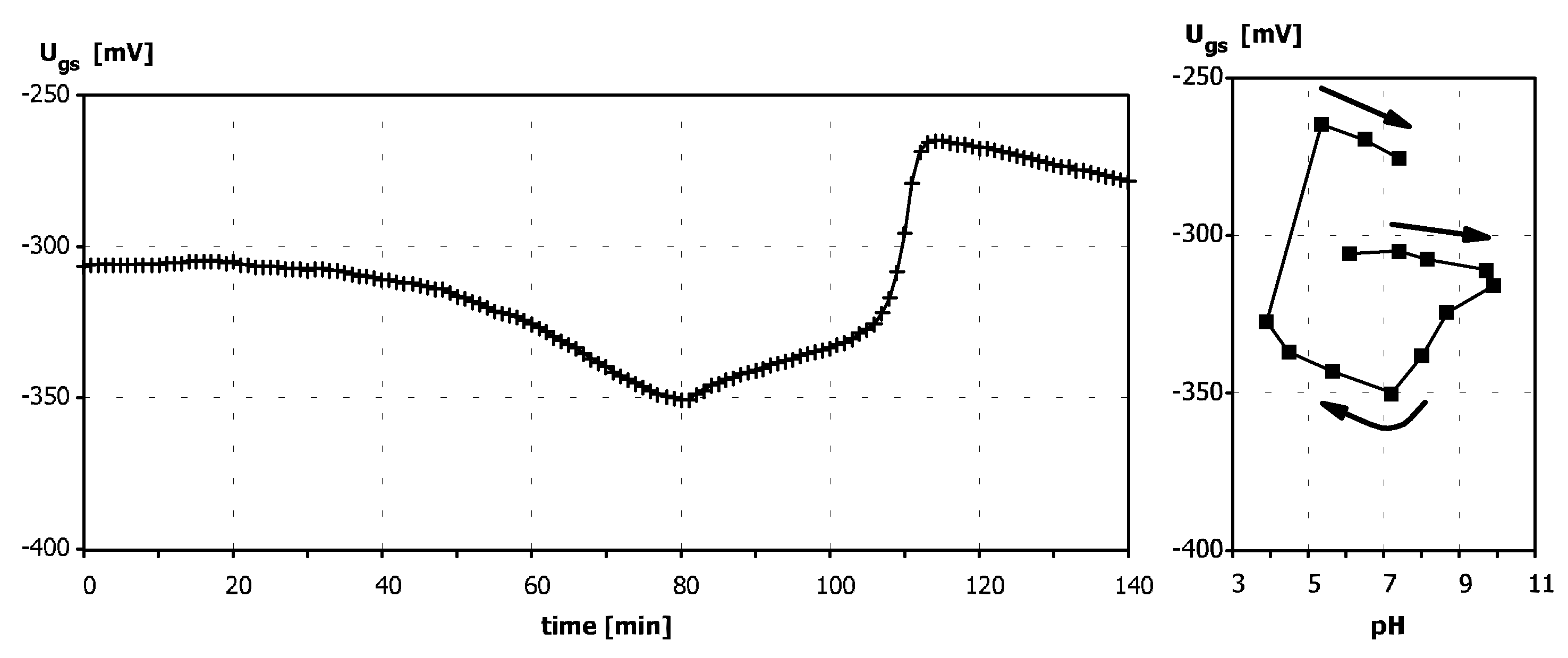
Application of ISFETs wth PolyHEMA Coatings to Construction of Ion-Selective Sensors
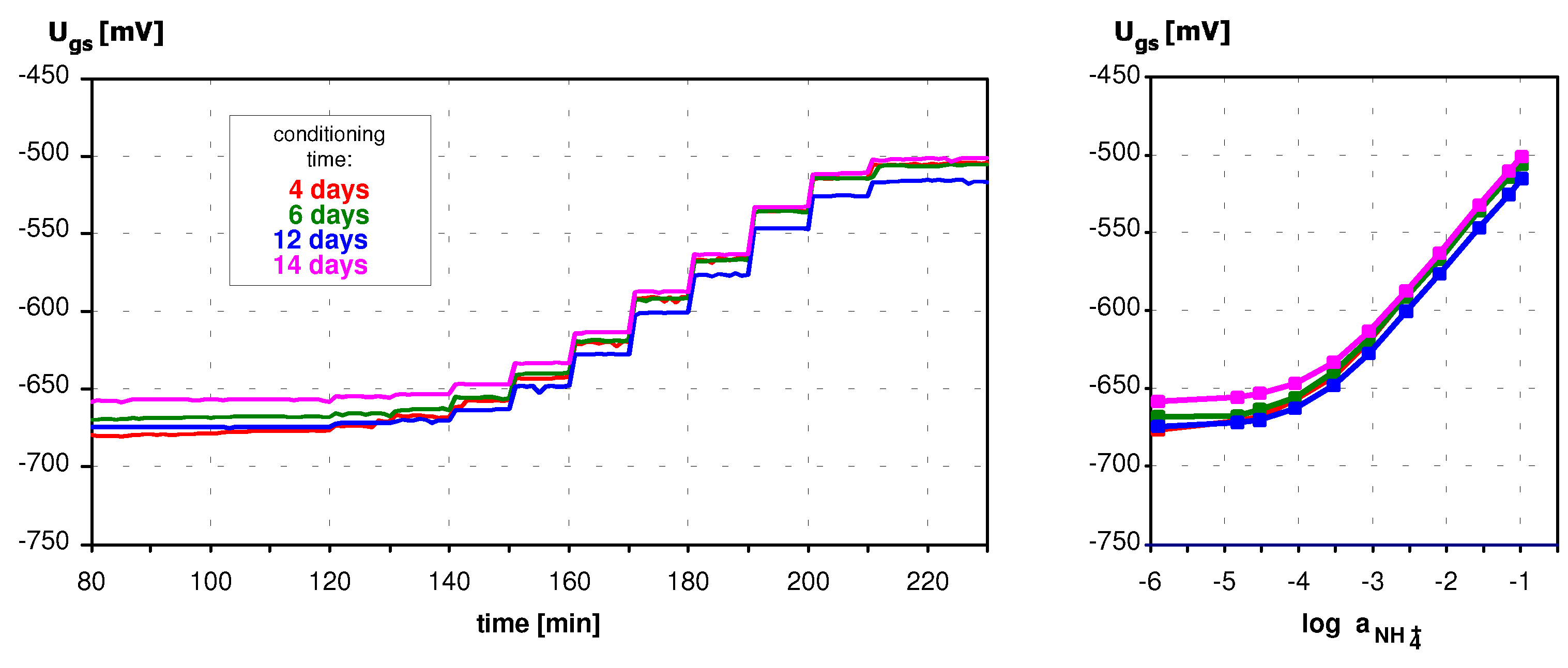
Conclusions
References
- Bergveld, P.; Sibbald, A. Analytical and biomedical applications of ion-sensitive field effect transducers; Elsevier Science Pub. B.V.: Amsterdam, 1988. [Google Scholar]
- Torbicz, W. Theory and properties of field effect transistors as biosensors; Ossolineum: Wroclaw, 1988. [Google Scholar]
- Pijanowska, D.; Torbicz, W. Long-term stability of Si3N4, Al2O3 and Ta2O5 gate pH-ISFETs. Proc. of SPIE - The Int. Society for Optical Engineering on Optoelectronic and Electronic Sensors, USA 1995, 2634, 210. [Google Scholar]
- Torbicz, W.; Pijanowska, D. ISFETs Instability Problems and Methods of their Minimization for Long-term pH Monitoring Purposes. Biocybernetics and Biomedical Engineering 1996, 16(1–2), 71–91. [Google Scholar]
- Sudhölter, E. J. R.; van der Wal, P. D.; Skowronska-Ptasinska, M.; van den Berg, A.; Bergveld, P.; Reinhoudt, D. N. Anal. Chim. Acta 1990, 230, 59.
- Dawgul, M.; Jazwinski, J.; Koszur, J.; Brzozka, Z.; Torbicz, W. Development of an intermediate hydrogel layer for chemically modified FET based on Si3N4. In Proc. of Eurosensors XI, The 11th European Conference on Solid State Transducers, Warsaw, Poland, September 21-24, 1997; 1275.
- Brzózka, Z.; Dawgul, M.; Pijanowska, D.; Torbicz, W. Development of NH4+-sensitive polymer membranes for long term performance microsensor. Proc. of SPIE - The Int. Society for Optical Engineering on Optoelectronic and Electronic Sensors 1996, 3054, 187–196. [Google Scholar]
- Brzózka, Z.; Dawgul, M.; Pijanowska, D.; Torbicz, W. Durable NH4+-sensitive CHEMFET. Sensors and Actuators B 1997, 44, 527–531. [Google Scholar]
- Pijanowska, D.; Torbicz, W. Long-term stability of Si3N4, Al2O3 and Ta2O5 gate pH-ISFETs. Proc. of SPIE - The Int. Society for Optical Engineering on Optoelectronic and Electronic Sensors, USA 1995, 2634, 210. [Google Scholar]
- Sample Availability:
© 2003 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Dawgul, M.; Pijanowska, D.G.; Krzyskow, A.; Kruk, J.; Torbicz, W. An influence of polyHEMA gate layer on properties of ChemFETs. Sensors 2003, 3, 146-159. https://doi.org/10.3390/s30600146
Dawgul M, Pijanowska DG, Krzyskow A, Kruk J, Torbicz W. An influence of polyHEMA gate layer on properties of ChemFETs. Sensors. 2003; 3(6):146-159. https://doi.org/10.3390/s30600146
Chicago/Turabian StyleDawgul, Marek, Dorota G. Pijanowska, Alfred Krzyskow, Jerzy Kruk, and Wladyslaw Torbicz. 2003. "An influence of polyHEMA gate layer on properties of ChemFETs" Sensors 3, no. 6: 146-159. https://doi.org/10.3390/s30600146
APA StyleDawgul, M., Pijanowska, D. G., Krzyskow, A., Kruk, J., & Torbicz, W. (2003). An influence of polyHEMA gate layer on properties of ChemFETs. Sensors, 3(6), 146-159. https://doi.org/10.3390/s30600146



