Chemiresistive Effect in Ti0.2V1.8C MXene/Metal Oxide Hetero-Structured Composites
Highlights
- Heterostructures of Ti0.2V1.8C MXenes with metal oxides have been synthesized.
- Ti0.2V1.8C MXene/metal oxide composites have been tested as chemiresistive elements.
- The synthesis protocol for Ti0.2V1.8C MXene/metal oxide composites could be further scaled when developing sensor prototype units.
- The Ti0.2V1.8C MXene/metal oxide composites are shown as promising, versatile materials for designing multisensor arrays.
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Materials Under Study
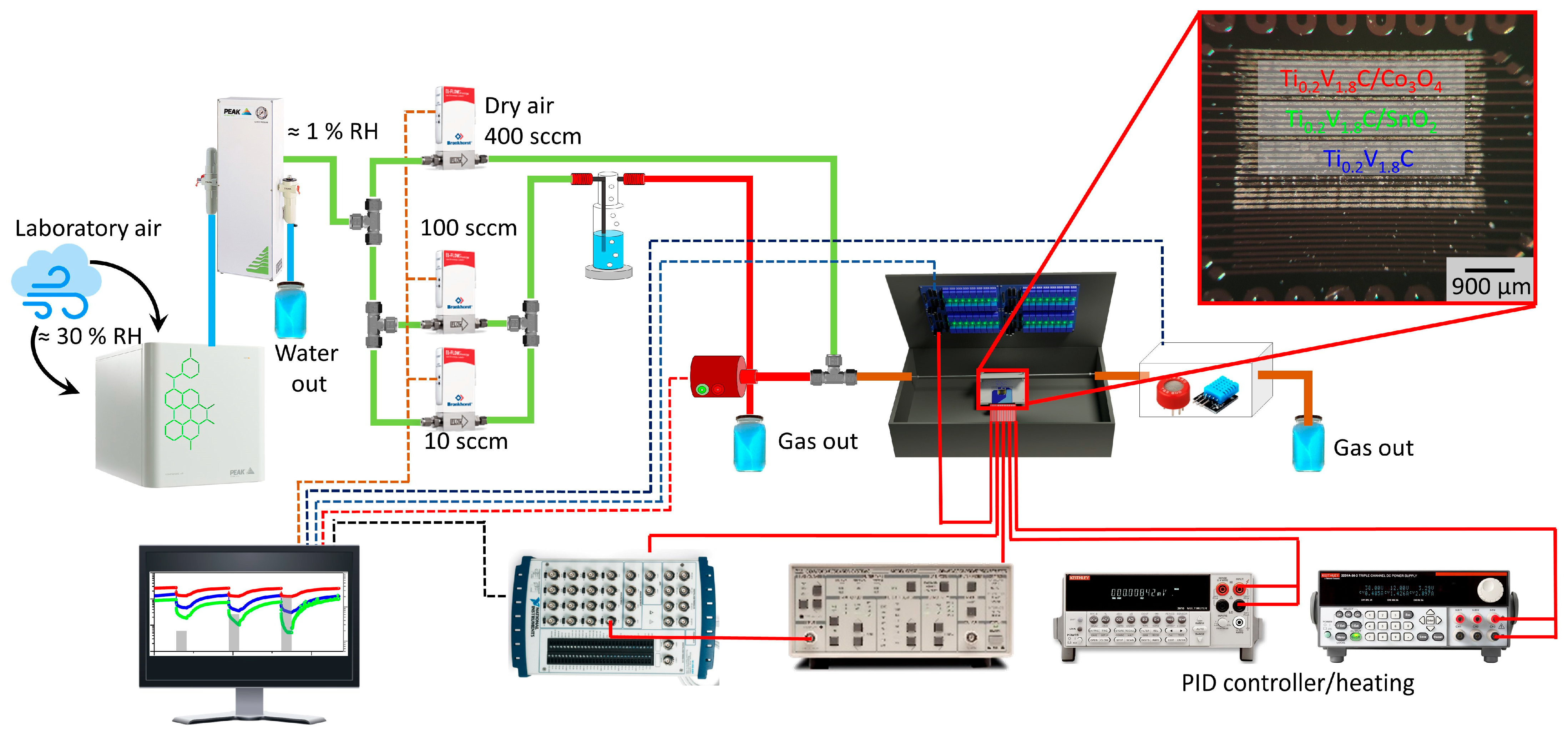
2.2. Gas-Sensing Characterization of On-Chip Sensor Elements
3. Results and Discussion
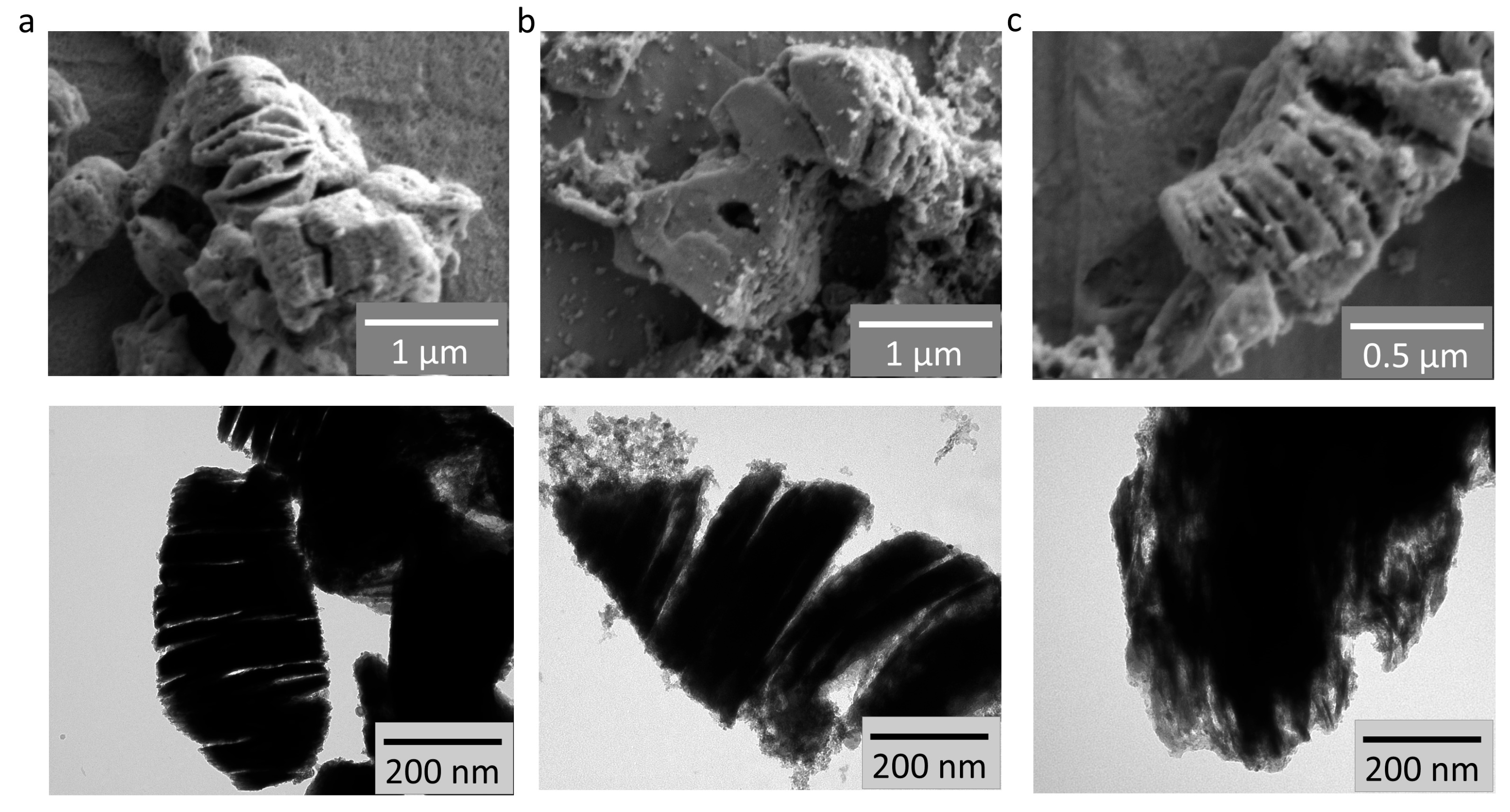
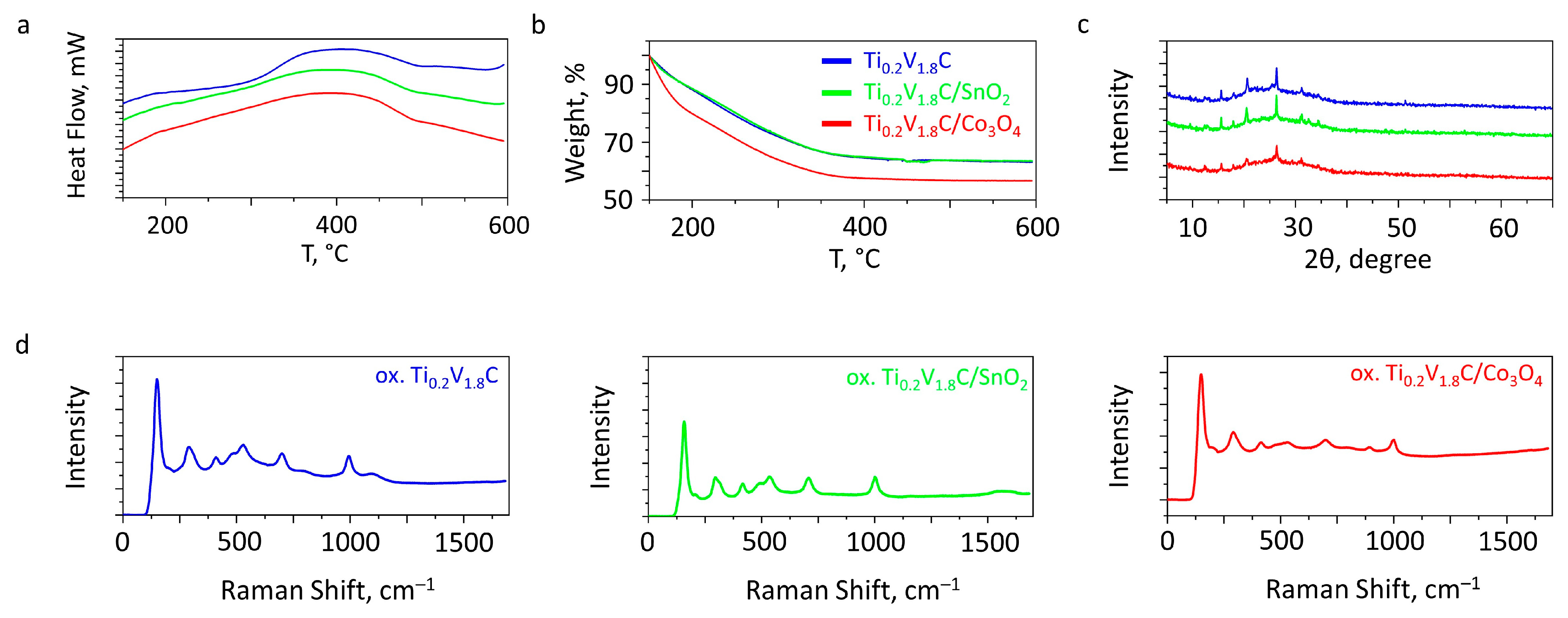
3.1. Results of Physical Characterization of the Materials
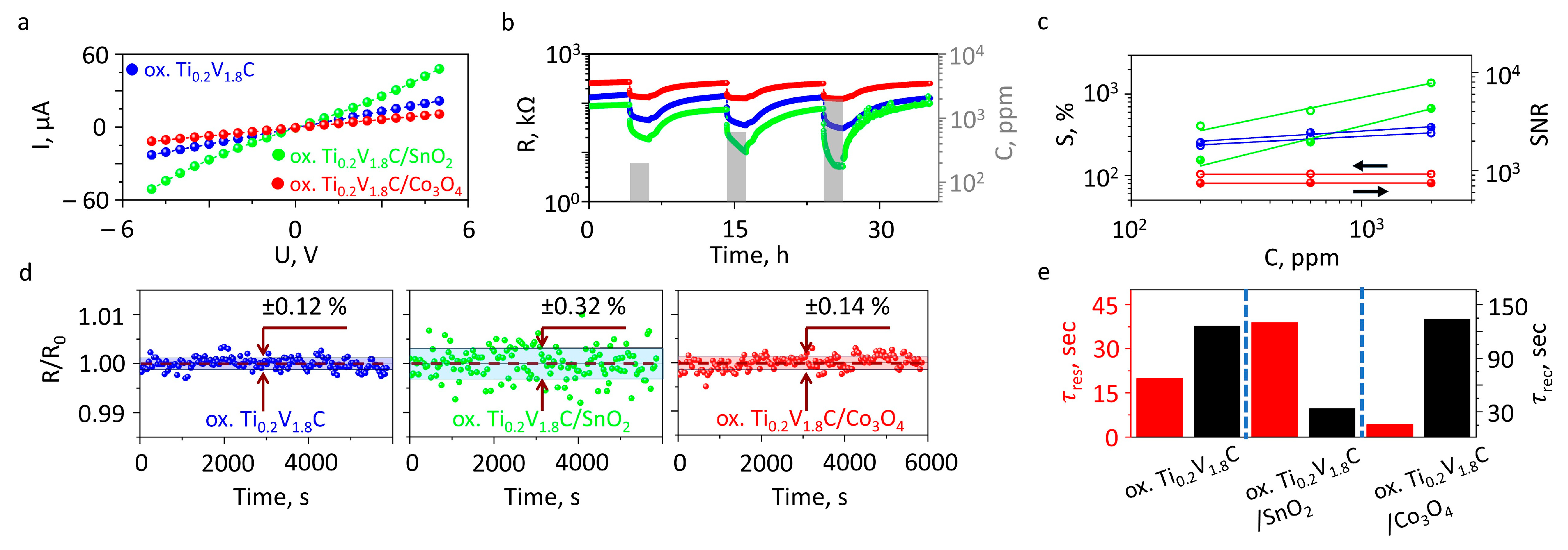
3.2. Results of Gas-Sensing Characterization of On-Chip Sensor Elements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, A.; Edwards, P. Validate personal air-pollution sensors. Nature 2016, 535, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Silva-Neto, H.A.; da Silva Sousa, D.; Duarte, L.C.; da Silveira Petruci, J.F.; Coltro, W.K.T. Recent developments on aerial lab-on-a-drone platforms for remote environmental monitoring: A review. Anal. Chim. Acta 2025, 1337, 343544. [Google Scholar] [CrossRef] [PubMed]
- Soroka, T.; Ravia, A.; Snitz, K.; Honigstein, D.; Weissbrod, A.; Gorodisky, L.; Weiss, T.; Perl, O.; Sobel, N. Humans have nasal respiratory fingerprints. Curr. Biol. 2025, 35, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Kim, D.-S.; Seo, J.H.; Chung, J.H.; Park, Z.; Kwon, K.C.; Ko, J.-K.; Ha, T.W.; Lee, J.-O.; Kim, G.-L.; et al. Artificial olfactory system enabled by ultralow chemical sensing variations of 1D SnO2 nanoarchitectures. Adv. Sci. 2025, 12, 2501293. [Google Scholar] [CrossRef]
- Khorramifar, A.; Karami, H.; Lvova, L.; Kolouri, A.; Łazuka, E.; Piłat-Rożek, M.; Łagód, G.; Ramos, J.; Lozano, J.; Kaveh, M.; et al. Environmental engineering applications of electronic nose systems based on MOX gas sensors. Sensors 2023, 2312, 5716. [Google Scholar] [CrossRef]
- Dutta, T.; Noushin, T.; Tabassum, S.; Mishra, S.K. Road map of semiconductor metal-oxide-based sensors: A review. Sensors 2023, 23, 6849. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Shynybekov, Y.; Soltabayev, B.; Yergaliuly, G.; Mentbayeva, A. The Cross-sensitivity of chemiresistive gas sensors: Nature, methods, and peculiarities: A systematic review. ACS Sens. 2024, 9, 6358–6371. [Google Scholar] [CrossRef]
- Korotcenkov, G. II–VI Semiconductor-based conductometric gas sensors: Is there a future for these sensors? Sensors 2024, 24, 3861. [Google Scholar] [CrossRef]
- Kumar, A.; Mazumder, J.T.; Joyen, K.; Favier, F.; Mirzaei, A.; Kim, J.-Y.; Kwoka, M.; Bechelany, M.; Jha, R.K.; Kumar, M.; et al. Defect engineering approaches for metal oxide semiconductor-based chemiresistive gas sensing. Coord. Chem. Rev. 2025, 541, 216836. [Google Scholar] [CrossRef]
- Qu, L.; Xu, Y.; Cui, W.; Wu, L.; Feng, Y.; Gu, Y.; Pan, H. Trends in conductive MOFs for sensing: A review. Anal. Chim. Acta 2025, 1336, 343307. [Google Scholar] [CrossRef]
- Kumar, S.; Mehdi, S.M.Z.; Seo, Y. 1D MXenes: Synthesis, properties, and applications. Small 2024, 20, 2405576. [Google Scholar] [CrossRef]
- Ding, G.; Li, H.; Zhao, J.; Zhou, K.; Zhai, Y.; Lv, Z.; Zhang, M.; Yan, Y.; Han, S.-T.; Zhou, Y. Nanomaterials for flexible neuromorphics. Chem. Rev. 2024, 124, 12738–12843. [Google Scholar] [CrossRef]
- Kissine, V.V.; Sysoev, V.V.; Voroshilov, S.A. Conductivity of SnO2 thin films in the presence of surface adsorbed species. Sens. Actuators B 2001, 79, 163–170. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Islam, M.; Shafi, M.; Ali, S.; Wang, H.-E. Developments and prospects of MXenes for energy storage and environmental sustainability. Coord. Chem. Rev. 2025, 540, 216797. [Google Scholar] [CrossRef]
- Bi, W.; Gao, G.; Li, C.; Wu, G.; Cao, G. Synthesis, properties, and applications of MXenes and their composites for electrical energy storage. Prog. Mater. Sci. 2024, 142, 101227. [Google Scholar] [CrossRef]
- Liu, X.; Xu, F.; Li, Z.; Liu, Z.; Yang, W.; Zhang, Y.; Fan, H.; Yang, H.Y. Design strategy for MXene and metal chalcogenides/oxides hybrids for supercapacitors, secondary batteries and electro/photocatalysis. Coord. Chem. Rev. 2022, 464, 214544. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, T.L.; Gorobtsov, P.Y.; Nagornov, I.A.; Korotcenkov, G.; Sysoev, V.V.; Kuznetsov, N.T. Application of titanium carbide MXenes in chemiresistive gas sensors. Nanomaterials 2023, 13, 850. [Google Scholar] [CrossRef]
- Kumar, P.; Raza, W.; Suganthi, S.; Khan, M.Q.; Ahmad, K.; Oh, T.H. Recent progress in MXenes-based materials for gas sensors and photodetectors. Chemosensors 2024, 12, 147. [Google Scholar] [CrossRef]
- Dananjaya, V.; Hansika, N.; Marimuthu, S.; Chevali, V.; Mishra, Y.K.; Grace, A.N.; Salim, N.; Abeykoon, C. MXenes and its composite structures: Synthesis, properties, applications, 3D/4D printing, and artificial intelligence; machine learning integration. Prog. Mater. Sci. 2025, 152, 101433. [Google Scholar] [CrossRef]
- Ahmad, N.; Rasheed, S.; Mohyuddin, A.; Fatima, B.; Nabeel, M.I.; Riaz, M.T.; Najam-ul-Haq, M.; Hussain, D. 2D MXenes and their composites; design, synthesis, and environmental sensing applications. Chemosphere 2024, 352, 141280. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Yan, F. Constructed MXene matrix composites as sensing material and applications thereof: A review. Anal. Chim. Acta 2024, 1288, 342027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Jian, M.; Jiang, Q.; Li, X. MXene key composites: A new arena for gas sensors. Nano-Micro Lett. 2024, 16, 209. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, Y.; Huang, C.; Gu, S.; Du, B.; Dong, Y.; Shi, Y.; Liu, H.; Horprathum, M.; Hu, W.; et al. MOF-derived NiO-SnO2/Ti3C2Tx composite materials for detection of ppb-Level NO2 at room temperature. ACS Appl. Nano Mater. 2025, 8, 8927–8936. [Google Scholar] [CrossRef]
- Mei, Z.; Song, Y.; Jiang, G.; Wu, D.; Li, G. SnO2/ZnO/Ti3C2Tx MXene nanocomposites for highly sensitive and stable ethanol sensing at low temperature. Sens. Actuators B 2024, 419, 136390. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Chang, X.; Gao, W.; Chen, X.; Niu, S.; Sun, S. Room temperature gas sensors for NH3 detection based on SnO2 films and lamellar-structured Ti3C2Tx MXene heterojunction nanocomposites. Appl. Surf. Sci. 2024, 660, 159976. [Google Scholar] [CrossRef]
- Sun, B.; Qin, F.; Jiang, L.; Gao, J.; Liu, Z.; Wang, J.; Zhang, Y.; Fan, J.; Kan, K.; Shi, K. Room-temperature gas sensors based on three-dimensional Co3O4/Al2O3@Ti3C2Tx MXene nanocomposite for highly sensitive NOx detection. Sens. Actuators B 2022, 368, 132206. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Glukhova, O.E.; Plugin, I.A.; Kolosov, D.A.; Nagornov, I.A.; Simonenko, T.L.; Varezhnikov, A.S.; Simonenko, E.P.; Sysoev, V.V.; Kuznetsov, N.T. The Ti0.2V1.8C MXene ink-prepared chemiresistor: From theory to tests with humidity versus VOCs. Chemosensors 2023, 11, 7. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Mokrushin, A.S.; Nagornov, I.A.; Dmitrieva, S.A.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Preparation and chemosensory properties of composite material Ti2CTx–10 mol % SnO2. Russ. J. Inorg. Chem. 2024, 69, 1587–1595. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Mokrushin, A.S.; Nagornov, I.A.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Chemosensory properties of nanocomposite Ti0.2V1.8CTx–V2O5–SnO2. Russ. J. Inorg. Chem. 2024, 69, 1454–1462. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, H. Recent advances in MXene gas sensors: Synthesis, composites, and mechanisms. npj 2D Mater. Appl. 2025, 9, 66. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Mokrushin, A.S.; Nagornov, I.A.; Dmitrieva, S.A.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Preparation and chemosensory properties of nanocomposite obtained by hydrothermal modification of Ti2CTx with hierarchically organized Co(CO3)0.5(OH)⋅0.11H2O. Russ. J. Inorg. Chem. 2024, 69, 1291–1300. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Varezhnikov, A.S.; Solomatin, M.A.; Struchkov, N.S.; Stolyarova, D.Y.; Ryzhkov, S.A.; Antonov, G.A.; Gabrelian, V.S.; Cherviakova, P.D.; et al. Toward On-Chip Multisensor Arrays for Selective Methanol and Ethanol Detection at Room Temperature: Capitalizing the Graphene Carbonylation. ACS Appl. Mater. Interfaces 2023, 15, 28370–28386. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, M.; Zou, J.; Guo, R.; Zeng, X.; Ding, W. Surfactant induced formation of flower-like V2O5 microspheres as cathode materials for rechargeable magnesium batteries. Ionics 2019, 25, 5889–5897. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Zhang, H.; Cao, J.; Wang, L.; Hu, Q. Preparation and methane adsorption of two-dimensional carbide Ti2C. Adsorption 2016, 22, 915–922. [Google Scholar] [CrossRef]
- Londoño-Calderón, C.L.; Vargas-Hernández, C.; Jurado, J.F. Desorption influence of water on structural, electrical properties and molecular order of vanadium pentoxide xerogel films. Rev. Mex. De Fis. 2010, 56, 411–415. [Google Scholar]
- Korotcenkov, G.; Tolstoy, V.P. Current trends in nanomaterials for metal oxide-based conductometric gas sensors: Advantages and limitations—Part 2: Porous 2D nanomaterials. Nanomaterials 2023, 13, 237. [Google Scholar] [CrossRef]
- Pazniak, H.; Plugin, I.A.; Sheverdyaeva, P.M.; Rapenne, L.; Varezhnikov, A.S.; Agresti, A.; Pescetelli, S.; Moras, P.; Kostin, K.B.; Gorokhovsky, A.V.; et al. Alcohol vapor sensor based on quasi-2D Nb2O5 derived from oxidized Nb2CTz MXenes. Sensors 2024, 24, 38. [Google Scholar] [CrossRef]
- Uma, S.; Shobana, M.K. Band structure and mechanism of semiconductor metal oxide heterojunction gas sensor. Inorg. Chem. Commun. 2024, 160, 111941. [Google Scholar] [CrossRef]
- Qian, L.; Rahmati, F.; Li, F.; Zhang, T.; Wang, T.; Zhang, H.; Yan, S.; Zheng, Y. Recent advances in 2D MXene-based heterostructures for gas sensing: Mechanisms and applications in environmental and biomedical fields. Nanoscale 2025, 17, 8975–8998. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chang, X.; Liu, X.; Zheng, W.; Zhang, J. Atomic surface engineering of MXene for stable hydrogen sensing at low temperature. Small 2025, 21, e10304. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Han, R.; Wu, Z.; Liu, Y.; Tan, X.; Meng, S.; Wang, Y.; Peng, S. Enhanced gas sensing performance of ethanol gas by the construction of dual Co3O4/ N-MX heterojunction structures. J. Alloys Compd. 2025, 1041, 183614. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, F.; Yang, Y.; Li, X.; Wang, H.; Sang, L.; Zhao, W.; Wang, T.; Jia, F.; Zhou, T.; et al. Gas-sensing mechanism and performances of Ti3C2Tx/CuO for H2S at room temperature. Chem. Eng. J. 2025, 519, 165339. [Google Scholar] [CrossRef]
- Yao, Y.; Xin, J.; Wang, W.; Yang, Y.; Shi, Q.; Wang, X.; Xie, L.; Kurajica, S.; Zhu, Z. Construction of 0D-2D heterostructure driven by MOF-derived Co3O4 and Ti3C2Tx MXene for ultra-sensitive hexanal detection. J. Alloys Compd. 2025, 1037, 182579. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-order chemical sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef]
- Goschnick, J.; Haeringer, D.; Kiselev, I. Multicomponent quantification with a novel method applied to gradient gas sensor microarray signal patterns. Sens. Actuators B 2007, 127, 237–241. [Google Scholar] [CrossRef]

| Analyte | MXene/Metal Oxide Composite | ||
|---|---|---|---|
| Oxidized Ti0.2V1.8C | Ti0.2V1.8C/SnO2 | Ti0.2V1.8C/Co3O4 | |
| Ammonia | 0.43 ± 0.17 | 0.18 ± 0.24 | 0.81 ± 0.09 |
| Methanol | 0.07 ± 0.002 | 0.32 ± 0.08 | 0.02 ± 0.002 |
| Ethanol | 0.15 ± 0.03 | 0.58 ± 0.07 | 0.001 ± 0.005 |
| Butanol | 0.20 ± 0.02 | 0.13 ± 0.05 | 0.06 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Plugin, I.A.; Simonenko, N.P.; Simonenko, E.P.; Simonenko, T.L.; Varezhnikov, A.S.; Solomatin, M.A.; Sysoev, V.V.; Kuznetsov, N.T. Chemiresistive Effect in Ti0.2V1.8C MXene/Metal Oxide Hetero-Structured Composites. Sensors 2026, 26, 496. https://doi.org/10.3390/s26020496
Plugin IA, Simonenko NP, Simonenko EP, Simonenko TL, Varezhnikov AS, Solomatin MA, Sysoev VV, Kuznetsov NT. Chemiresistive Effect in Ti0.2V1.8C MXene/Metal Oxide Hetero-Structured Composites. Sensors. 2026; 26(2):496. https://doi.org/10.3390/s26020496
Chicago/Turabian StylePlugin, Ilia A., Nikolay P. Simonenko, Elizaveta P. Simonenko, Tatiana L. Simonenko, Alexey S. Varezhnikov, Maksim A. Solomatin, Victor V. Sysoev, and Nikolay T. Kuznetsov. 2026. "Chemiresistive Effect in Ti0.2V1.8C MXene/Metal Oxide Hetero-Structured Composites" Sensors 26, no. 2: 496. https://doi.org/10.3390/s26020496
APA StylePlugin, I. A., Simonenko, N. P., Simonenko, E. P., Simonenko, T. L., Varezhnikov, A. S., Solomatin, M. A., Sysoev, V. V., & Kuznetsov, N. T. (2026). Chemiresistive Effect in Ti0.2V1.8C MXene/Metal Oxide Hetero-Structured Composites. Sensors, 26(2), 496. https://doi.org/10.3390/s26020496









