A Systematic Review of Surface Electromyography in Sarcopenia: Muscles Involved, Signal Processing Techniques, Significant Features, and Artificial Intelligence Approaches
Abstract
1. Introduction
2. Materials and Methods
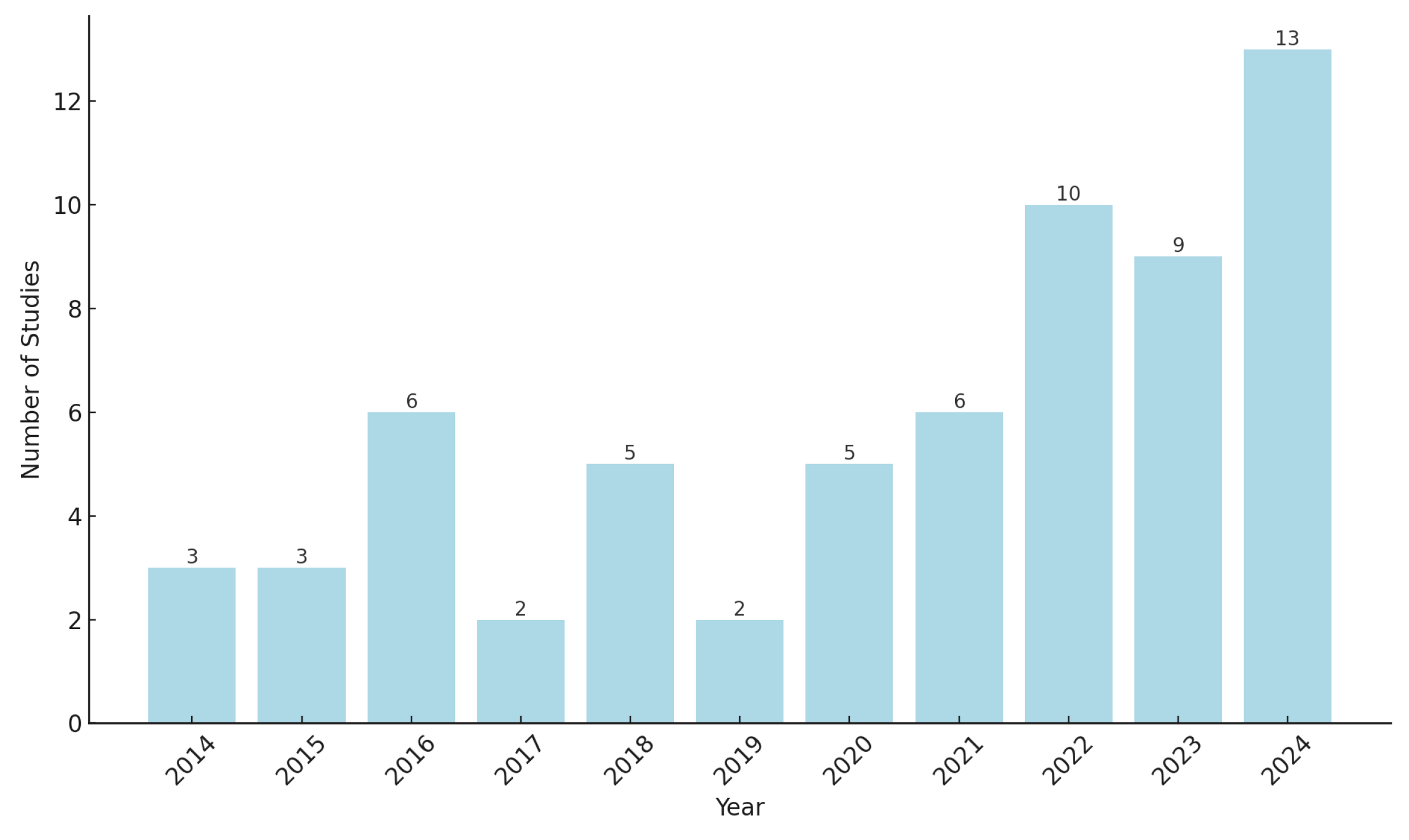
2.1. Article Selection, Inclusion and Exclusion Criteria
- -
- Peer-reviewed articles published in indexed journals.
- -
- Conference papers, included due to their contribution to emerging research in the field.
- -
- Studies using sEMG for analysis, detection or assessment of sarcopenia- related parameters.
- -
- Studies conducted on human participants.
- -
- Articles published in languages other than English.
- -
- Articles for which full text was not available.
- -
- Studies that did not mention sarcopenia, related parameters, or sEMG.
- -
- Studies primarily evaluating nutritional interventions or focusing on unrelated conditions
- -
- Studies involving non-skeletal muscles.
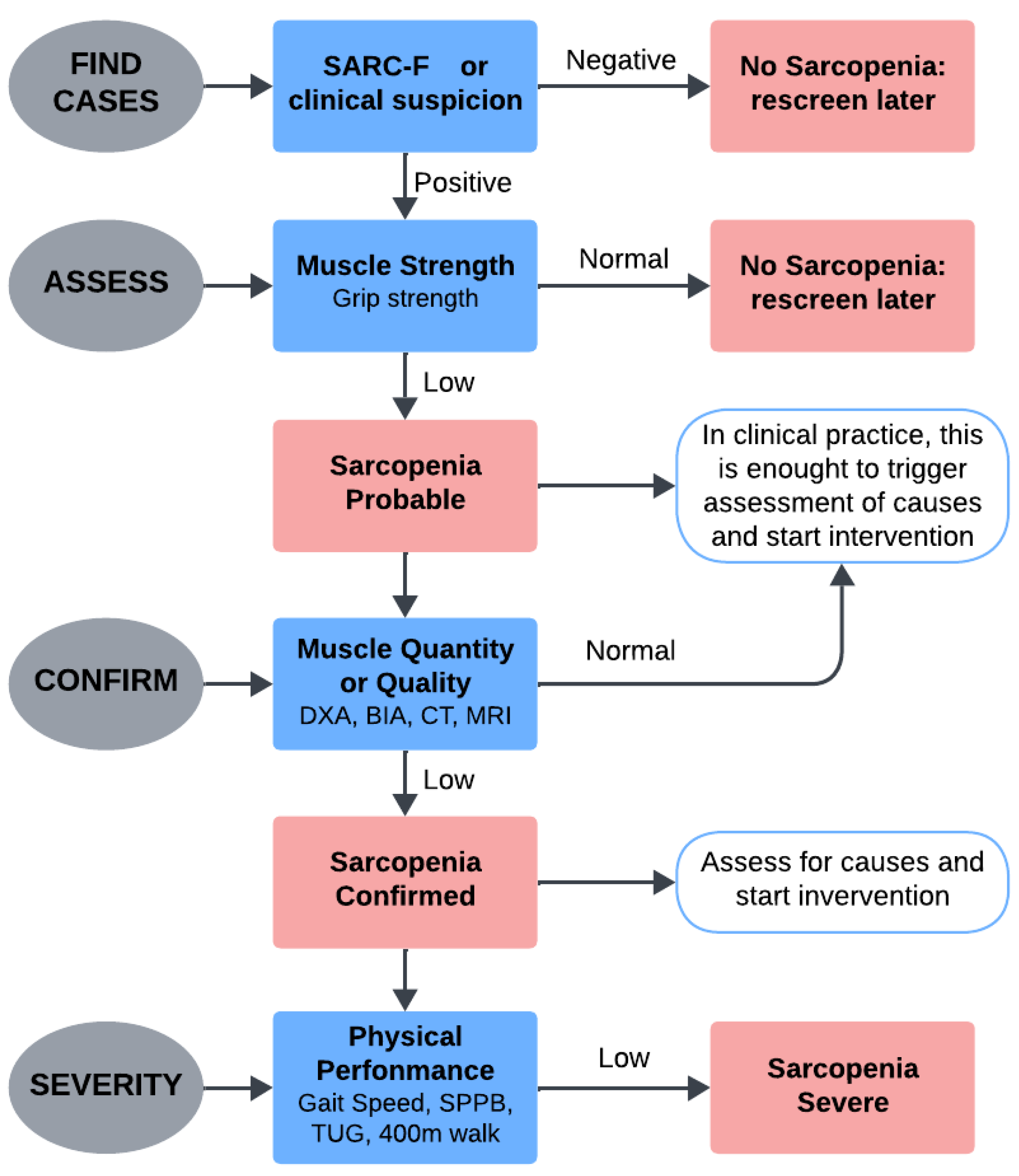
2.2. Overview on sEMG in Sarcopenia Detection
- -
- Non-invasive and Real-Time Monitoring: sEMG enables non-invasive, real-time analysis of muscle activity during both static and dynamic tasks, offering insights into natural movement conditions [21].
- -
- -
3. Muscles and Motor Tasks for sEMG Signal Acquisition
3.1. Commonly Analyzed Muscles
3.1.1. Lower Limb Muscles
3.1.2. Upper Limb Muscles
3.1.3. Core and Back Muscles
3.2. Motor Tasks
3.2.1. Isometric Exercises
3.2.2. Dynamic Exercises
3.3. Muscles and Motor Tasks Used in Other Studies
4. sEMG Recording Techniques
4.1. Standards for Electrodes Placement
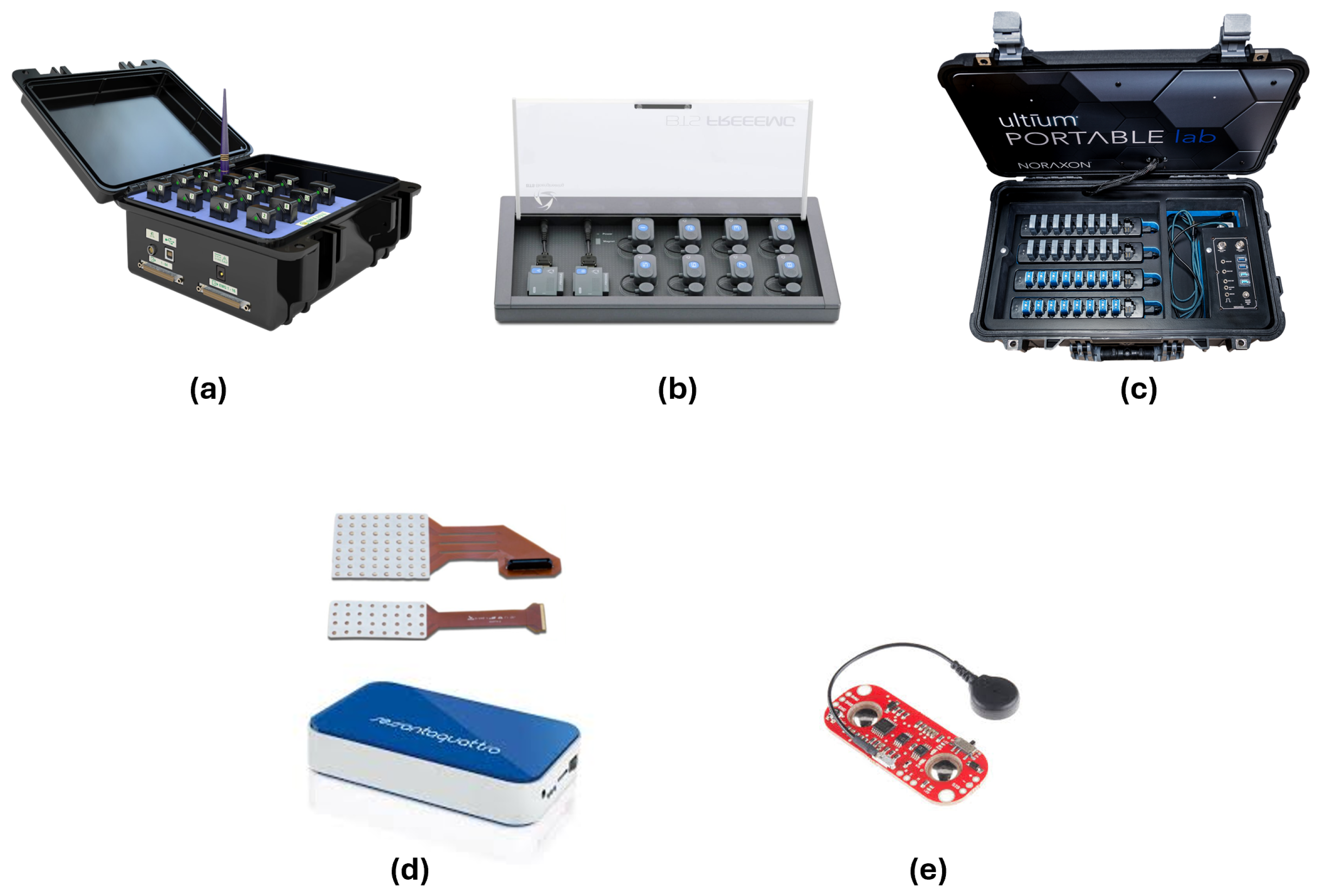
4.2. sEMG Devices Used in Sarcopenia Studies
- -
- -
- -
- -
- Custom-Made Flexible Multichannel Electrode System, (Zhejiang University, Hangzhou, China) used in [43]. This system enables the acquisition of sEMG signals during EF and extension, providing high-resolution data for ML applications.
- -
- -
- -
- -
5. Signal Processing Methods
5.1. Filtering and Preprocessing
Analysis of the Selected Studies
5.2. Feature Extraction and Selection
Analysis of the Selected Articles
6. Statistical Analysis and Artificial Intelligence Approaches for Sarcopenia
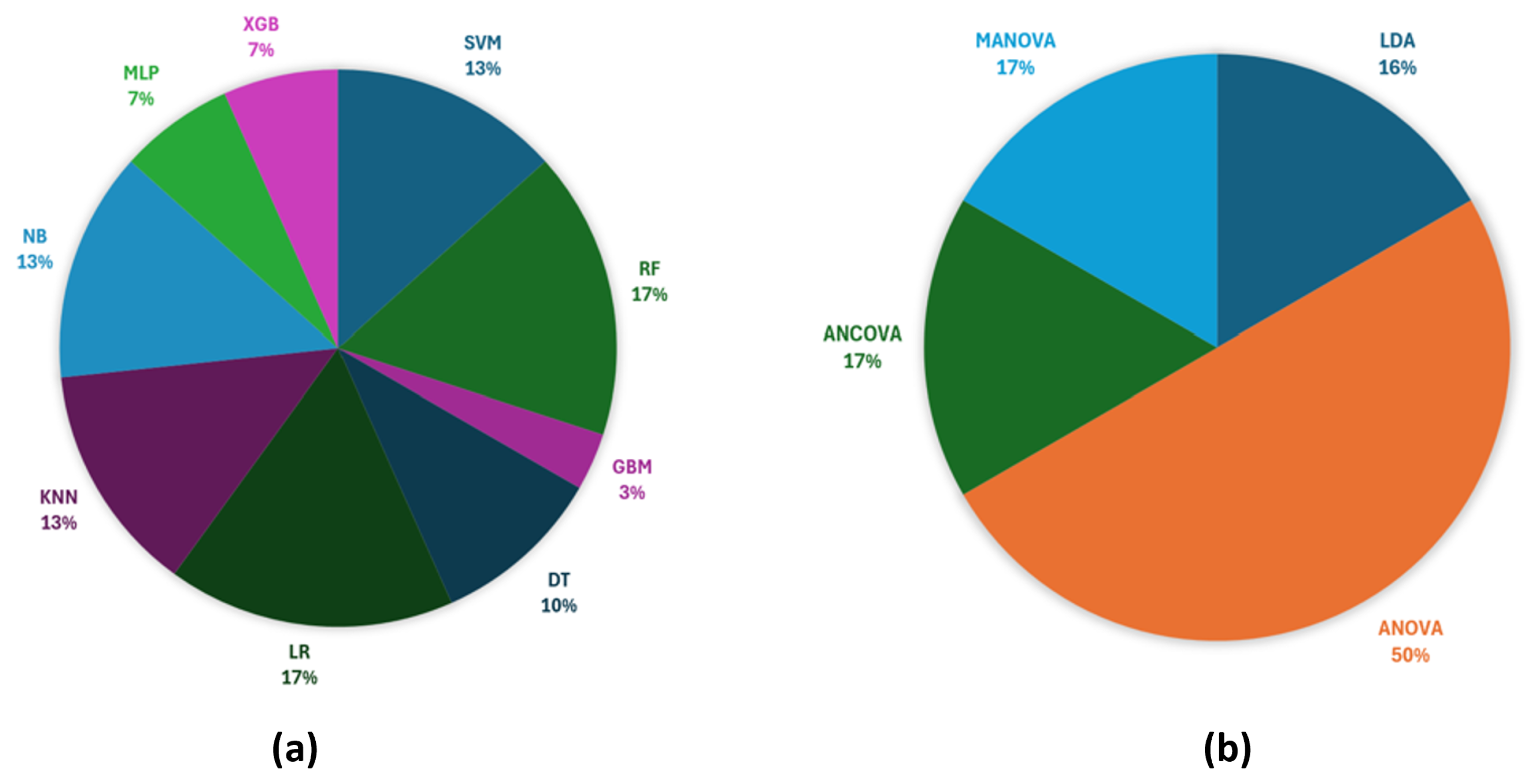
6.1. Analysis in the Selected Studies
6.2. Interpretability and Clinical Feasibility of AI Models
7. Other Studies
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EWGSOP | European Working Group on Sarcopenia in Older People |
| AWGS | Asian Working Group for Sarcopenia |
| IWGS | International Working Group on Sarcopenia |
| FNIH | Foundation of National Institutes of Health |
| DXA | Dual-energy X-ray Absorptiometry |
| BIA | Bioelectrical Impedance Analysis |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| SPPB | Short Physical Performance Battery |
| sEMG | surface Electromyography |
| AI | Artificial Intelligence |
| SA | Statistical Analysis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ML | Machine Learning |
| VL | Vastus Lateralis |
| GL | Gastrocnemius Lateralis |
| TA | Tibialis Anterior |
| RF | Rectus Femoris |
| BF | Biceps Femoris |
| GM | Gluteus Maximus |
| HD-sEMG | High-Density sEMG |
| BRA | Brachioradialis |
| FCR | Flexor Carpi Radialis |
| FDS | Flexor Digitorum Superficialis |
| FCU | Flexor Carpi Ulnaris |
| ECU | Extensor Carpi Ulnaris |
| ED | Extensor Digitorum |
| BB | Biceps Brachii |
| TB | Triceps Brachii |
| L5 | Multifidus |
| L1 | Iliocostalis Lumborum |
| MVC | Maximum Voluntary Contraction |
| SVC | Sustained Voluntary Contraction |
| EF | Elbow Flexion |
| EE | Elbow Extension |
| KF | Knee Flexion |
| KE | Knee Extension |
| IED | Inter-electrode Distance |
| IZ | Innervation Zones |
| SENIAM | Surface EMG for Non-invasive Assessment of Muscles |
| EMD | Empirical Mode Decomposition |
| ICA | Independent Component Analysis |
| RMS | Root Mean Square |
| MAV | Mean Absolute Value |
| iEMG | Integrated EMG |
| WL | Waveform Length |
| ZC | Zero Crossing |
| SSC | Slope Sign Change |
| CWT | Continuous Wavelet Transform |
| WE | Wavelet Entropy |
| IAV | Integrated Absolute Value |
| MPF | Mean Power Frequency |
| MF | Median Frequency |
| AIF | Averaged Instantaneous Frequency |
| IMFs | Intrinsic Mode Functions |
| SE | Sample Entropy |
| mRMR | Minimum Redundancy Maximum Relevance |
| RQA | Recurrence Quantification Analysis |
| BW | Bandwidth |
| SMR | Spectral Moment Ratio |
| SampEn | Sample Entropy |
| SpecEn | Spectral Entropy |
| HFD | Higuchi Fractal Dimension |
| CSD | Center Shape Distance |
| LSD | Left Shape Distance |
| RSD | Right Shape Distance |
| PCA | Principal Component Analysis |
| ACR | Amplitude Contribution Ratio |
| CCR | Co-contraction Ratio |
| MCI | Muscular Contraction Intensity |
| MCD | Muscle Contraction Dynamics |
| SA | Statistical Analysis |
| SVM | Support Vector Machine |
| RF | Random Forest |
| GBM | Gradient Boosting Machine |
| SHAP | SHapley Additive exPlanations |
| DT | Decision Tree |
| LR | Logistic Regression |
| KNN | K-Nearest Neighbors |
| NB | Naive Bayes |
| MLP | Multi-layer Perceptron |
| XGB | Extreme Gradient Boosting |
| LDA | Linear Discriminant Analysis |
| ASM | Appendicular Skeletal Muscle Mass |
| ICDMC | Incenter-Circumcenter Distance of Muscle Coordination |
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Cawthon, P.M. Sarcopenia definition: The position statements of the sarcopenia definition and outcomes consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Wang, C.; Tao, W.; Dou, Q.; Yang, Y. Sarcopenia as a predictor of hospitalization among older people: A systematic review and meta-analysis. BMC Geriatr. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Bruyère, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Zamboni, M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala Maren, S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagonosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Ackermans, L.L.; Rabou, J.; Basrai, M.; Schweinlin, A.; Bischoff, S.C.; Cussenot, O.; Blokhuis, T.J. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin. Nutr. ESPEN 2022, 48, 36–44. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS ONE 2019, 12, e0169548. [Google Scholar] [CrossRef]
- Borzuola, R.; Giombini, A.; Torre, G.; Campi, S.; Albo, E.; Bravi, M.; Macaluso, A. Central and peripheral neuromuscular adaptations to ageing. J. Clin. Med. 2020, 9, 741. [Google Scholar] [CrossRef]
- Lorusso, L.; Esposito, L.; Sancarlo, D.; D’Onofrio, G. Sarcopenia: Technological Advances in Measurement and Rehabilitation. In Frailty and Sarcopenia-Recent Evidence and New Perspectives; IntechOpen: London, UK, 2022. [Google Scholar]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering, and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar]
- Hung, Y.T.; Liu, B.; Lin, Y.C. AI Classification System on Sarcopenia for Elderly. In Proceedings of the 2023 IEEE 5th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (ECBIOS), Tainan, Taiwan, 2–4 June 2023; pp. 123–126. [Google Scholar]
- Junquera-Godoy, I.; Gomis-Tena, J.; Saiz, J.; Martinez-de-Juan, J.L.; Carot-Sierra, J.M.; Prats-Boluda, G. Evaluation of Surface Electromyography Parameters as Possible Biomarkers for the Assessment of Sarcopenia. In Proceedings of the 2023 IEEE 23rd International Conference on Bioinformatics and Bioengineering (BIBE), Virtual, 4–6 December 2023; pp. 285–291. [Google Scholar]
- Ma, Y.; Yang, B.; Zan, P.; Zhang, Y. Comparative Analysis of Classification Methods for Diagnosing Myasthenia Gravis based on Lumbar Electromyography. In Proceedings of the 2023 12th International Conference on Computing and Pattern Recognition, Qingdao, China, 27–29 October 2023; pp. 65–71. [Google Scholar]
- Adrian, E.D.; Bronk, D.W. The discharge of impulses in motor nerve fibres: Part II. The frequency of discharge in reflex and voluntary contractions. J. Physiol. 1929, 67, 119–151. [Google Scholar]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar]
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Non-Invasive Applications; John Wiley and Sons: Hoboken, NJ, USA, 2004; Volume 11. [Google Scholar]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar]
- Garcia, M.C.; Vieira, T.M.M. Surface electromyography: Why, when and how to use it. Rev. Andal. Med. Del Deporte 2011, 4, 17–28. [Google Scholar]
- Häkkinen, K.; Newton, R.U.; Gordon, S.E.; McCormick, M.; Volek, J.S.; Nindl, B.C.; Kraemer, W.J. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J. Gerontol. Ser. Biol. Sci. Med. Sci. 1998, 53, B415–B423. [Google Scholar]
- Ebenbichler, G.; Habenicht, R.; Ziegelbecker, S.; Kollmitzer, J.; Mair, P.; Kienbacher, T. Age-and sex-specific effects in paravertebral surface electromyographic back extensor muscle fatigue in chronic low back pain. GeroScience 2020, 42, 251–269. [Google Scholar]
- Re, R.; Scano, A.; Amata, O.; Spinelli, L.; Tomba, A.; Brambilla, C.; Contini, D. A personalized clinical assessment: Multi-sensor approach for understanding musculoskeletal health in the frail population. BioMedical Eng. OnLine 2024, 23, 91. [Google Scholar]
- Hirono, T.; Takeda, R.; Nishikawa, T.; Okudaira, M.; Kunugi, S.; Yoshiko, A.; Ueda, S.; Yoshimura, A.; Watanabe, K. Motor unit firing patterns in older adults with low skeletal muscle mass. Arch. Gerontol. Geriatr. 2024, 116, 105151. [Google Scholar]
- Piasecki, M.; Ireland, A.; Piasecki, J.; Stashuk, D.W.; Swiecicka, A.; Rutter, M.K.; McPhee, J.S. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J. Physiol. 2018, 596, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Rescio, G.; Manni, A.; Siciliano, P.; Caroppo, A. Comparative analysis of supervised classifiers for the evaluation of sarcopenia using a sEMG-based platform. Sensors 2022, 22, 2721. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Lee, D.; Jamsrandoj, A.; Soylu, N.N.; Jung, D.; Kim, J.; Mun, K.R. sEMG-based Sarcopenia risk classification using empirical mode decomposition and machine learning algorithms. Math. Biosci. Eng. 2024, 21, 2901–2921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huo, Y.; Yin, W.; Xiang, J. Postural balance disorders in sarcopenia based on surface electromyography. Heliyon 2024, 10, e24116. [Google Scholar] [CrossRef]
- Imrani, L.; Boudaoud, S.; Lahaye, C.; Moreau, C.; Ghezal, M.; Ben Manaa, S.; Doulazmi, M.; Laforêt, J.; Marin, F.; Kinugawa, K. High-density Surface Electromyography as Biomarker of Muscle Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78, 25–33. [Google Scholar] [CrossRef]
- Li, N.; Ou, J.; He, H.; He, J.; Zhang, L.; Peng, Z.; Jiang, N. Exploration of a machine learning approach for diagnosing sarcopenia among Chinese community-dwelling older adults using sEMG-based data. J. NeuroEng. Rehabil. 2024, 21, 69. [Google Scholar] [CrossRef]
- Jin, Z.; Jian, Y.; Qin, C.; Lu, Y.; Chen, J.; Ling, X.; Xu, K. Wearable Multichannel Electromyography Sensors for Homecare Sarcopenia Diagnosis. IEEE Sensors J. 2024, 24, 33361–33370. [Google Scholar] [CrossRef]
- He, H.; Wu, X.; Li, N.; Jiang, Y.; He, J.; Jiang, N. Multi-channel EMG manifestations of upper-extremity muscle coordination imbalance among community-dwelling sarcopenic seniors. BioMed. Eng. OnLine 2024, 23, 115. [Google Scholar] [CrossRef]
- Sung, J.H.; Baek, S.H.; Park, J.W.; Rho, J.H.; Kim, B.J. Surface Electromyography-Driven Parameters for Representing Muscle Mass and Strength. Sensors 2023, 23, 5490. [Google Scholar] [CrossRef]
- Song, K.; Shin, H.E.; Park, W.; Lee, D.; Jang, J.; Shim, G.Y.; Won, C.W. Digital Biomarker for Muscle Function Assessment Using Surface Electromyography with Electrical Stimulation and a Non-Invasive Wearable Device. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3048–3058. [Google Scholar] [CrossRef]
- Kienbacher, T.; Habenicht, R.; Starek, C.; Mair, P.; Wolf, M.; Paul, B.; Ebenbichler, G. The potential use of spectral electromyographic fatigue as a screening and outcome monitoring tool of sarcopenic back muscle alterations. J. Neuroeng. Rehabil. 2014, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ke, X.; Jiang, C.; Song, W.; Feng, J.; Zhou, H.; Lan, F. Effects of 12 weeks of Tai Chi on neuromuscular responses and postural control in elderly patients with sarcopenia: A randomized controlled trial. Front. Neurol. 2023, 14, 1167957. [Google Scholar]
- Ebenbichler, G.; Habenicht, R.; Blohm, P.; Bonato, P.; Kollmitzer, J.; Mair, P.; Kienbacher, T. The Back Muscle Surface Electromyography-Based Fatigue Index: A Digital Biomarker of Human Neuromuscular Aging? Bioengineering 2023, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Botter, A.; Barone, U. Detection and conditioning of surface EMG signals. In Surface Electromyography: Physiology, Engineering, and Applications; IEEE: Piscataway, NJ, USA, 2016; pp. 1–37. [Google Scholar]
- Mesin, L.; Merletti, R.; Rainoldi, A. Surface EMG: The issue of electrode location. J. Electromyogr. Kinesiol. 2016, 19, 719–726. [Google Scholar]
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J. Neurosci. Methods 2004, 134, 37–43. [Google Scholar] [CrossRef]
- Sacco, I.C.; Gomes, A.A.; Otuzi, M.E.; Pripas, D.; Onodera, A.N. A method for better positioning bipolar electrodes for lower limb EMG recordings during dynamic contractions. J. Neurosci. Methods 2009, 180, 133–137. [Google Scholar]
- Beretta Piccoli, M.; Rainoldi, A.; Heitz, C.; Wüthrich, M.; Boccia, G.; Tomasoni, E.; Barbero, M. Innervation zone locations in 43 superficial muscles: Toward a standardization of electrode positioning. Muscle Nerve 2014, 49, 413–421. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede Roessingh Res. Dev. 2007, 10, 8–12. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar]
- Delsys, Inc. Available online: https://delsys.com/trigno/ (accessed on 19 February 2025).
- BTS Bioengineering. Available online: https://www.btsbioengineering.com/it/products/freeemg/?gad_source=1&gclid=CjwKCAiAn9a9BhBtEiwAbKg6fngw25fIt4AD0RpiBi-OOqPTm24GL1S4cu-aoIMEKxXHIkDg-ADzmBoCkRoQAvD_BwE (accessed on 19 February 2025).
- Noraxon. Available online: https://www.noraxon.com/our-products/ultium-emg/ (accessed on 19 February 2025).
- OTBioelettronica. Available online: https://otbioelettronica.it/sessantaquattro/ (accessed on 19 February 2025).
- Myoware. Available online: https://myoware.com/products/muscle-sensor/ (accessed on 19 February 2025).
- Fialkoff, B.; Hadad, H.; Santos, D.; Simini, F.; David, M. Hand grip force estimation via EMG imaging. Biomed. Signal Process. Control 2022, 74, 103550. [Google Scholar]
- Nicolet Biomedical. Available online: http://www.nicolet-med.com/index.asp (accessed on 19 February 2025).
- TMSi. Available online: https://www.tmsi.com/products/textile-hd-emg-grids/ (accessed on 19 February 2025).
- Chowdhury, R.H.; Reaz, M.B.; Ali, M.A.B.M.; Bakar, A.A.; Chellappan, K.; Chang, T.G. Surface electromyography signal processing and classification techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chatterjee, A.; Saha, S.; Mukherjee, D.; Sen, R.; Banerjee, S.; Mandal, D. EMG Signal Acquisition and Processing for Feature Extraction And Detection of Disease. J. Eng. Technol. Manag. 2024, 19, 20. [Google Scholar]
- Merletti, R.; Conte, L.R.L. Surface EMG signal processing during isometric contractions. J. Electromyogr. Kinesiol. 1997, 7, 241–250. [Google Scholar] [PubMed]
- Gohel, V.; Mehendale, N. Review on electromyography signal acquisition and processing. Biophys. Rev. 2020, 12, 1361–1367. [Google Scholar]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar]
- Spiewak, C. A comprehensive study on EMG feature extraction and classifiers. Open Access J. Biomed. Eng. Biosci. 2018, 1, 1. [Google Scholar] [CrossRef]
- Watanabe, K.; Kouzaki, M.; Ogawa, M.; Akima, H.; Moritani, T. Relationships between muscle strength and multi-channel surface EMG parameters in eighty-eight elderly. Eur. Rev. Aging Phys. Act. 2018, 15, 3. [Google Scholar]
- Wu, R.; Delahunt, E.; Ditroilo, M.; Lowery, M.; De Vito, G. Effects of age and sex on neuromuscular-mechanical determinants of muscle strength. Age 2016, 38, 57. [Google Scholar]
- Orssatto, L.B.R.; Borg, D.N.; Blazevich, A.J.; Sakugawa, R.L.; Shield, A.J.; Trajano, G.S. Intrinsic motoneuron excitability is reduced in soleus and tibialis anterior of older adults. GeroScience 2021, 43, 2719–2735. [Google Scholar]
- Wu, R.; De Vito, G.; Lowery, M.M.; O’Callaghan, B.; Ditroilo, M. Age-related fatigability in knee extensors and knee flexors during dynamic fatiguing contractions. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2022, 62, 102626. [Google Scholar]
- Ou, J.; Li, N.; He, H.; He, J.; Zhang, L.; Jiang, N. Detecting muscle fatigue among community-dwelling senior adults with shape features of the probability density function of sEMG. J. Neuroeng. Rehabil. 2024, 21, 196. [Google Scholar]


| Database | Query |
|---|---|
 | TITLE-ABS-KEY (“surface electromyography” OR “sEMG” OR “surface EMG” OR “Superficial EMG” OR “Superficial Electromyography” OR “s-EMG” OR “Surface-based EMG”) AND TITLE-ABS-KEY (“sarcopenia” OR “presarcopenia” OR “muscle strength” OR “muscle mass” OR “muscle quality” OR “neuromuscular activation” OR “Age-related muscle loss” OR “Muscle wasting” OR “Age-associated sarcopenia” OR “Muscle atrophy” OR “muscle mass loss” OR “Muscle strength testing” OR “Handgrip dynamometry” OR “Grip strength”) AND TITLE-ABS-KEY (“elderly” OR “aging population” OR “older people” OR “older adults” OR “older individuals” OR “elderly population”) |
 | ((surface electromyography[Title/Abstract]) OR (sEMG[Title/Abstract]) OR (surface EMG[Title/Abstract]) OR (Superficial EMG[Title/Abstract]) OR (Superficial Electromyography[Title/Abstract]) OR (s-EMG[Title/Abstract]) OR (Surface-based EMG[Title/Abstract])) AND ((sarcopenia[Title/Abstract]) OR (presarcopenia[Title/Abstract]) OR (muscle strength[Title/Abstract]) OR (muscle mass[Title/Abstract]) OR (muscle quality[Title/Abstract]) OR (neuromuscular activation[Title/Abstract]) OR (Age-related muscle loss[Title/Abstract]) OR (Muscle wasting[Title/Abstract]) OR (Age-associated sarcopenia[Title/Abstract]) OR (Muscle atrophy[Title/Abstract]) OR (muscle mass loss[Title/Abstract]) OR (Muscle strength testing[Title/Abstract]) OR (Handgrip dynamometry[Title/Abstract]) OR (Grip strength[Title/Abstract])) AND ((elderly[Title/Abstract]) OR (aging population[Title/Abstract]) OR (older people[Title/Abstract]) OR (older adults[Title/Abstract]) OR (older individuals[Title/Abstract]) OR (elderly population[Title/Abstract])) |
 | (((“Document Title”:sEMG) OR (“Document Title”:“surface electromiography”) OR (“Document Title”:“surface EMG”) OR (“Document Title”:“Superficial EMG”) OR (“Document Title”:“Superficial Electromyography”) OR (“Document Title”:“Surface-based EMG”) OR (“Document Title”:“s-EMG”)) AND ((“Document Title”:“sarcopenia”) OR (“Document Title”:“presarcopenia”) OR (“Document Title”:“muscle strenght”) OR (“Document Title”:“muscle mass”) OR (“Document Title”:“muscle quality”) OR (“Document Title”:“neuromuscular activation”) OR (“Document Title”:“age-related muscle loss”) OR (“Document Title”:“Muscle wasting”) OR (“Document Title”:“Age-associated sarcopenia”) OR (“Document Title”:“Muscle atrophy”) OR (“Document Title”: “muscle mass loss”) OR (“Document Title”:“Muscle strength testing”) OR (“Document Title”:“Handgrip dynamometry”) OR (“Document Title”:“Grip strength”)) AND ((“Document Title”:“elderly”) OR (“Document Title”:“aging population”) OR (“Document Title”:“older people”) OR (“Document Title”:“older adults”) OR (“Document Title”:“older individuals”) OR (“Document Title”:“elderly population”)) AND ((“Abstract”:sEMG) OR (“Abstract”:“surface electromiography”) OR (“Abstract”:“surface EMG”) OR (“Abstract”:“Superficial EMG”)) AND ((“Abstract”:“sarcopenia”) OR (“Abstract”:“presarcopenia”) OR (“Abstract”:“muscle strenght”) OR (“Abstract”:“muscle mass”) OR (“Abstract”:“muscle quality”) OR (“Abstract”:“neuromuscular activation”) OR (“Abstract”:“age-related muscle loss”) OR (“Abstract”:“Muscle wasting”) OR (“Abstract”:“Age-associated sarcopenia”) OR (“Abstract”:“Muscle atrophy”) OR (“Abstract”: “muscle mass loss”) OR (“Abstract”:“Muscle strength testing”) OR (“Abstract”: “Handgrip dynamometry”) OR (“Abstract”:“Grip strength”)) AND ((“Abstract”:“elderly”) OR (“Abstract”:“aging population”) OR (“Abstract”:“older people”) OR (“Abstract”:“older adults”) OR (“Abstract”:“older individuals”) OR (“Abstract”:“elderly population”))) |
| Ref. | Muscles | Motor Tasks |
|---|---|---|
| Hirono et al. [36] | VL | MVC of knee extensors |
| Pasecki et al. [37] | VL, TA | MVC of knee extensors |
| Leone et al. [38] | GL, TA | Sit-to-Stand test, gait speed test (5 m) |
| Hung et al. [25] | Not explicitly specified, but focused on lower limb muscles | Rehabilitation exercises prescribed by physiotherapists for lower limbs |
| Kumar et al. [39] | RF, BF, TA, and GL | Normal walking, fast walking, standard squat, wide squat |
| Zhang et al. [40] | GM, RF, BF, TA, GL | Static standing posture |
| Imrani et al. [41] | RF | 3 times Sit-To-Stand (chair rising) |
| Godoy et al. [26] | RF, BF | Sit-To-Stand test for 30 s without using hands |
| Li et al. [42] | BRA, FCR, FDS, FCU, E CU, ED | Contractions at 20% and 50% of MVC during handgrip tasks |
| Jin et al. [43] | BB | MVC and SVC during EF, EE |
| He et al. [44] | BRA, FCR, FDS, FCU, ECU, ED | Handgrip contractions at 20% and 50% of MVC |
| Sung et al. [45] | BB, TB, RF, and BF | MVC during EF, EE, KF, KE |
| Ma et al. [27] | L5, L1 | Lumbar movements (forward bends, lateral bends), maximum handgrip test, Sit-to-Stand test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, A.; Carluccio, A.M.; Caroppo, A.; Manni, A.; Rescio, G. A Systematic Review of Surface Electromyography in Sarcopenia: Muscles Involved, Signal Processing Techniques, Significant Features, and Artificial Intelligence Approaches. Sensors 2025, 25, 2122. https://doi.org/10.3390/s25072122
Leone A, Carluccio AM, Caroppo A, Manni A, Rescio G. A Systematic Review of Surface Electromyography in Sarcopenia: Muscles Involved, Signal Processing Techniques, Significant Features, and Artificial Intelligence Approaches. Sensors. 2025; 25(7):2122. https://doi.org/10.3390/s25072122
Chicago/Turabian StyleLeone, Alessandro, Anna Maria Carluccio, Andrea Caroppo, Andrea Manni, and Gabriele Rescio. 2025. "A Systematic Review of Surface Electromyography in Sarcopenia: Muscles Involved, Signal Processing Techniques, Significant Features, and Artificial Intelligence Approaches" Sensors 25, no. 7: 2122. https://doi.org/10.3390/s25072122
APA StyleLeone, A., Carluccio, A. M., Caroppo, A., Manni, A., & Rescio, G. (2025). A Systematic Review of Surface Electromyography in Sarcopenia: Muscles Involved, Signal Processing Techniques, Significant Features, and Artificial Intelligence Approaches. Sensors, 25(7), 2122. https://doi.org/10.3390/s25072122








