Research on a Small Oil Detection Instrument Combining Micro-Distillation Range Analyzer and Raman Spectroscopy Technology
Abstract
1. Introduction
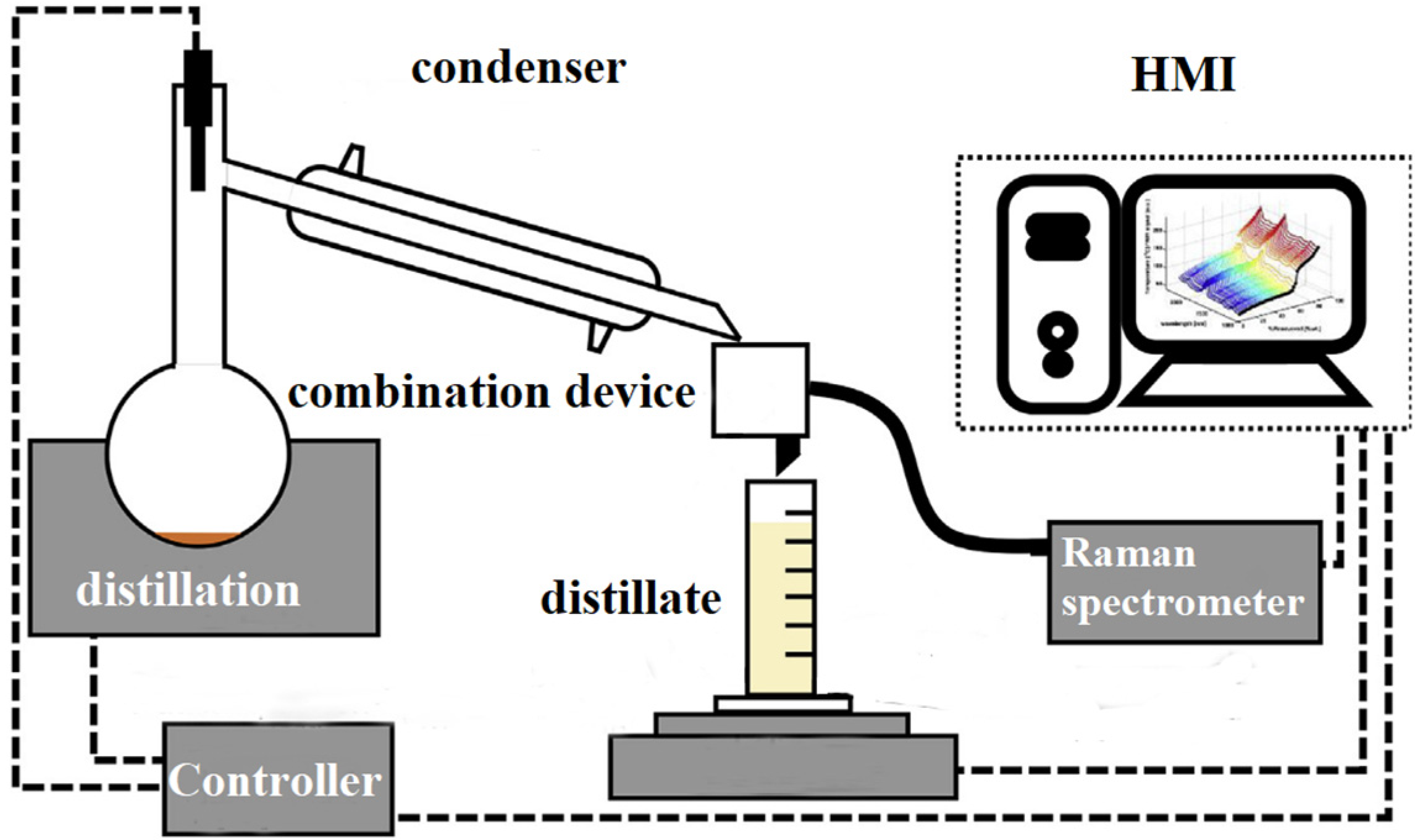
2. Design and Preparation of Instruments
2.1. Hardware Design
2.1.1. Self-Developed Micro-Distillation Instrument
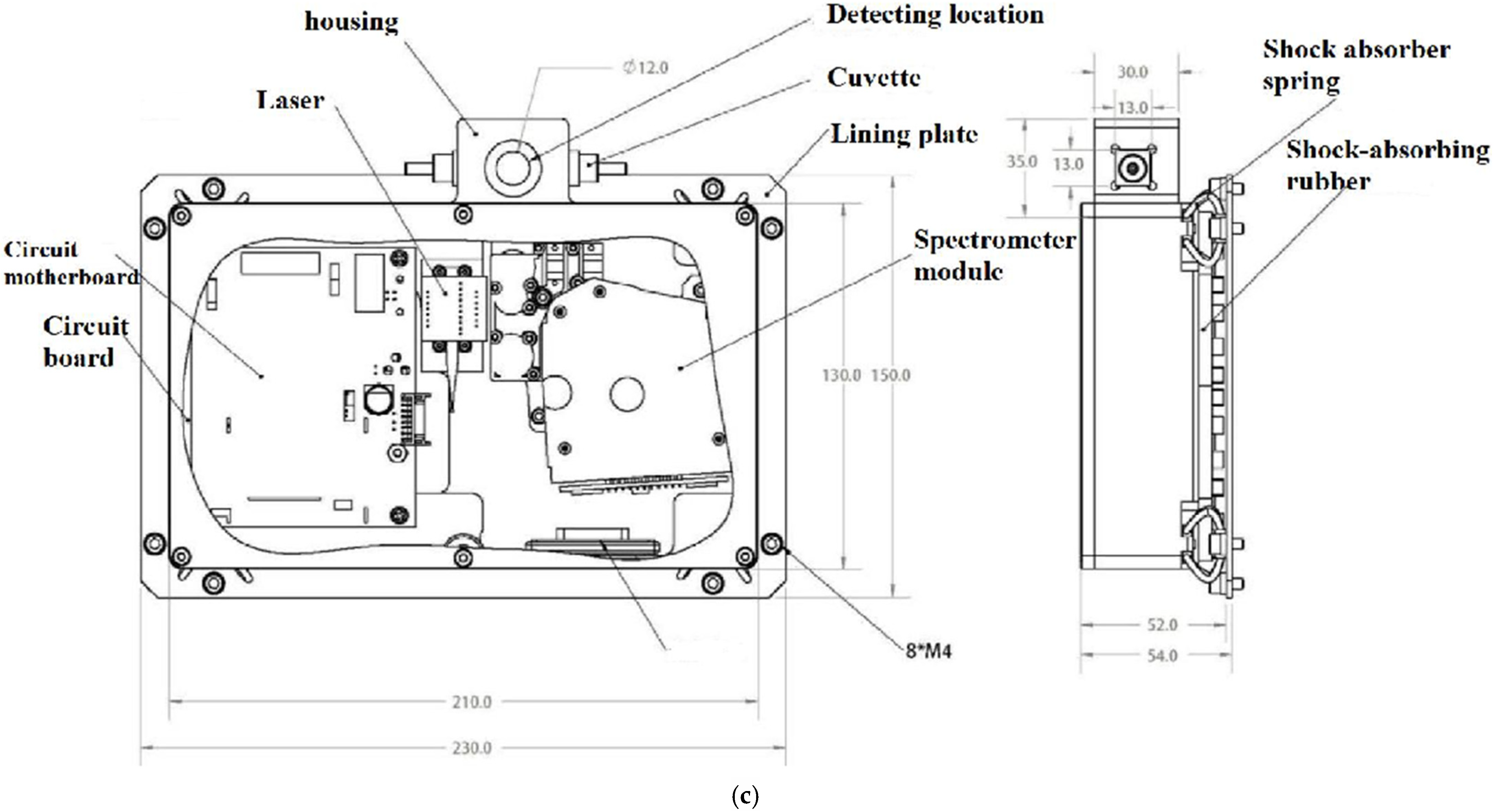
2.1.2. Self-Developed Raman Spectrometer
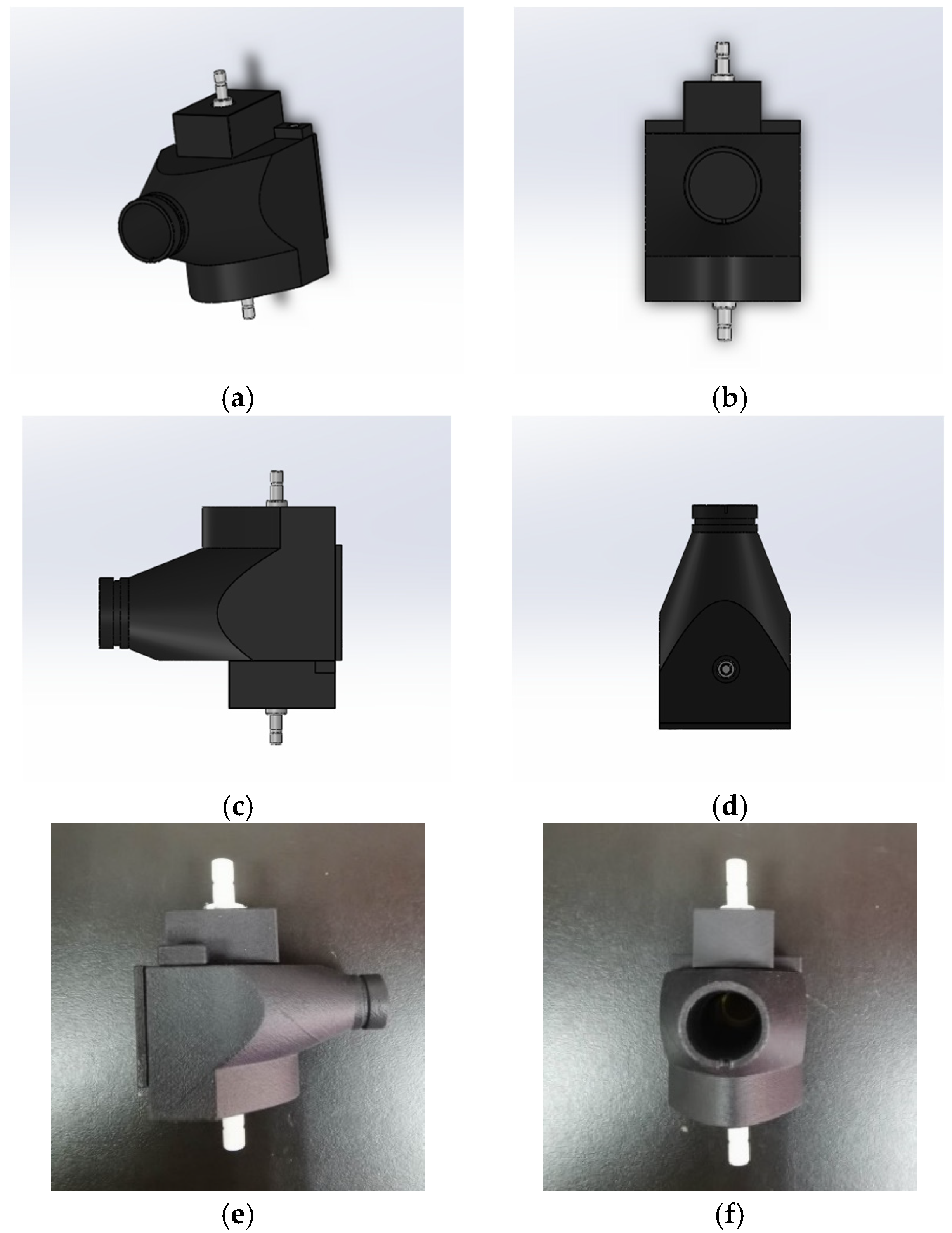
2.1.3. Combination Module Design
2.2. Software Design
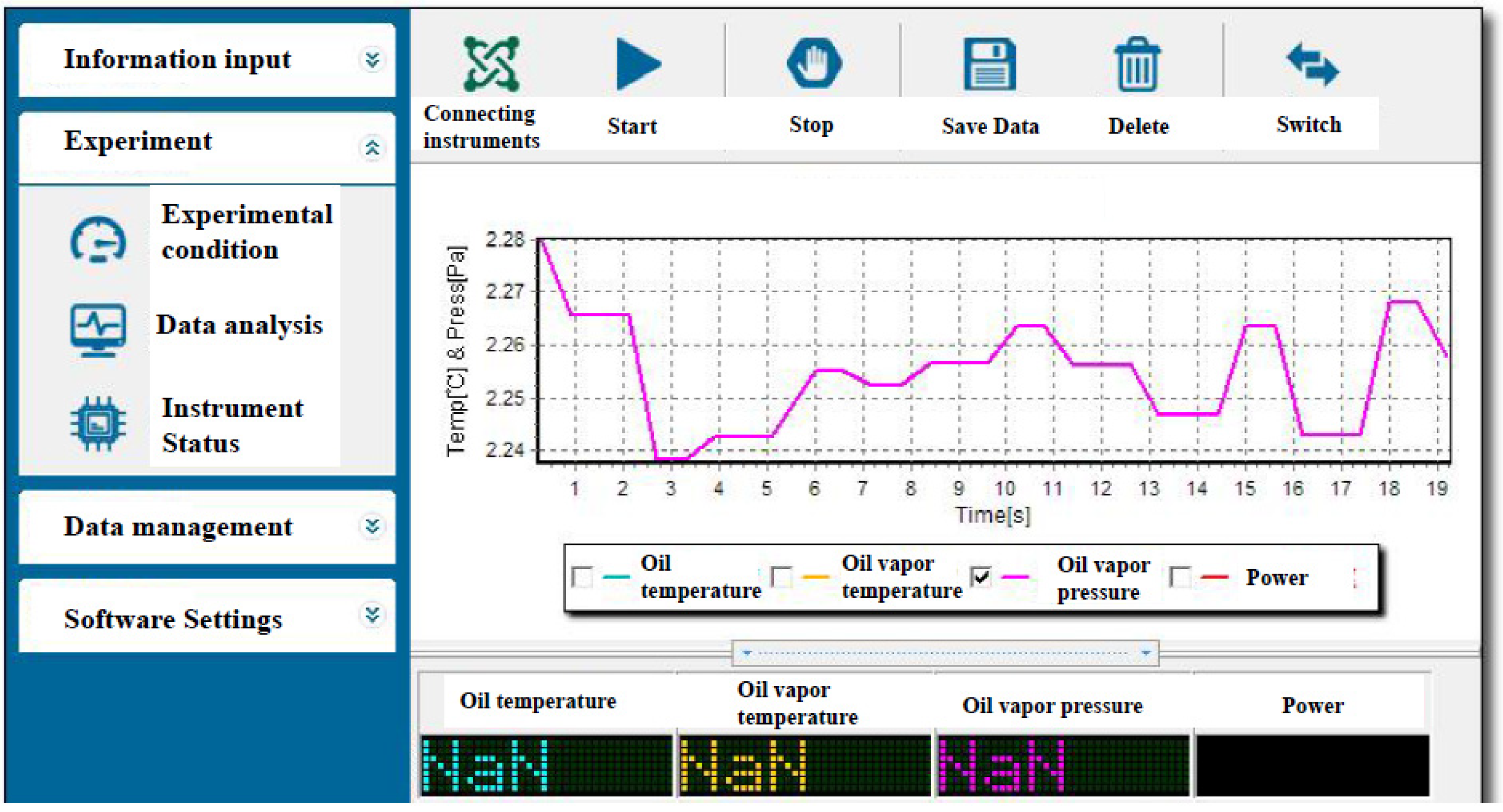
2.2.1. Micro-Distillation Instrument Software
2.2.2. Raman Spectrometer Software
3. Data Processing Methods
3.1. Savitzy–Golay Smoothing Processing
3.2. Baseline Correction
3.3. Normalization Processing
4. Experimental Details
4.1. Materials
4.2. Experimental Methods
4.3. Data Processing Experiment
4.4. Joint Testing Experiment
5. Results and Discussion
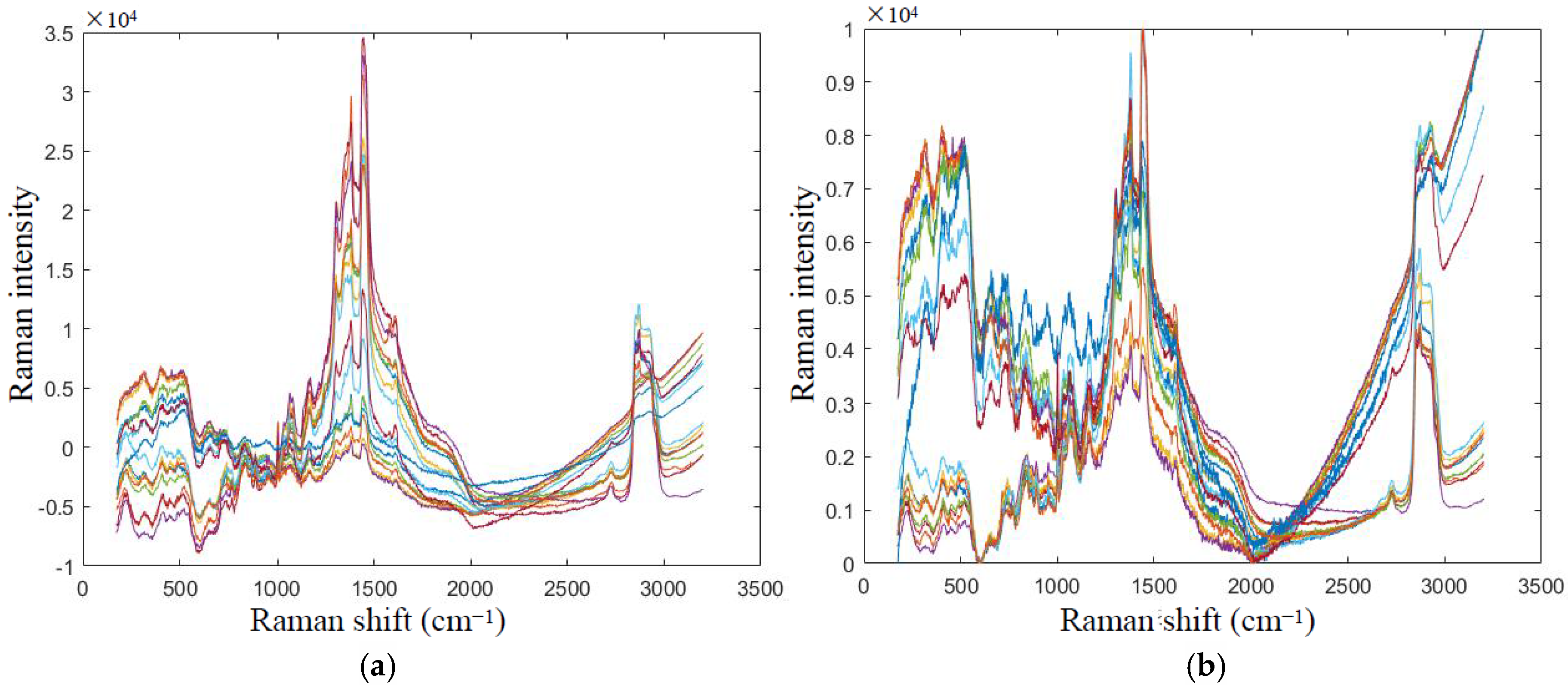
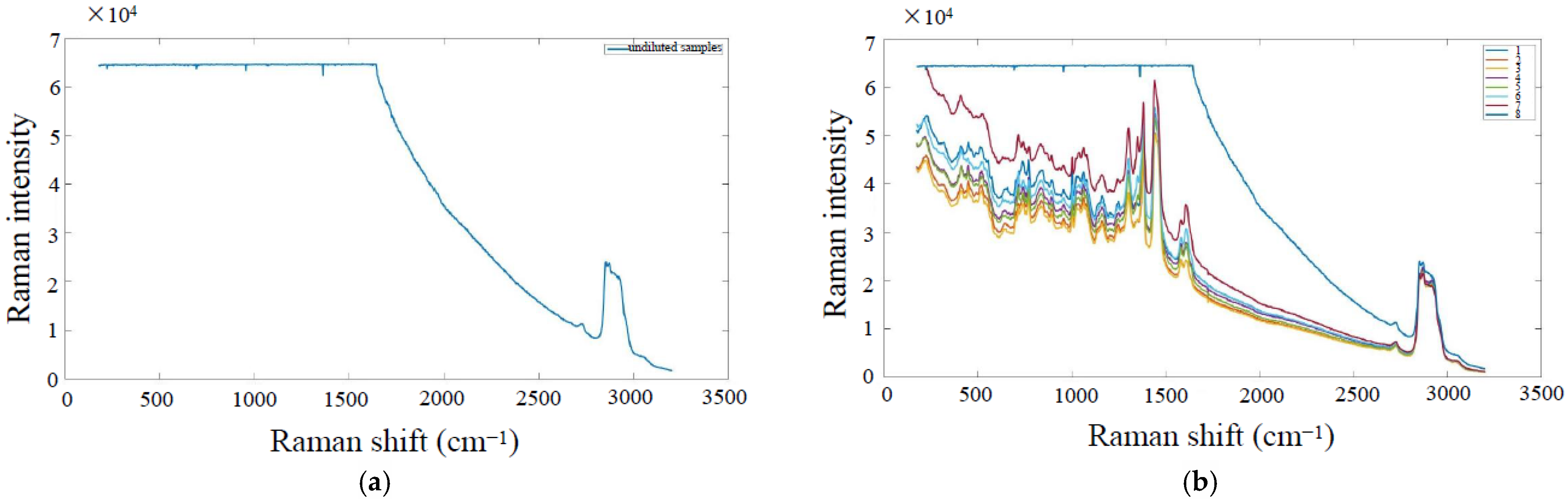
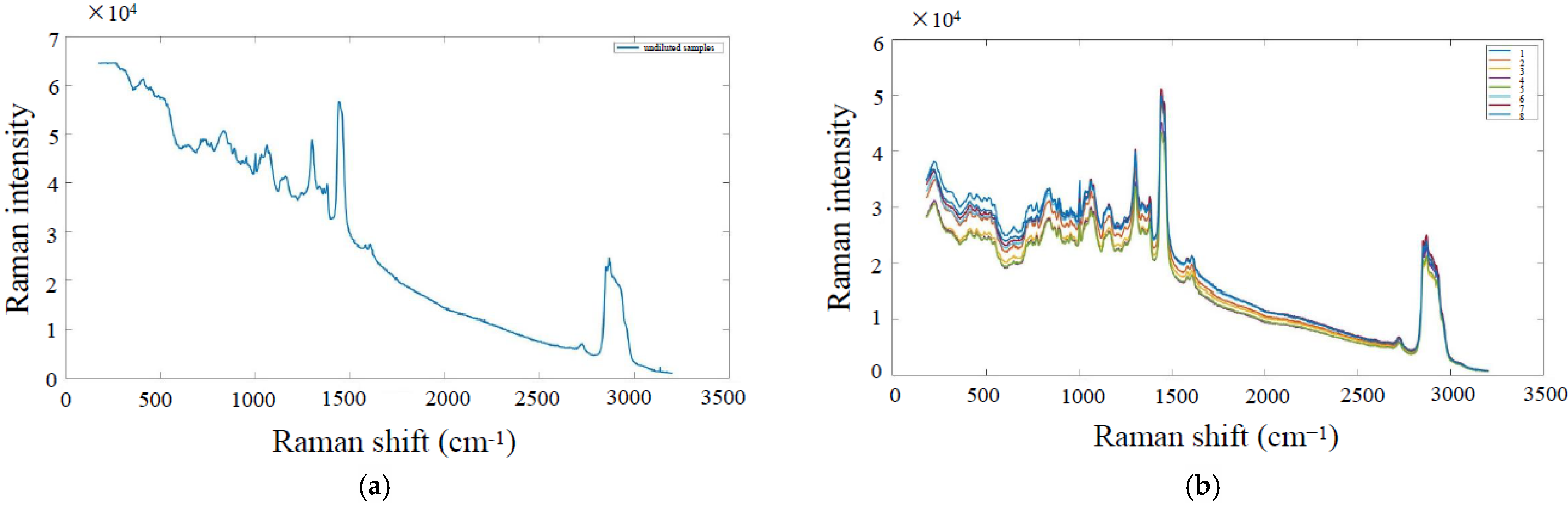
5.1. Data Smoothing
5.2. Baseline Correction and Normalization Processing
5.3. Joint Testing System Test Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Yuan, Y.; Xia, C.; Alahmadi, T.A.; Alharbi, S.A.; Sekar, M.; Pugazhendhi, A. Production of waste tyre pyrolysis oil as the replacement for fossil fuel for diesel engines with constant hydrogen injection via air intake manifold. Fuel 2024, 355, 129458. [Google Scholar] [CrossRef]
- Balamurugan, T.; Arun, A.; Sathishkumar, G.B. Biodiesel derived from corn oil–A fuel substitute for diesel. Renew. Sustain. Energy Rev. 2018, 94, 772–778. [Google Scholar] [CrossRef]
- Kumar, V.; Choudhary, A.K. Prediction of the Performance and emission characteristics of diesel engine using diphenylamine Antioxidant and ceria nanoppaper additives with biodiesel based on machine learning. Energy 2024, 301, 131746. [Google Scholar] [CrossRef]
- Nabi, M.N.; Hussam, W.K.; Rashid, A.B.; Islam, J.; Islam, S.; Afroz, H.M.M. Notable improvement of fuel properties of waste tire pyrolysis oil by blending a novel pumpkin seed oil–biodiesel. Energy Rep. 2022, 8, 112–119. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Faisal, F.; Rasul, M.G.; Schaller, D.; Khan, M.; Dexter, R. Automobile fuels (diesel and petrol) from plastic pyrolysis oil—Production and characterisation. Energy Rep. 2022, 8, 730–735. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Francisco, J.; Bautista, F.M.; Romero, A.A.; Luna, D. Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions. Energies 2024, 17, 1322. [Google Scholar] [CrossRef]
- Filgueiras, T.C.G.M.; De Sousa, E.A.; Freire, W.A.C.; Pinheiro, M.G.A.; Saavedra, R.d.S.; Lameira, P.I.D.; Rocha, H.M.Z.; Nogueira, M.F.M. Combustion analysis of ternary mixtures of diesel oil, biodiesel, and refined soybean oil: A view of cylinder pressure. Acta Scientiarum. Technol. 2024, 46, e61751. [Google Scholar] [CrossRef]
- Gayathri, K.; Anandavelu, K. A neo emission reduction approach for minimizing atmospheric air pollutants using ternary fuel with pentanol and seaweed anti- oxidant in multi-cylinder diesel engine. Glob. Nest J. 2023, 25, 16–22. [Google Scholar]
- Zhu, L.; Zhai, H.L.; Zhao, B.Q. Rapid determination of the key temperatures in diesel distillation process based on near-infrared spectroscopy. Infrared Phys. Technol. 2023, 131, 104644. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Argirov, G.; Vasilev, S.; Yordanov, D. Validation of Diesel Fraction Content in Heavy Oils Measured by High Temperature Simulated Distillation and Physical Vacuum Distillation by Performance of Commercial Distillation Test and Process Simulation. Appl. Sci. 2022, 12, 11824. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- GB/T 6536-2010; Petroleum Products—Standard Test Method for Distillation at Atmospheric Pressure. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (AQSIQ) and Standardization Administration of China (SAC): Beijing, China, 2011.
- ASTM D86-07a; Standard Test Method for Distillation of Petroleum Products at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2007.
- Zhang, M.; Guo, Y.; Hu, F.; Liu, Z.; Shi, J.; He, X. Research on marine oil spill pollution detection based on laser Raman spectroscopy. Proc. SPIE 2020. [Google Scholar] [CrossRef]
- Kostina, Y.V.; Rusakova, O.Y.; Mikhalitsyn, L.A.; Bondarenko, G.N. Use of Raman Spectroscopy in Analysis of Crude Oils, Petroleum Products, Oil-Bearing Rocks, and Petrochemical Process Catalysts (A Review). Pet. Chem. 2023, 63, 1–30. [Google Scholar] [CrossRef]
- Li, J.; Ming, T.F.; Sun, Y.L.; Tian, H.X.; Sheng, C.X. Research on Raman Spectroscopy Detection Method for Lubricating Oil Contaminated by Coolant. Spectrosc. Spectr. Anal. 2021, 41, 817–821. [Google Scholar]
- de Lima, C.J.; Silveira, L.; Rosa, V.D.S.; Lucindo, M.A.A.; Pacheco, M.T.T. Assembly and characterization of an optical fiber probe for use in a portable Raman spectrometer. Instrum. Sci. Technol. 2024, 1–18. [Google Scholar] [CrossRef]
- Gloerfelt-Tarp, F.; Mieog, J.C.; Bigland, M.; Wheeler, S.; Palmer, W.M.; Kretzschmar, T. Predicting tea tree oil distillate composition using portable spectrometric technology. J. Raman Spectrosc. 2022, 53, 771–784. [Google Scholar] [CrossRef]
- Naz, S.; Ahmed, W.; Ramadan, M.F. Raman spectroscopy characterization and hepato-protective traits of bioactive phytochemicals in Cannabis sativa essential oil fractions. Chem. Pap. 2024, 78, 2415–2430. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, H.; Ding, Q.; Che, X.; Bi, Z.; Wang, L. Research on Small-Scale Detection Instrument for Drinking Water Combined Laser Spectroscopy and Conductivity Technology. Sensors 2023, 23, 2985. [Google Scholar] [CrossRef]
- Quintanilla, M.E.P.; Abdunabiev, S.; Allegretti, M.; Merlone, A.; Musacchio, C.; Pasero, E.G.A.; Tordella, D.; Canavero, F. Innovative Mini Ultralight Radioprobes to Track Lagrangian Turbulence Fluctuations Within Warm Clouds: Electronic Design. Sensors 2021, 21, 1351. [Google Scholar] [CrossRef]
- Hradecká, I.; Velvarská, R.; Jaklová, K.D.; Vráblík, A. Rapid determination of diesel fuel properties by near-infrared spectroscopy. Infrared Phys. Technol. 2021, 119, 103933. [Google Scholar] [CrossRef]
- ASTM D7345-21; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure (Micro Method). ASTM International: West Conshohocken, PA, USA, 2021.
- Mazet, V.; Carteret, C.; Brie, D.; Idier, J.; Humbert, B. Background removal from spectra by designing and minimising a non-quadratic cost function. Chemom. Intell. Lab. Syst. 2005, 76, 121–133. [Google Scholar]
- Mazet, V.; Brie, D.; Idier, J. Baseline spectrum estimation using half-quadratic minimization. In Proceedings of the European Signal Processing Conference, Vienna, Austria, 6–10 September 2004; IEEE: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- GB 19147-2016; Automotive Diesel Fuel. Standards Press of China: Beijing, China, 2016.
- Wu, H.; Volponi, J.V.; Singh, S. Single-Cell Diesel Mining on Microalgae: Direct and Quantitative Monitoring of Microalgal Oil Production In Vivo by Raman Spectroscopy. Biophys. J. 2010, 98, 744a. [Google Scholar] [CrossRef][Green Version]
- Aiguo, O.Y.; Yu, Z.; Tianyi, T.; Yande, L. Research on Density, Viscosity, and Ethanol Content Analysis of Ethanol Diesel Fuel Based on Raman Spectroscopy. Spectrosc. Spectr. Anal. 2018, 38, 1772–1778. [Google Scholar]




| Number | Oil Type | Number | Oil Type | Number | Oil Type |
|---|---|---|---|---|---|
| No. 1 | −10# diesel oil 1 | No. 6 | 0# diesel oil 1 | No. 11 | Sri Lanka Diesel |
| No. 2 | −10# diesel oil 2 | No. 7 | 0# diesel oil 2 | No. 12 | Moroccan diesel |
| No. 3 | −10# diesel oil 3 | No. 8 | 0# diesel oil 3 | No. 13 | Anqing Petrochemical 5# Diesel |
| No. 4 | Mix diesel oil 1 | No. 9 | 0# diesel oil 4 | No. 14 | Angola Diesel |
| No. 5 | Mix diesel oil 2 | No. 10 | 0# diesel oil 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Zhao, C.; Zuo, X.; Shu, J.; Hua, W.; Guan, L.; Gu, K. Research on a Small Oil Detection Instrument Combining Micro-Distillation Range Analyzer and Raman Spectroscopy Technology. Sensors 2025, 25, 2130. https://doi.org/10.3390/s25072130
Yan H, Zhao C, Zuo X, Shu J, Hua W, Guan L, Gu K. Research on a Small Oil Detection Instrument Combining Micro-Distillation Range Analyzer and Raman Spectroscopy Technology. Sensors. 2025; 25(7):2130. https://doi.org/10.3390/s25072130
Chicago/Turabian StyleYan, Hao, Chen Zhao, Xiuli Zuo, Jianhua Shu, Weixing Hua, Liang Guan, and Kecheng Gu. 2025. "Research on a Small Oil Detection Instrument Combining Micro-Distillation Range Analyzer and Raman Spectroscopy Technology" Sensors 25, no. 7: 2130. https://doi.org/10.3390/s25072130
APA StyleYan, H., Zhao, C., Zuo, X., Shu, J., Hua, W., Guan, L., & Gu, K. (2025). Research on a Small Oil Detection Instrument Combining Micro-Distillation Range Analyzer and Raman Spectroscopy Technology. Sensors, 25(7), 2130. https://doi.org/10.3390/s25072130






