Abstract
This paper presents a ZnO-Pt/Ru sensor prepared by a two-step hydrothermal method with in situ-grown ZnO nanorods and doped with Pt and Ru elements by immersion sintering. Characterization results showed that Pt and Ru were successfully modified on the surface of ZnO nanorods. ZnO-Pt/Ru achieved a response of 25–50 ppm H2S at the optimum operating temperature of 198 °C. In addition, the lower limit of H2S detection of ZnO-Pt/Ru reached 50 ppb with a response of about 10%, indicating a wide concentration detection range. Due to the good catalytic properties of Pt, the recovery characteristics of ZnO at high concentrations of H2S were significantly improved. The response time of ZnO-Pt/Ru (30 s) was also significantly shorter than pristine ZnO (56 s), with excellent selectivity. As far as the gas-sensitive enhancement mechanism is concerned, at the macroscopic level, the ZnO surface was modified by Pt and Ru, and this special structure of ZnO-Pt/Ru significantly increased the specific surface area. At the microscopic level, the PN junction formed between Pt/Ru and ZnO provided abundant holes for electron migration.
1. Introduction
Hydrogen sulfide (H2S) is a highly toxic, corrosive, and flammable gas with a rotten egg odor. It is typically derived from organic matter such as crude oil, natural gas, food, and the bacterial decomposition of human and animal waste. Exposure to high concentrations of H2S may cause physical discomfort, including a sore throat, coughing, eye irritation, etc. At concentrations as high as 1000 ppm, it can even cause immediate death [1].
Currently, the main methods for detecting H2S include chemical resistance and gas sensitivity, electrochemical detection based on redox reactions, gas chromatography, and optical methods [2]. However, most of these detection techniques typically have drawbacks such as complex structures and high costs [3]. For example, gas chromatography is unsuitable for on-site monitoring due to its complicated operation process and expensive and large equipment [4]. In recent years, gas sensors based on metal oxide semiconductors (MOs) have attracted significant attention due to their high response, low cost, and other advantages [5,6].
Typical metal oxides include CuO, WO3, ZnO, and SnO2, all widely studied for H2S gas-sensing detection. ZnO, as an N-type semiconductor, is characterized by a wide bandgap energy of approximately 3.3 eV. It also has advantages such as high electron mobility, non-toxicity, thermal stability, and chemical stability. The fabrication and testing of H2S sensors based on various ZnO nanostructures has been widely studied.
For example, Cu-doped ZnO films were deposited on glass substrates by Shewale et al. using spray pyrolysis technology, and the response to 20 ppm H2S at 260 °C reached 0.38 [7]. Xuan et al. prepared a ZnO nanoparticle sensor in situ on a gas-sensitive electrode through a one-step simple sol-gel method, which had a response of 0.75–750 ppb H2S at room temperature [8]. Niyanta et al. successfully fabricated CuO-coated ZnO nanowires on Si/SiO2 substrates via a hydrothermal method using thermal evaporation, which had a high response of 40–10 ppm H2S at 200 °C [9]. A flower-like hierarchical ZnO structure, characterized by rapid response, was synthesized by Fan et al. through a combination of electrospinning and hydrothermal methods [10]. Wu et al. prepared MOF ZIF-8/ZnO composite structures via the template method with a detection limit as low as 50 ppb, showing excellent selectivity to H2S compared to other reducing gases [11].
Although MOs have been widely applied in gas-sensing detection, their sensing performance still requires improvement. One of the ways to improve sensor performance is the modification of the MO surface using metal sensitizers such as Pt, Ru, Pd, Ag, etc. For example, Kruefu et al. studied Ru-loaded WO3 synthesized via a hydrothermal/immersion method, which exhibited excellent selectivity and response capabilities toward H2S [12]. ZnO nanorods were functionalized and modified with Pt by Yu et al. using a simple immersion-calcination method, achieving a lower detection limit [13]. Su et al. showed that Pd functionalization significantly reduced the operating temperature and increased the operating range compared to SnO2 [14].
Bimetals consist of two precious metals: Pt/Pd, Ag/Pt, and Au/Pd. Compared to single metals, bimetals typically exhibit superior gas-sensing properties due to the synergistic effects between components. For example, Pd/Pt nanoparticles were uniformly modified on networked SnO2 nanowires by Choi et al. using sequential radiative analysis, which reduced the response and recovery times of the sensor for NO2 [15]. Chen et al. used the wet impregnation method to load Pt and Ru onto the surface of WO3 nanoparticles prepared by tungstic acid pyrolysis to enhance the sensitivity of WO3 nanowire sensors to acetone [16]. Pd/Au-loaded ZnO nanowires were fabricated by Shen et al. using a simple mixed solution heating evaporation method, improving the selectivity and reproducibility for NO2 [17]. Pd/Pt bimetal-modified In2O3 hollow spheres were synthesized by Jiang et al. using solvothermal and in situ reduction methods, enabling trace H2S detection with excellent selectivity and anti-interference capabilities [18].
Platinum and ruthenium are both noble metals with excellent catalytic properties and potential synergies between them. Therefore, a bimetallic Pt and Ru-modified ZnO gas sensor for H2S detection was fabricated via a two-step hydrothermal growth method in this study. The structure and morphology of the composite material were analyzed using scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). Currently, there are few studies on Pt and Ru as co-doped materials, and the in situ hydrothermal growth method is different from traditional coatings. It also promotes the connection between doped elements and ZnO while increasing the specific surface area of ZnO nanorods. Subsequent gas-sensing tests showed that the sensor showed a high response (15 ppm/790%) at high concentrations and a very low detection limit (50 ppb), with a wide concentration detection range, and demonstrated good recovery and rapid response performance. These results were attributed to chemical sensitization and electronic sensitization mechanisms.
2. Materials and Methods
2.1. Preparation of Sensing Materials
Chemicals: Zn (CH3COO)2·2H2O (CAS number 5970-45-6, 99%), C6H12N4 (CAS number 100-97-0, 99%), Zn (NO3)2·6H2O (CAS number 10196-18-6, 99%), anhydrous ethanol (CAS number 64-17-5, >99.7%), H2PtCl6 (CAS number 18497-13-7, analytical grade, 37%), and RuCl3 (CAS number 14898-67-0, 98%).
All chemicals were purchased from Shanghai McLin Biochemical Technology Co., Ltd. (Shanghai, China) and Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
2.2. Synthesis of ZnO-Pt/Ru Nanorod
ZnO seed layer preparation: First, the alumina substrates were immersed in acetone, anhydrous ethanol, and deionized water, respectively, and ultrasonically cleaned for 10 min. The alumina substrates were immersed in a 0.01 mol/L Zn (CH3COO)2·2H2O ethanol mixed solution for 4 min, then placed in a vacuum drying oven (DZF6090, Changzhou Henglong Instrument Co., Changzhou, China) at 80 °C for 5 min of annealing, repeated four times. Finally, the alumina substrates were heated in a muffle furnace (SGM28, Luoyang Sigma High-Temperature Furnace Co., Luoyang, China) at a rate of 2 °C/min to 400 °C, where they were kept for 2 h and then cooled naturally to room temperature.
Preparation of ZnO Growth Precursor Solution: 0.2102 g of C6H12N4 and 0.4464 g of Zn (NO3)2·6H2O were dissolved in 50 mL of deionized water, and the concentration was 30 mmol/L. The pre-sintered alumina substrates and the precursor solution were placed in a Teflon-lined hydrothermal autoclave and heated at 95 °C for 3 h. After natural cooling, the substrates were removed and washed with deionized water and anhydrous ethanol three times, respectively. The cleaned alumina substrates were aged at a DC voltage of 5 V for 3 days, and then used as the original ZnO sensor.
Pt/Ru doping: An ethanol solution of 0.06 M H2PtCl6 and 0.03 M RuCl3 was prepared. The original alumina substrates with ZnO nanorods were immersed in the two solutions for 10 min, followed by calcination at 500 °C for 3 h. In this way, ZnO nanorods doped with Pt and Ru were obtained (ZnO-Ru, ZnO-Pt).
A total of 0.06 M H2PtCl6, 0.03 M RuCl3, and anhydrous ethanol were added to a beaker. The mixture was stirred on a magnetic stirrer for 10 min. The remaining preparation process was the same as described above. Thus, ZnO doped with both Pt and Ru (ZnO-Pt/Ru) was successfully prepared. The overall preparation process is shown in Figure 1. Regarding the selection of Pt and Ru doping amounts, a review of relevant literature indicated that excessive doping decreases sensing performance. After comprehensive consideration, Pt doping was set at 0.5 wt% [19,20,21] and Ru doping at 0.25 wt% [22,23,24].
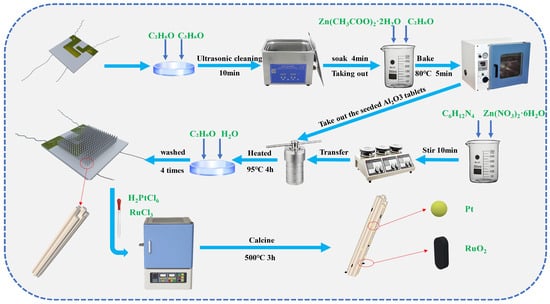
Figure 1.
Schematic of the fabrication process for ZnO-Pt/Ru and other gas sensors.
2.3. Characterization of Sensing Materials
The crystal structure of the samples was analyzed using X-ray diffraction (XRD, Rigaku Smart Lab, Rigaku Corporation, Tokyo, Japan, Cu Kα radiation, λ = 1.5406 Å) in the 2θ range of 10–80°. The surface morphology and elemental composition of the samples were observed using a field-emission scanning electron microscope (SEM, TESCAN MIRA LMS, Tescan Co., Ltd., Brno, Czech Republic; Zeiss Sigma 500, Oberkochen, Germany) with an energy-dispersive X-ray spectroscopy (EDS) detector, operating at an acceleration voltage of 15 kV and 3 kV, respectively. The valence states of each element and the chemical composition of the samples were analyzed using X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha XPS system, Thermo Fisher Scientific, Waltham, MA, USA) with Al Kα radiation. The specific surface area and pore size of the samples were determined using a porosity analyzer (Micromeritics 3Flex, Norcross, GA, USA), and the nitrogen adsorption/desorption isotherms were determined at 468 K. The microstructure and crystallinity of the samples were further investigated by transmission electron microscopy (TEM, JEM-F200, JEOL Ltd., Tokyo, Japan) and energy-dispersive spectroscopy (EDS, JEM-F200, JEOL Ltd., Tokyo, Japan).
2.4. Gas-Sensing Testing Environment
The test environment consisted of a 10 L sealed gas chamber, a data acquisition card, and a computer terminal. The data acquisition card was used to collect the resistance value of the sensor in the air and gas to be tested. The test method used static measurements, where the concentration of the target gas in the gas chamber was controlled by introducing a quantitative volume of the target gas into an air-filled 10 L gas chamber. For example, to measure a 1 ppm gas, if a 1000 ppm standard gas is used as the gas source, 10 mL of the gas source must be injected into the gas chamber. During the test, the response value was calculated according to the formula (Ra − Rg)/Rg. In gas-sensitive characterization, the response time is defined as T90: the time required for the resistance to change by 90% from the start of the response to the end of the response. The recovery time is defined as RT90: the time required for the resistance to change by 90% from the time of removal from the target gas environment.
3. Results and Discussion
3.1. Characterization of Material
Figure 2 shows the XRD spectra of the original ZnO, ZnO-Ru, ZnO-Pt, ZnO-Pt/Ru, and ZnO standard PDF card (PDF#98-000-0483). The main peaks of all four materials corresponded to the (101), (100), and (002) of ZnO. It was observed that ZnO predominantly grows along the (101) direction in all samples.
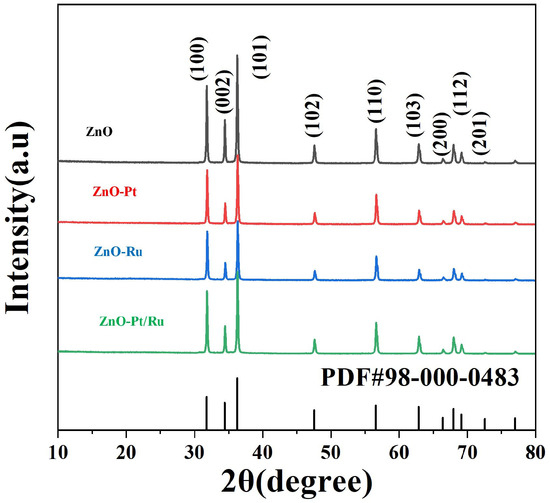
Figure 2.
X-ray diffraction patterns of pure ZnO, ZnO-Ru, ZnO-Pt, and ZnO-Pt/Ru.
XPS was used to detect the elemental composition and chemical states of the materials. As shown in the full spectrum in Figure 3a, Pt and Ru were detected in the sample. The C1s peak at 284.8 eV was used as a baseline for calibration. Figure 3b shows the main peak of C1s at 284.8 eV. After convolution fitting, three peaks were observed at 284.76 eV, 286.36 eV, and 288.7 eV, corresponding to C-C, C-O, and C=O, respectively.
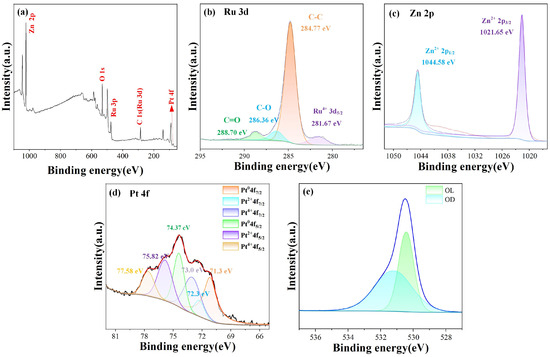
Figure 3.
XPS spectra of ZnO-Pt/Ru: (a) Survey; (b) Ru 3d; (c) Zn 2p; (d) Pt 4f; (e) O1s.
Additionally, because the 3d orbitals of Ru overlap significantly with the C1s peak, peak deconvolution was performed. The peak located at 281.67 eV corresponds to the 3d5/2 state of Ru [25]. Figure 3c shows the Zn 2p spectrum of the ZnO-Pt/Ru composites. The Zn 2p orbitals were spin-split into Zn 2p1/2 and Zn 2p3/2, with binding energies of 1021.65 eV and 1044.58 eV. The double peak distance was 22.93 eV, indicating the presence of Zn2+ and confirming the formation of ZnO [26]. Figure 3d shows the Pt 4f spectrum, which can be deconvoluted into three pairs of peaks [27,28]. The Pt 4f 7/2 and 4f 5/2 peaks at 71.1 and 74.4 eV correspond to Pt0+, while the peaks at 72.2 eV and 75.8 eV indicate the formation of Pt2+. The fitted peaks at 73.0 and 77.6 eV correspond to Pt4+, indicating the presence of Pt and its oxide, PtOx. The O1s spectrum shown in Figure 3e is deconvoluted into two peaks at 530.4 eV and 531.2 eV, which was attributed to lattice oxygen and adsorbed oxygen, respectively [29].
Figure 4a shows the SEM image of ZnO where the ZnO nanorods of ZnO nanorods are mainly concentrated at 747 ± 356 nm in diameter and 5.849 ± 2.713 um in length. The cross-section exhibits a unique hexagonal structure. The cross-stacked nanorods increase the contact area with the target gas and enhance the adsorption and desorption of gases. Figure 4b is a partially enlarged view of a single rod. It can be observed that the structure inside a single nanorod consists of multiple smaller nanorods. Figure 4c shows that the nanorods with a length of 4–6 µm are cross-arranged and form a relatively regular ZnO film on the substrate. Compared to traditional coating methods, this approach increases the voids, allowing more space for gas interaction with the material. Figure 4e–g show the EDS spectra of ZnO. It can be observed that the distribution of Zn and O is roughly consistent with the distribution of ZnO nanorods in the images and there are no impurity elements.
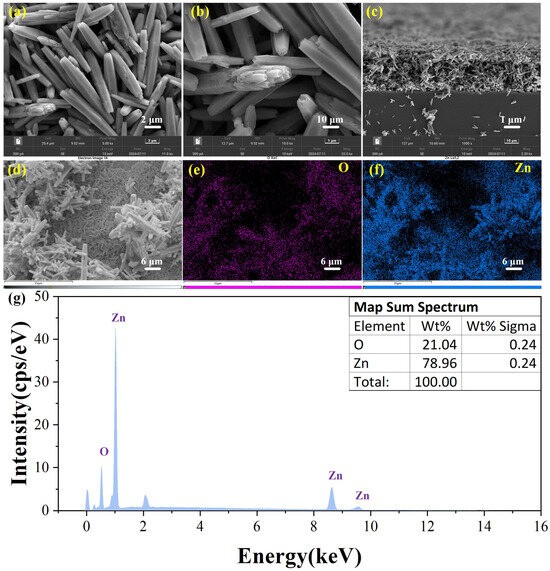
Figure 4.
SEM images of (a,b) ZnO with different magnifications; cross-sectional side view under the silicon substrate of (c) pure ZnO; EDS elemental mapping profiles of (e) O and (f) Zn in pure ZnO from (d); (g) EDS spectrum of ZnO.
Figure 5a–c shows SEM images of ZnO-Pt, ZnO-Ru, and ZnO-Pt/Ru respectively. Figure 5d shows the EDS pattern of ZnO-Pt/Ru. As shown in Figure 5d, Pt and Ru in ZnO-Pt/Ru are successfully doped with an expected doping ratio of 0.52 wt% and 0.23 wt%, respectively. Although Pt and Ru are successfully doped, the material structure that distinguishes ZnO nanorods is not clearly observed in Figure 5a–c. To investigate further, we used TEM to characterize ZnO and ZnO-Pt/Ru.
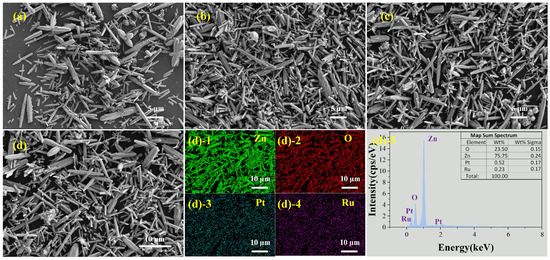
Figure 5.
SEM images of (a) ZnO-Pt, (b) ZnO-Ru and (c) ZnO-Pt/Ru; EDS analysis of ((d)-1–(d)-5) from (d).
The adsorption–desorption isotherms of ZnO and ZnO-Pt/Ru in an N2 atmosphere were measured using a BET analyzer. Their specific surface areas and pore size distributions were analyzed. As shown in Figure 6a,b, the isotherms of both materials exhibit similar curve shapes, classified as type IV isotherms according to the IUPAC classification. These isotherms exhibit the following characteristics: in the low relative pressure region (p/po < 0.3), the adsorption quantity remains low and the curve is relatively flat, indicating the predominance of monolayer adsorption. In the medium relative pressure range (p/po ≈ 0.3–0.8), the adsorption amount gradually increases, suggesting the occurrence of multi-layer adsorption. At high relative pressures (p/po > 0.8), the adsorption quantity rises sharply, and a distinct hysteresis loop appears near p/po ≈ 1.0, signifying capillary condensation. The presence of a noticeable hysteresis loop between the adsorption and desorption curves further confirms the existence of a well-defined porous structure in the materials.
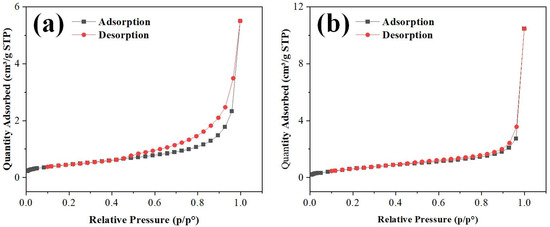
Figure 6.
N2 adsorption and desorption isotherms of (a) ZnO and (b) ZnO-Pt/Ru composites.
By comparing Figure 6a,b, it can be observed that ZnO-Pt/Ru has a higher adsorption capacity than ZnO, suggesting that ZnO-Pt/Ru likely possesses a larger specific surface area and a wider pore size distribution. To further verify this hypothesis, the specific surface areas and pore sizes of both materials were analyzed, yielding values of 1.6777 m2/g and 19.782 nm for ZnO, and 2.6451 m2/g and 22.584 nm for ZnO-Pt/Ru, respectively. Compared to pure ZnO, ZnO-Pt/Ru demonstrates significant enhancements in both specific surface area and average pore diameter. Therefore, the incorporation of Pt and Ru effectively increases the specific surface area of ZnO, thereby enhancing the interaction between the gas-sensitive material and target gas molecules and improving its adsorption and desorption capabilities.
Figure 7 shows TEM images of ZnO nanorods and ZnO-Pt/Ru. From Figure 7a–c, it can be seen that the diameter of the original ZnO nanorods ranges from 400 nm to 600 nm. After magnifying the surface of ZnO-Pt/Ru, some particles can be observed randomly distributed on the surface of the ZnO nanorods, indicating that Pt and Ru have been successfully doped on the ZnO nanorod surface. In Figure 7d, the original ZnO nanorods exhibit lattice spacings of 0.247 nm and 0.2605 nm, which belong to the (101) and (002) planes of ZnO, respectively. The lattice spacings measured in Figure 7e were 0.236 nm and 0.227 nm, which belong to the (110) plane and (111) plane of Pt, respectively. Similarly, in Figure 7f, the measured lattice spacing of 0.224 nm was attributed to the (200) plane of RuO2.
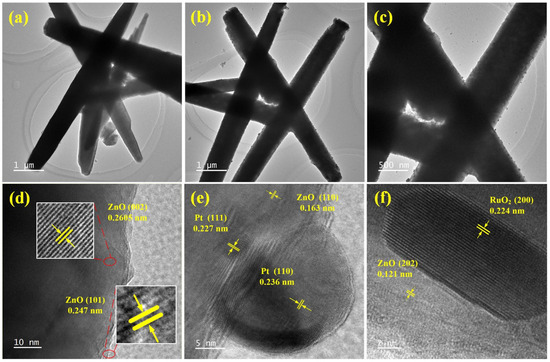
Figure 7.
TEM images of (a) ZnO, (b,c) ZnO-Pt/Ru at different magnifications; HRTEM images of (d) ZnO, (e,f) ZnO-Pt/Ru.
In the HADDF image (Figure 8a), the surface particles can be clearly observed. The EDS spectrum obtained from the HADDF image element mapping is shown in Figure 8b–e, indicating the existence of both Pt and Ru. The elemental composition is approximately Pt 0.36%, Ru 0.1%, Zn 55.05%, and O 44.58%.
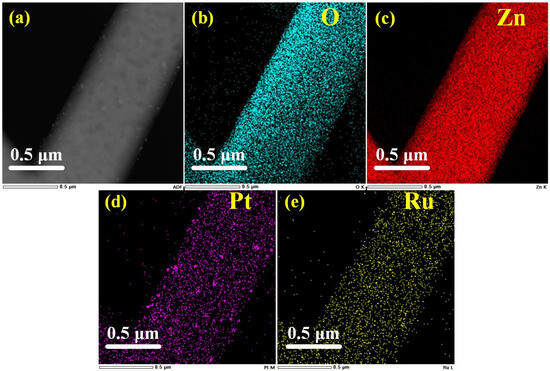
Figure 8.
HADDF image of (a) ZnO-Pt/Ru and EDS elemental mapping profiles of (b) O, (c) Zn, (d) Pt and (e) Ru.
3.2. Gas-Sensing Performance
The operating temperature plays a crucial role in the redox reactions involved in the gas adsorption process. It significantly impacts the practical application of sensors. To determine the optimal operating temperature, different voltages were applied to the heating electrodes of the alumina-based sensors, adjusting the corresponding temperatures to 130 °C, 165 °C, 198 °C, and 249 °C. The response of four sensors to 10 ppm H2S was measured at these four temperatures (Figure 9a–d). Among all the sensors, the ZnO-Pt/Ru sensor exhibited superior sensitivity across different operating temperatures. Therefore, the optimal operating temperature of ZnO-Pt/Ru was selected as the overall operating temperature. Notably, the ZnO-Pt/Ru sensor demonstrated the highest gas response at 198 °C. Although all four sensors exhibited shorter recovery times at 249 °C, their responses were significantly lower than those at other temperatures. Excessively high operating temperatures may also shorten the sensor’s lifespan. Therefore, 198 °C was selected as the optimum operating temperature.
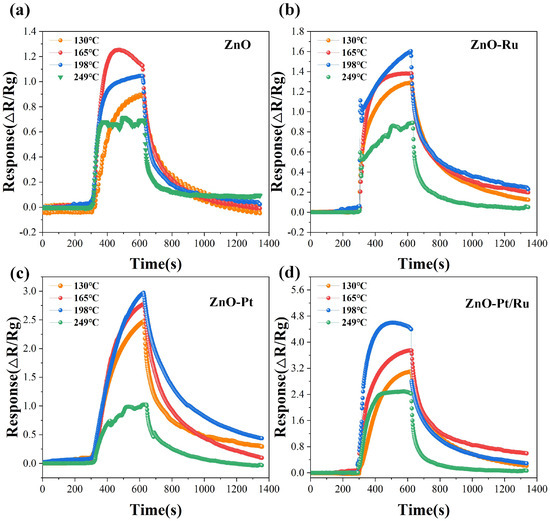
Figure 9.
Gas responses of (a) ZnO, (b) ZnO-Ru, (c) ZnO-Pt, and (d) ZnO-Pt/Ru sensors to 10 ppm H2S at various operating temperatures.
Figure 10a shows the responses of the four sensors at optimal operating temperatures. At low concentrations, the ZnO-Pt/Ru response increases steadily in proportion to the concentration gradient, while the responses of the other three sensors do not increase significantly. ZnO-Pt/Ru exhibits a 10% response at 50 ppb H2S, indicating that ZnO-Pt/Ru has good H2S low concentration detection capabilities.
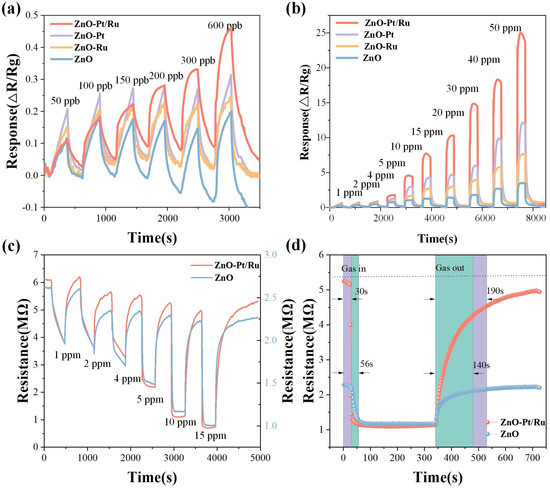
Figure 10.
Gas responses of ZnO, ZnO-Ru, ZnO-Pt, and ZnO-Pt/Ru sensors to (a) 50–600 ppb H2S, (b) 1–50 ppm H2S at the temperatures of 198 °C, (c) dynamic resistance curves of ZnO-Pt/Ru and ZnO sensors with increasing H2S concentration at 198 °C, (d) response and recovery of ZnO sensors and ZnO-Pt/Ru sensors toward 15 ppm H2S.
Figure 10b shows the dynamic response of the four sensors at 198 °C under H2S concentrations ranging from 1 to 50 ppm. It can be observed that at higher concentrations, the ZnO-Ru sensor exhibited approximately double the response performance than the original ZnO. However, with the increase in test duration and H2S gas concentration, the ZnO-Ru response cannot be fully recovered. Additionally, it can be clearly observed that ZnO-Pt/Ru shows a significantly higher response to H2S gas than the original ZnO, especially at high concentrations. Under a 50 ppm H2S atmosphere, the ZnO-Pt/Ru response was nearly eight times that of the original ZnO (3.5), and the H2S response was also better than that of ZnO-Ru and ZnO-Pt. Figure 10c shows the dynamic resistance changes of ZnO-Pt/Ru and original ZnO under exposure to 1, 2, 4, 5, 10, and 15 ppm H2S. It can be observed that after exposure to H2S gas, the resistance of the gas-sensitive material suddenly increases, reflecting the N-type semiconductor characteristics of ZnO. Moreover, the resistance of ZnO-Pt/Ru increased significantly due to the trace doping of Pt and Ru. In addition, during the desorption of H2S, all gas-sensitive materials exhibited varying degrees of the drift phenomenon. The baseline resistance of the original ZnO showed a downward drift, indicating the phenomenon of incomplete recovery. However, although the drift of ZnO-Pt/Ru was also observed, the extent of the drift was significantly reduced compared to the original ZnO.
Figure 10d shows the resistance variation curves of ZnO-Pt/Ru and the original ZnO at a concentration of 15 ppm. The figure demonstrates that while the response intensity of ZnO-Pt/Ru to 15 ppm H2S was close to 8, the response speed was still faster than the corresponding original ZnO. In addition, due to its high response intensity, the recovery time of ZnO-Pt/Ru was longer than that of the original ZnO.
Figure 11a shows the relationship between the responses of the four sensors and H2S gas concentration. With the increase in H2S concentration, the ZnO-Pt/Ru sensor’s response significantly increased, while the responses of the other sensors increased slowly. Compared to ZnO (R2 = 0.9801), ZnO-Ru (R2 = 0.9901), and ZnO-Pt (R2 = 0.9839), the ZnO-Pt/Ru sensor exhibited a better linear relationship (R2 = 0.9944). Its response follows the equation y = −0.313 + 0.496x. Figure 11b shows the dynamic response curves of the four materials to 10 ppm H2S at 198 °C. Over six consecutive measurements, all of the sensors exhibited good repeatable dynamic recovery characteristics, indicating that all four materials had excellent repeatability.
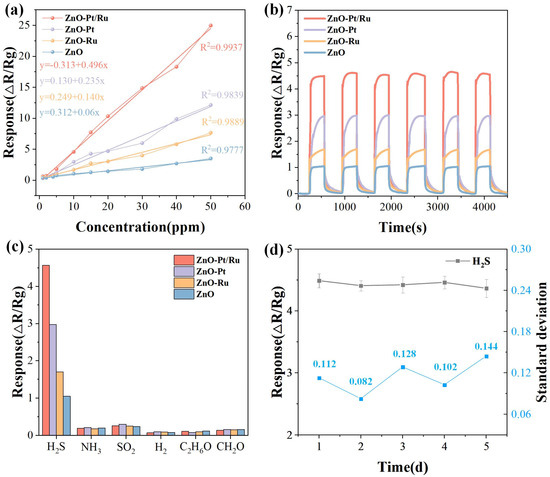
Figure 11.
(a) Fitting curves as a function of H2S concentration in logarithmic coordinates; (b) repeatability of the ZnO, ZnO-Ru, ZnO-Pt, and ZnO-Pt/Ru sensors toward 10 ppm H2S at 198 °C and (c) selectivity of ZnO, ZnO-Ru, ZnO-Pt, and ZnO-Pt/Ru sensors to various target gases; (d) response of the ZnO-Pt/Ru sensors to 10 ppm H2S over 5 days.
The response of the four sensors to 10 ppm of H2S, NH3, SO2, H2, C2H6O, and CH2O was tested (Figure 11c). The ZnO-Pt/Ru sensor had the largest response to H2S and a very low response to other gases, showing good selectivity.
In order to characterize the stability of the sensor in the experiment, we tested five sets of H2S gas response data each day for a period of five days. As shown in Figure 11d, the standard deviation values fluctuated slightly between 0.082 and 0.144. These small variations indicate that the ZnO-Pt/Ru sensor’s response to 10 ppm H2S remained basically smooth and did not show significant fluctuations, indicating excellent stability.
The above results indicate that the ZnO-Pt/Ru sensor developed in this study exhibits superior gas-sensing properties to most ZnO-based sensors (Table 1).

Table 1.
Performance comparison of ZnO-Pt/Ru Gas Sensor with Other H2S Gas Sensors.
3.3. Gas-Sensing Mechanism
For N-type MO gas sensors, such as ZnO, the charge carriers were free electrons. Oxygen in the air is adsorbed to the surface of ZnO. Simultaneously, the free electrons in the conduction band of ZnO flow toward the O2 molecules adsorbed on the ZnO surface. The O2 on the ZnO surface gradually transforms into various chemically adsorbed oxygen species, such as O2− and O22− [19,31]. This process can be explained by Equations (1)–(3). At this time, the concentration of charge carriers in the conduction band of ZnO decreases, forming an electron depletion layer, which leads to an increase in the resistance of ZnO. When ZnO is transferred from the air and atmosphere to H2S, the adsorbed oxygen on the ZnO surface undergoes a redox reaction with H2S, releasing free electrons into the conduction band of ZnO. As a result, the carrier concentration increases, the depletion layer narrows, and the resistance decreases, which was consistent with the results in Figure 10c,d. This process is expressed in Equations (4) and (5):
In addition, another mechanism, known as sulfidation and desulfurization, occurs during the adsorption of H2S by ZnO [35,36]. Studies have shown that ZnO undergoes a sulfidation process at temperatures above 150 °C in the presence of H2S, as shown in Equations (6)–(8). The resulting ZnS further contributes to a decrease in the resistance of ZnO.
Since the desulfurization reaction typically requires temperatures above 300 °C, but the working temperature of the sensors in this study was below 300 °C, the desulfurization process was unlikely to occur under these conditions. This conclusion also explains the phenomenon of resistance drift of ZnO-Pt/Ru from H2S to air in Figure 10c.
In this study, the gas-sensing performance of ZnO-Pt/Ru was significantly superior to that of the original ZnO primarily due to the presence of Pt and Ru nanoparticles and their respective sensitization effects. The analysis of XPS peak fitting and TEM crystal plane spacing revealed that the form of Pt in the composite was both elemental Pt and PtOx. The work function of Pt (5.65 eV) is greater than that of ZnO (3.30 eV), resulting in the formation of a Schottky junction between Pt and ZnO. A potential barrier exists at the interface between Pt and ZnO, causing free electrons in the conduction band of ZnO to transfer to Pt nanoparticles.
As shown in Figure 12, this process increases the space charge area, which effectively thickens the electron depletion layer in ZnO. As a result, the resistance of ZnO will increase further, creating a more obvious resistance change. This principle is also consistent with the observation in Figure 10d, where the resistance of ZnO-Pt/Ru is higher than that of the original ZnO.
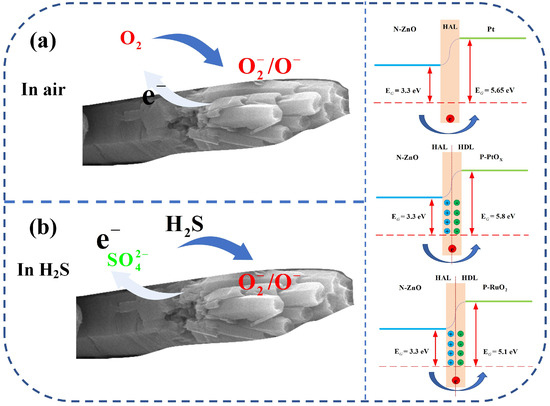
Figure 12.
Schematic diagrams of the gas-sensing mechanism for ZnO-Pt/Ru under different gas atmospheres (a) O2 and (b) H2S.
RuO2 and PtOx were formed during calcination in a muffle furnace. Both are P-type semiconductors. A PN junction is formed between ZnO and RuO2 (PtOx) due to differences in their work functions [37]. Electrons transfer from ZnO to RuO2 (PtOx) until their Fermi levels are equal. This process is accompanied by the bending of the energy band at the interface, forming an electron depletion layer at the interface junction and ultimately promoting the thickening of the electron depletion layer [38,39] and enhances gas-sensing performance by promoting the adsorption of chemically adsorbed oxygen. The improved performance of ZnO-Pt/Ru is attributed to the functionalization of Pt and the electronic sensitization mechanism at the PN junction of PtOx and RuO2. This mechanism accelerates the movement of charge carriers during the reaction between the target gas and the sensor, significantly enhancing H2S sensing performance.
4. Conclusions
ZnO nanorods were synthesized on alumina ceramic substrates via a hydrothermal growth method. Subsequently, a certain amount of Pt and Ru were doped into the in situ-grown ZnO nanorods by a drip calcination method, successfully verifying the presence of the doped elements. Compared to the original ZnO and ZnO samples doped with Pt or Ru separately, ZnO-Pt/Ru exhibited a significantly improved response at the optimal operating temperature of 198 °C, which is relatively low compared to conventional ZnO operating temperatures. This improvement reduced sensor energy consumption and extended sensor lifetime. Additionally, ZnO-Pt/Ru showed fast response and good recovery characteristics, with a linear relationship in the 1–50 ppm concentration range, good repeatability, and a high response of nearly 26 at 50 ppm. Moreover, ZnO-Pt/Ru demonstrated excellent selectivity, which was attributed to the electronic sensitization and chemical sensitization mechanisms introduced by doped Pt and Ru, efficiently distinguishing the reducing gas H2S.
Author Contributions
Methodology, J.Z.; formal analysis, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, B.T.; validation, B.T.; data curation, C.Z.; supervision, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant no. 62271176) and the China National Basic Enhancement Program (2022-JCJQ-JJ-0438).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, F.I.M.; Awwad, F.; Greish, Y.E.; Mahmoud, S.T. Hydrogen Sulfide (H2S) Gas Sensor: A Review. IEEE Sens. J. 2019, 19, 2394–2407. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. Trac-Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Li, W.; Shahbazi, M.; Xing, K.; Tesfamichael, T.; Motta, N.; Qi, D.-C. Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure. Nanomaterials 2022, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Krokowski, D.; Guan, B.J.; Bederman, I.; Majumder, M.; Parisien, M.; Diatchenko, L.; Kabil, O.; Willard, B.; Banerjee, R.; et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. eLife 2015, 4, e10067. [Google Scholar] [CrossRef]
- Hsueh, T.-J.; Wu, S.-S. Highly sensitive Co3O4 nanoparticles/MEMS NO2 gas sensor with the adsorption of the Au nanoparticles. Sens. Actuators B Chem. 2021, 329, 129201. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhu, X.; Li, Y.; Cai, W. Bilayer Au nanoparticle-decorated WO3 porous thin films: On-chip fabrication and enhanced NO2 gas sensing performances with high selectivity. Sens. Actuators B Chem. 2019, 280, 192–200. [Google Scholar] [CrossRef]
- Shewale, P.S.; Patil, V.B.; Shin, S.W.; Kim, J.H.; Uplane, M.D. H2S gas sensing properties of nanocrystalline Cu-doped ZnO thin films prepared by advanced spray pyrolysis. Sens. Actuators B Chem. 2013, 186, 226–234. [Google Scholar] [CrossRef]
- Xuan, J.-Y.; Zhao, G.-D.; Shi, X.-B.; Geng, W.; Li, H.-Z.; Sun, M.-L.; Jia, F.-C.; Tan, S.-G.; Yin, G.-C.; Liu, B. In-situ fabrication of ZnO nanoparticles sensors based on gas-sensing electrode for ppb-level H2S detection at room temperature. Chin. Phys. B 2021, 30, 020701. [Google Scholar] [CrossRef]
- Datta, N.; Ramgir, N.; Kaur, M.; Ganapathi, S.K.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Selective H2S sensing characteristics of hydrothermally grown ZnO-nanowires network tailored by ultrathin CuO layers. Sens. Actuators B Chem. 2012, 166, 394–401. [Google Scholar] [CrossRef]
- Fan, C.; Sun, F.; Wang, X.; Huang, Z.; Keshvardoostchokami, M.; Kumar, P.; Liu, B. Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials 2019, 9, 1277. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, S.; Gong, Y.; Gong, Y.; Wu, W.; Mao, Z.; Liu, Q.; Hu, S.; Long, X. MOF-SMO hybrids as a H2S sensor with superior sensitivity and selectivity. Sens. Actuators B Chem. 2019, 292, 32–39. [Google Scholar] [CrossRef]
- Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Ultra-sensitive H2S sensors based on hydrothermal/impregnation-made Ru-functionalized WO3 nanorods. Sens. Actuators B Chem. 2015, 215, 630–636. [Google Scholar] [CrossRef]
- Yu, A.; Li, Z.; Yi, J. Selective detection of parts-per-billion H2S with Pt-decorated ZnO nanorods. Sens. Actuators B Chem. 2021, 333, 129545. [Google Scholar] [CrossRef]
- Su, Y.; Chen, P.; Wang, P.; Ge, J.; Hu, S.; Zhao, Y.; Xie, G.; Liang, W.; Song, P. Pd-loaded SnO2 hierarchical nanospheres for a high dynamic range H2S micro sensor. RSC Adv. 2019, 9, 5987–5994. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, S.S. Bimetallic Pd/Pt nanoparticle-functionalized SnO2 nanowires for fast response and recovery to NO2. Sens. Actuators B Chem. 2013, 181, 446–453. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Sun, B.; He, J.; Kang, S.; Hua, Z.-Q.; Tian, C. Surface modification of WO3 nanoparticles with Pt and Ru for VOCs sensors. Chin. J. Anal. Chem. 2022, 50, 100143. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhong, X.; Li, G.; Han, C.; Wei, D.; Li, S. Bimetallic Au/Pd nanoparticles decorated ZnO nanowires for NO2 detection. Sens. Actuators B Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, T.; Sun, J.; Quan, H.; Zhou, T. Pd/Pt-Bimetallic-Nanoparticle-Doped In2O3 Hollow Microspheres for Rapid and Sensitive H2S Sensing at Low Temperature. Nanomaterials 2023, 13, 668. [Google Scholar] [CrossRef]
- Duan, X.; Xu, D.; Jia, W.; Sun, B.; Li, R.; Yan, R.; Zhao, W. Pt and black phosphorus co-modified flower-like WS2 composites for fast NO2 gas detection at low temperature. Nanoscale 2024, 16, 2478–2489. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, S.-H.; Liu, C.-X.; Wu, R.-J. Fabrication of Pd-Pt/ZnO for High Sensitive Gaseous Formaldehyde Sensor. J. Nanosci. Nanotechnol. 2018, 18, 1682–1687. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Kruefu, V.; Inpan, U.; Leangtanom, P.; Arkarvipath, C.; Kongpark, P.; Phokharatkul, D.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Enhanced Gas-Sensing Performances of Ru-Loaded p-Type Co3O4 Nanoparticles. Phys. Status Solidi A-Appl. Mater. Sci. 2018, 215, 1701015. [Google Scholar] [CrossRef]
- Xu, J.; Han, J.; Zhang, Y.; Sun, Y.A.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuators B Chem. 2008, 132, 334–339. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Qu, F.; Liu, S.; Lin, C.-T.; Du, S.; Chen, Y.; Meng, F.; Yang, M. ZnO nanoflowers modified with RuO2 for enhancing acetone sensing performance. Nanotechnology 2020, 31, 115502. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.; Yang, J.; Sun, Y.; Lu, G. Acetone sensors with high stability to humidity changes based on Ru-doped NiO flower-like microspheres. Sens. Actuators B Chem. 2020, 313, 127965. [Google Scholar] [CrossRef]
- Patil, V.L.; Vanalakar, S.A.; Tarwal, N.L.; Patil, A.P.; Dongale, T.D.; Kim, J.H.; Patil, P.S. Construction of Cu doped ZnO nanorods by chemical method for Low temperature detection of NO2 gas. Sens. Actuators A Phys. 2019, 299, 111611. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Cho, J.S.; Yoon, J.-W.; Na, C.W.; Lee, C.-S.; Ahn, J.H.; Kang, Y.C.; Lee, J.-H. Extremely sensitive ethanol sensor using Pt-doped SnO2 hollow nanospheres prepared by Kirkendall diffusion. Sens. Actuators B Chem. 2016, 234, 353–360. [Google Scholar] [CrossRef]
- Guo, L.; Xie, N.; Wang, C.; Kou, X.; Ding, M.; Zhang, H.; Sun, Y.; Song, H.; Wang, Y.; Lu, G. Enhanced hydrogen sulfide sensing properties of Pt-functionalized α-Fe2O3 nanowires prepared by one-step electrospinning. Sens. Actuators B Chem. 2018, 255, 1015–1023. [Google Scholar] [CrossRef]
- Hu, K.; Li, Y.; Ge, C.; Bai, L.; Liu, G.; Qiao, G.; Kang, S.G.; Kim, E.J.; Wang, M. Room-temperature ppb-level NO2 sensitivity of three-dimensional ordered macroporous Au-loaded SnO2 under intermittent UV light irradiation. Sens. Actuators B Chem. 2023, 387, 133786. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kini, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S gas sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Nimbalkar, A.R.; Patil, M.G. Synthesis of ZnO thin film by sol-gel spin coating technique for H2S gas sensing application. Phys. B-Condens. Matter 2017, 527, 7–15. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Tamboli, M.S.; Patil, S.S.; Dateer, R.B.; Mulla, I.S.; Choi, H.; Suryavanshi, S.S. Microwave-Epoxide-Assisted Hydrothermal Synthesis of the CuO/ZnO Heterojunction: A Highly Versatile Route to Develop H2S Gas Sensors. Acs Omega 2020, 5, 8587–8595. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, V.; Sureshkumar, S.; Rao, T.S.; Sridhar, T.M. Impedance Spectroscopy-Based Reduced Graphene Oxide-Incorporated ZnO Composite Sensor for H2S Investigations. Acs Omega 2019, 4, 9976–9982. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Shinde, A.B.; Kulkarni, S.G.; Maiti, N.; Koinkar, P.M.; Sonawane, K.M. Gas sensing performance of Al doped ZnO thin film for H2S detection. J. Alloys Compd. 2018, 748, 6–11. [Google Scholar] [CrossRef]
- Motaung, D.E.; Mhlongo, G.H.; Bolokang, A.S.; Dhonge, B.P.; Swart, H.C.; Ray, S.S. Improved sensitivity and selectivity of pristine zinc oxide nanostructures to H2S gas: Detailed study on the synthesis reaction time. Appl. Surf. Sci. 2016, 386, 210–223. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.; Gong, M. Hydrothermal growth of ZnO nanoscrewdrivers and their gas sensing properties. Nanotechnology 2007, 18, 185601. [Google Scholar] [CrossRef]
- Wendel, P.; Periyannan, S.; Jaegermann, W.; Klein, A. Polarization dependence of ZnO Schottky barriers revealed by photoelectron spectroscopy. Phys. Rev. Mater. 2020, 4, 084604. [Google Scholar] [CrossRef]
- Jang, J.-S.; Choi, S.-J.; Kim, S.-J.; Hakim, M.; Kim, I.-D. Rational Design of Highly Porous SnO2 Nanotubes Functionalized with Biomimetic Nanocatalysts for Direct Observation of Simulated Diabetes. Adv. Funct. Mater. 2016, 26, 4740–4748. [Google Scholar] [CrossRef]
- Zhang, S.; Yange, M.; Liang, K.; Turak, A.; Zhang, B.; Meng, D.; Wang, C.; Qu, F.; Cheng, W.; Yang, M. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B Chem. 2019, 290, 59–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).