Vis/NIR Spectroscopy and Chemometrics for Non-Destructive Estimation of Chlorophyll Content in Different Plant Leaves
Abstract
Highlights
- The MSD method serves as a tool for selecting a representative calibration dataset in leaves.
- The leaves’ sensitive bands were in the ranges of 500–640 nm and 740–1100 nm.
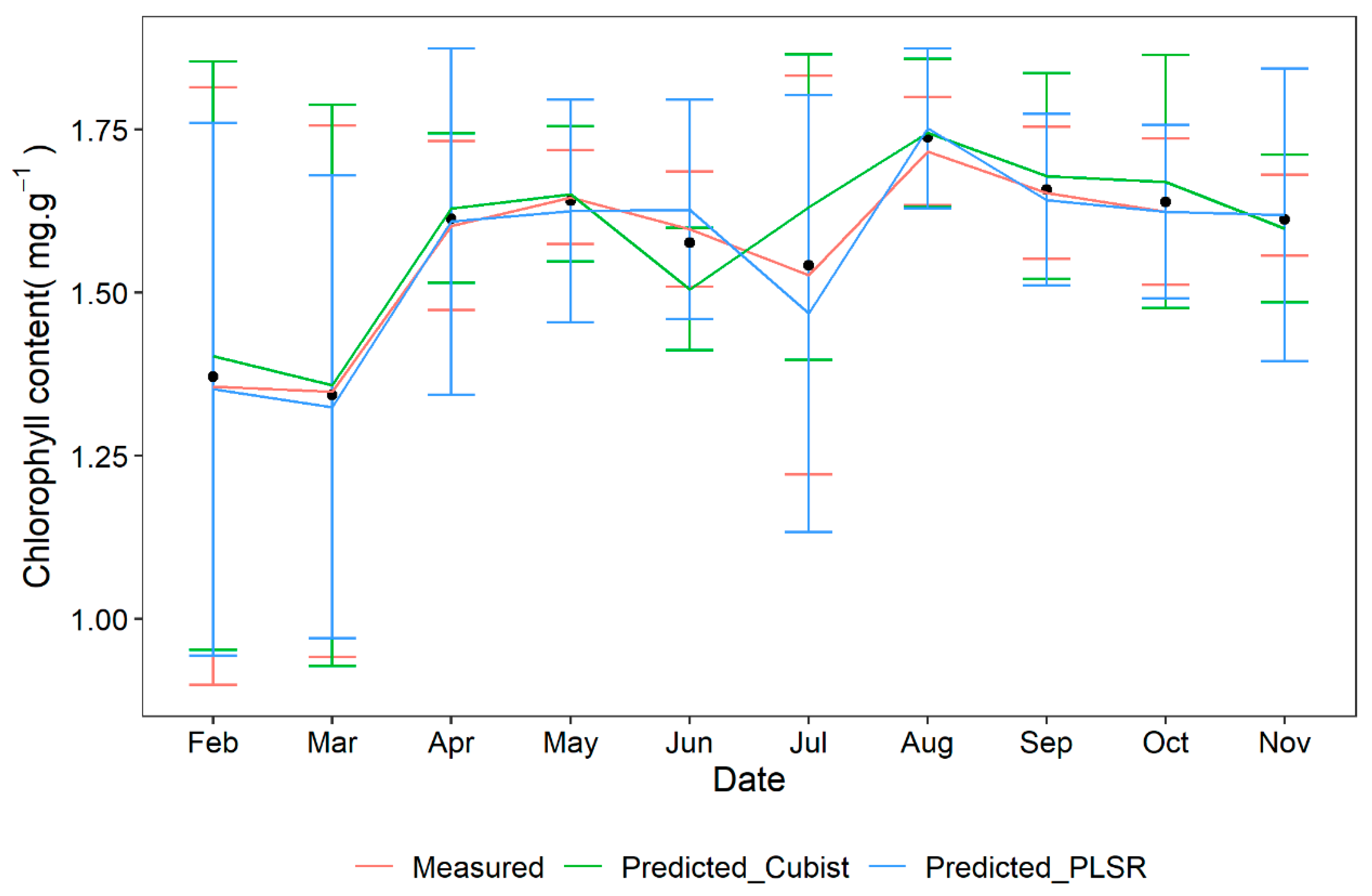
- Cubist achieved a higher determination coefficient than PLSR.
- Vis/NIR can be used for assessing drought’s impact on plants’ chlorophyll.
- MSD method can be useful for selecting a representative calibration dataset in leaves.
- Vis-NIR are the invaluable tool for assessing drought impacts on plant health and productivity.
Abstract
1. Introduction
| Methods | The Main Processes | Advantages | Disadvantages |
|---|---|---|---|
| Spectrophotometer method [24] | Using the different solvents | Simple and quick | Long extraction time (24h) and solvents with high toxicity destroy the leaves |
| Fluorometry [25] | Sample preparation, solvent extraction, measurement, data Analysis | High sensitivity, quick | Cost, sensitivity to light conditions, using solvent |
| HPLC [26] | Pigment extraction, Mobile phase configuration, Chromatographic condition test, Drawing of working curves | High sensitivity, good Resolution | Heavy workload, complicated steps, long extracted time, or strict extraction conditions |
| Optoacoustic spectrometry [27] | Measure the amount of light absorbed by a sample | Simple and rapid inexpensive and easy to operate | Low accuracy, easily affected by temperature and light intensity |
| Vis/NIR spectroscopy | Spectral measurement, data analysis | Non-destructive, Rapid, portable, scalability | Depends on the quality of the calibration model |
2. Materials and Methods
2.1. Leaf Sampling, Spectral Measurement, and Lab Chlorophyll Content Measurement
2.2. The Reprehensive Calibration Samples Selected
2.3. Models
3. Results
3.1. Statistics for Sampling Information and Spectroscopy Analysis
3.2. The Optimal Number of Subset Samples in the PC Space of the Vis/NIR Data
3.3. Model to Predict the Chlorophyll Content of Five Types of Plant Leaves
4. Discussion
4.1. The Selected Representative Calibration Dataset
4.2. The Principles of Prediction of Chlorophyll Content Using Vis/NIR Spectra
5. Conclusions
- (1)
- The MSD method serves as a tool for selecting a representative calibration dataset in leaves.
- (2)
- The sensitive bands of leaves were in the ranges of 500–640 nm and 740–1100 nm.
- (3)
- Cubist achieved a higher determination coefficient than PLSR.
- (4)
- Vis/NIR spectroscopy has proven to be effective in estimating chlorophyll content across five different types of leaves over various months.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleem, A.; Anwar, S.; Nawaz, T.; Fahad, S.; Saud, S.; Ur Rahman, T.; Khan, M.N.R.; Nawaz, T. Securing a sustainable future: The climate change threat to agriculture, food security, and sustainable development goals. J. Umm Al-Qura Univ. Appl. Sci. 2024, 1–17. [Google Scholar] [CrossRef]
- Siirila-Woodburn, E.R.; Rhoades, A.M.; Hatchett, B.J.; Huning, L.S.; Szinai, J.; Tague, C.; Nico, P.S.; Feldman, D.R.; Jones, A.D.; Collins, W.D. A low-to-no snow future and its impacts on water resources in the western United States. Nat. Rev. Earth Environ. 2021, 2, 800–819. [Google Scholar] [CrossRef]
- Li, S.; Lu, S.; Wang, X.; Chen, Z.; Li, B.; Zhao, N.; Xu, X. Drought timing and degradation status determine the grassland sensitivity to simulated drought events. Agric. Ecosyst. Environ. 2025, 378, 109312. [Google Scholar] [CrossRef]
- Chi, W.; Nan, Q.; Liu, Y.; Dong, D.; Qin, Y.; Li, S.; Wu, W. Stress resistance enhancing with biochar application and promotion on crop growth. Biochar 2024, 6, 43. [Google Scholar] [CrossRef]
- Brunette, E.; Wang, L.; Wassenaar, T.D. Groundwater abstraction and woodland mortality: Lessons from Namibia. J. Arid. Environ. 2024, 222, 105154. [Google Scholar] [CrossRef]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Chang. Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Chen, L.; Jiang, Y.; Bu, H.; Jiang, Y.; Li, P.; Cao, C. Physiological mechanism of drought-resistant rice coping with drought stress. J. Plant Growth Regul. 2022, 41, 2638–2651. [Google Scholar] [CrossRef]
- Shevela, D.; Bjorn, L.O. Photosynthesis: Solar Energy for Life; World Scientific Publishing: Singapore, 2018. [Google Scholar]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-based chlorophyll content estimation by evaluating vegetation index responses under different crop coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- De Cannière, S.; Herbst, M.; Vereecken, H.; Defourny, P.; Jonard, F. Constraining water limitation of photosynthesis in a crop growth model with sun-induced chlorophyll fluorescence. Remote Sens. Environ. 2021, 267, 112722. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Zhou, Y.; Liu, X.; Wu, J.; Tan, W.; Xu, C.; Xiong, X. Comparison between Satellite Derived Solar-Induced Chlorophyll Fluorescence, NDVI and kNDVI in Detecting Water Stress for Dense Vegetation across Southern China. Remote Sens. 2024, 16, 1735. [Google Scholar] [CrossRef]
- Shi, S.; Xu, L.; Gong, W.; Chen, B.; Chen, B.; Qu, F.; Tang, X.; Sun, J.; Yang, J. A convolution neural network for forest leaf chlorophyll and carotenoid estimation using hyperspectral reflectance. Int. J. Appl. Earth Obs. Geoinf. 2022, 108, 102719. [Google Scholar] [CrossRef]
- Berhe, M.; You, J.; Dossa, K.; Li, D.; Zhou, R.; Zhang, Y.; Wang, L. Examining chlorophyll extraction methods in sesame genotypes: Uncovering leaf coloration effects and anatomy variations. Plants 2024, 13, 1589. [Google Scholar] [CrossRef]
- Steidle Neto, A.J.; Lopes, D.C.; Pinto, F.A.C.; Zolnier, S. Vis/NIR spectroscopy and chemometrics for non-destructive estimation of water and chlorophyll status in sunflower leaves. Biosyst. Eng. 2017, 155, 124–133. [Google Scholar] [CrossRef]
- Gamon, J.; Surfus, J. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Liu, J.; Han, J.; Chen, X.; Shi, L.; Zhang, L. Nondestructive detection of rape leaf chlorophyll level based on Vis-NIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117202. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Z.; Li, Y.; Zhao, G.; Zhu, S.; Fu, W.; Peng, T.; Zhao, Q.; Svanberg, S.; Hu, J. Vis/NIR reflectance spectroscopy for hybrid rice variety identification and chlorophyll content evaluation for different nitrogen fertilizer levels. R. Soc. Open Sci. 2019, 6, 191132. [Google Scholar] [CrossRef]
- Rasooli Sharabiani, V.; Soltani Nazarloo, A.; Taghinezhad, E.; Veza, I.; Szumny, A.; Figiel, A. Prediction of winter wheat leaf chlorophyll content based on VIS/NIR spectroscopy using ANN and PLSR. Food Sci. Nutr. 2023, 11, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Tomasetto, F.; Yan, W.; Tan, Z.; Liu, J.; Jiang, J. Non-destructive Measurements of Toona sinensis Chlorophyll and Nitrogen Content Under Drought Stress Using Near Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 809828. [Google Scholar] [CrossRef]
- Ong, P.; Jian, J.; Li, X.; Yin, J.; Ma, G. Visible and near-infrared spectroscopic determination of sugarcane chlorophyll content using a modified wavelength selection method for multivariate calibration. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123477. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Zhang, L.; Zan, O.; Yan, X.; Feng, K. Spectral detection of leaf carbon and nitrogen as a proxy for remote assessment of photosynthetic capacity for wheat and maize under nitrogen stress. Comput. Electron. Agric. 2024, 224, 109174. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.; Castro-García, S.; Blanco-Roldán, G.L.; Agüera-Vega, J.; Gil-Ribes, J.A. Non-destructive determination of impact bruising on table olives using Vis–NIR spectroscopy. Biosyst. Eng. 2012, 113, 371–378. [Google Scholar] [CrossRef]
- Ulissi, V.; Antonucci, F.; Benincasa, P.; Farneselli, M.; Tosti, G.; Guiducci, M.; Tei, F.; Costa, C.; Pallottino, F.; Pari, L. Nitrogen concentration estimation in tomato leaves by VIS-NIR non-destructive spectroscopy. Sensors 2011, 11, 6411–6424. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yao, X.; Jia, W.; Tian, Y.; Ni, J.; Cao, W.; Zhu, Y. Comparison and intercalibration of vegetation indices from different sensors for monitoring above-ground plant nitrogen uptake in winter wheat. Sensors 2013, 13, 3109–3130. [Google Scholar] [CrossRef]
- Zheng-Shu, S.; Xian-Zheng, Z. Comparison of several methods for determining plant chlorophyll content. Plant Physiol. Commun. 1989, 5, 77–78. [Google Scholar]
- Lichtenthaler, H.K.; Hak, R.; Rinderle, U. The chlorophyll fluorescence ratio F690/F730 in leaves of different chlorophyll content. Photosynth. Res. 1990, 25, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC determination of chlorophyll and carotenoid pigments in processed green pea cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef]
- Adams, M.; Beadle, B.; King, A.; Kirkbright, G. Analytical optoacoustic spectrometry. Part II. Ultraviolet and visible optoacoustic spectra of some inorganic.; biochemical and phytochemical samples. Analyst 1976, 10, 553–561. [Google Scholar] [CrossRef]
- Gu, D.D.; Wang, W.-Z.; Hu, J.-D.; Zhang, X.-M.; Wang, J.-B.; Wang, B.-S. Nondestructive determination of total chlorophyll content in maize using three-wavelength diffuse reflectance. J. Appl. Spectrosc. 2016, 83, 541–547. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.; Schmidt, K.; Behrens, T.; Van Wesemael, B.; Demattê, J.A.M.; Scholten, T. Sampling optimal calibration sets in soil infrared spectroscopy. Geoderma 2014, 226–227, 140–150. [Google Scholar] [CrossRef]
- Yang, M.; Chen, S.; Guo, X.; Shi, Z.; Zhao, X. Exploring the potential of vis-NIR spectroscopy as a covariate in soil organic matter mapping. Remote Sens. 2023, 15, 1617. [Google Scholar] [CrossRef]
- Roger, J.-M.; Chauchard, F.; Bellon-Maurel, V. EPO–PLS external parameter orthogonalisation of PLS application to temperature-independent measurement of sugar content of intact fruits. Chemom. Intell. Lab. Syst. 2003, 66, 191–204. [Google Scholar] [CrossRef]
- Chauchard, F.; Cogdill, R.; Roussel, S.; Roger, J.M.; Bellon-Maurel, V. Application of LS-SVM to non-linear phenomena in NIR spectroscopy: Development of a robust and portable sensor for acidity prediction in grapes. Chemom. Intell. Lab. Syst. 2004, 71, 141–150. [Google Scholar] [CrossRef]
- Henderson, B.L.; Bui, E.N.; Moran, C.J.; Simon, D.A.P. Australia-wide predictions of soil properties using decision trees. Geoderma 2005, 124, 383–398. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.; Wadoux, A.C.; Franceschini, M.H.; Terra, F.; Marques, K.P.P.; Sayão, V.M.; Demattê, J.A.M. Robust soil mapping at the farm scale with vis–NIR spectroscopy. Eur. J. Soil Sci. 2019, 70, 378–393. [Google Scholar] [CrossRef]
- Fernàndez-Martínez, J.; Joffre, R.; Zacchini, M.; Fernández-Marín, B.; García-Plazaola, J.I.; Fleck, I. Near-infrared reflectance spectroscopy allows rapid and simultaneous evaluation of chloroplast pigments and antioxidants, carbon isotope discrimination and nitrogen content in Populus spp. leaves. For. Ecol. Manag. 2017, 399, 227–234. [Google Scholar] [CrossRef]
- Daniel, C. The role of visible and infrared spectroscopy combined with chemometrics to measure phenolic compounds in grape and wine samples. Molecules 2015, 20, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Lee, W. Determination of significant wavelengths and prediction of nitrogen content for citrus. Trans. ASAE 2005, 48, 455–461. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Jiang, J.; Liu, J. Spectroscopic determination of leaf chlorophyll content and color for genetic selection on Sassafras tzumu. Plant Methods 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Curcio, J.A.; Petty, C.C. The near infrared absorption spectrum of liquid water. JOSA 1951, 41, 302–304. [Google Scholar] [CrossRef]
- Hassan, H.; Fan Mingtao, F.M.; Zhang Tingjing, Z.T.; Yang Kun, Y.K. Prediction of total phenolics and flavonoids contents in Chinese wild rice (Zizania latifolia) using FT-NIR spectroscopy. Am. J. Food Technol. 2015, 10, 109–117. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ju, W.; Chen, B.; Chen, J.; Croft, H.; Mickler, R.A.; Yang, F. Estimation of leaf photosynthetic capacity from leaf chlorophyll content and leaf age in a subtropical evergreen coniferous plantation. J. Geophys. Res. Biogeosciences 2020, 125, e2019JG005020. [Google Scholar] [CrossRef]
- Shao, W.; Su, X.; Lu, J.; Liu, J.; Yang, Z.; Mei, C.; Liu, C.; Lu, J. Urban resilience of Shenzhen city under climate change. Atmosphere 2021, 12, 537. [Google Scholar] [CrossRef]


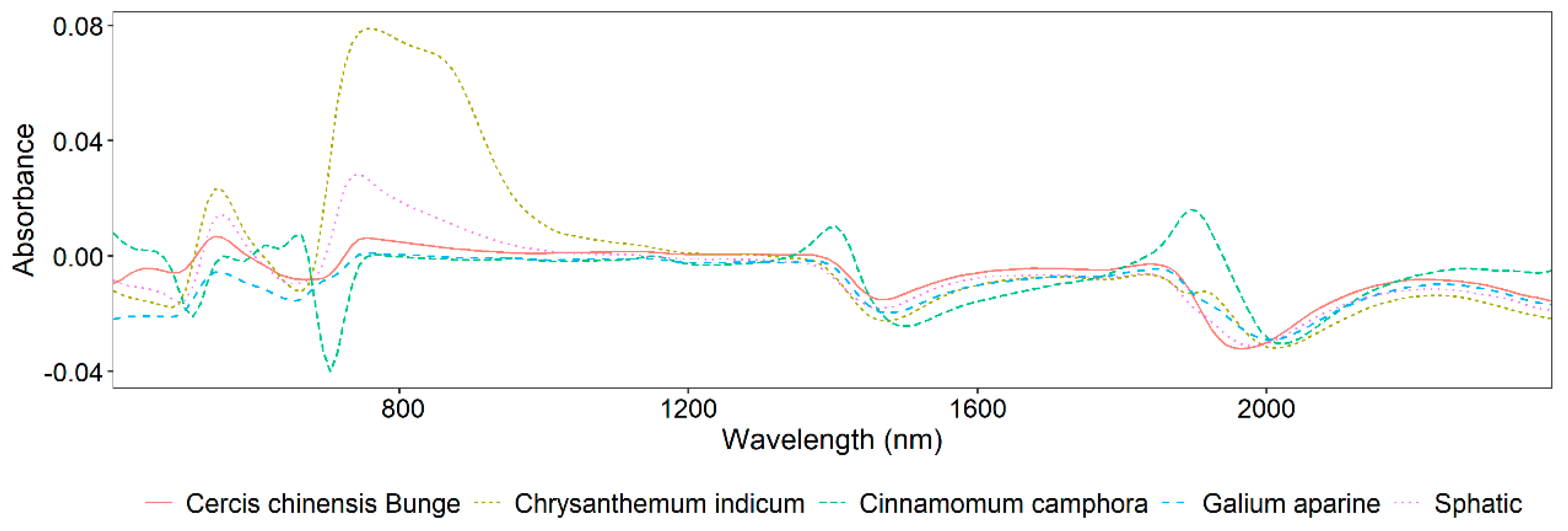

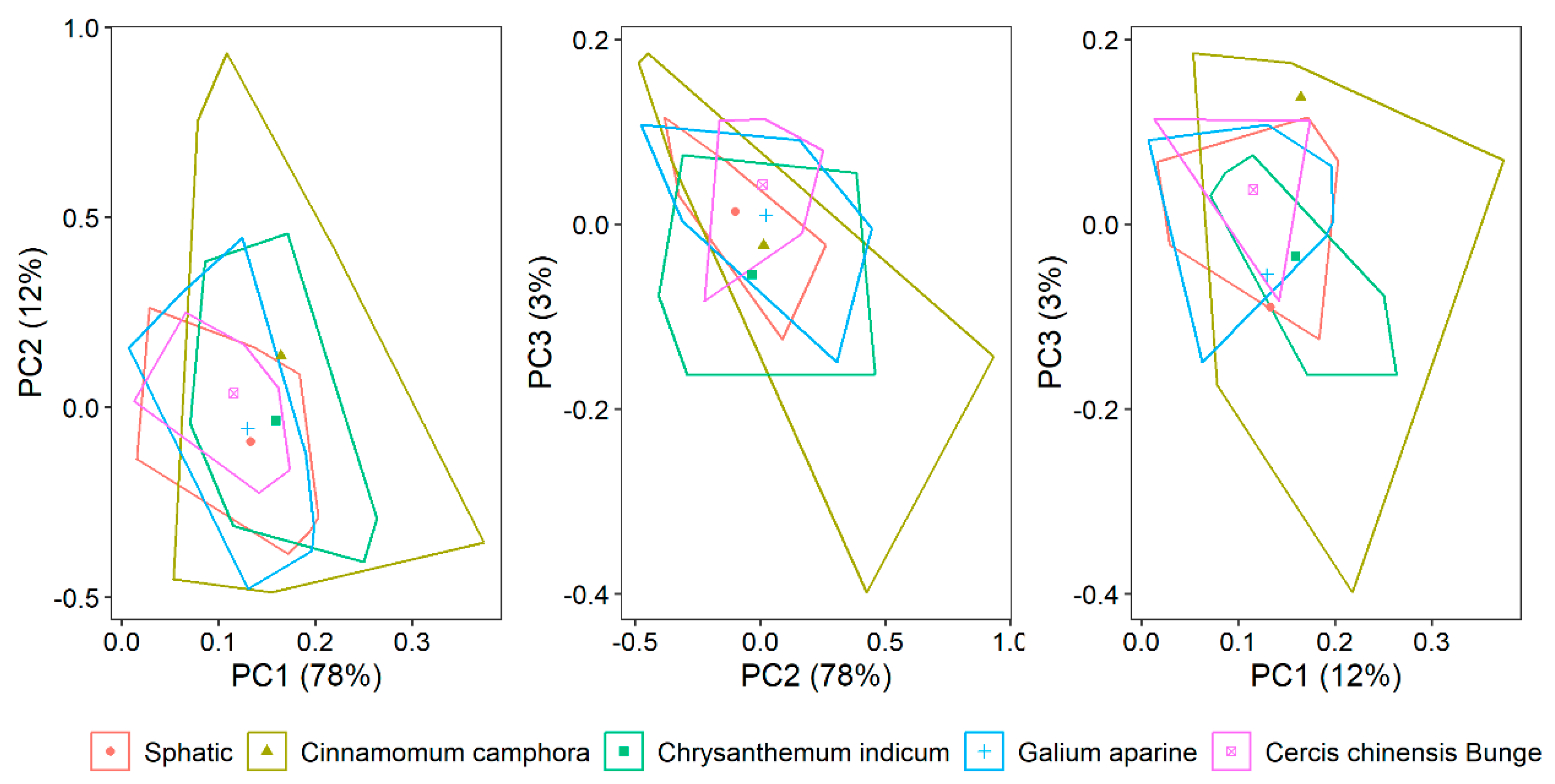
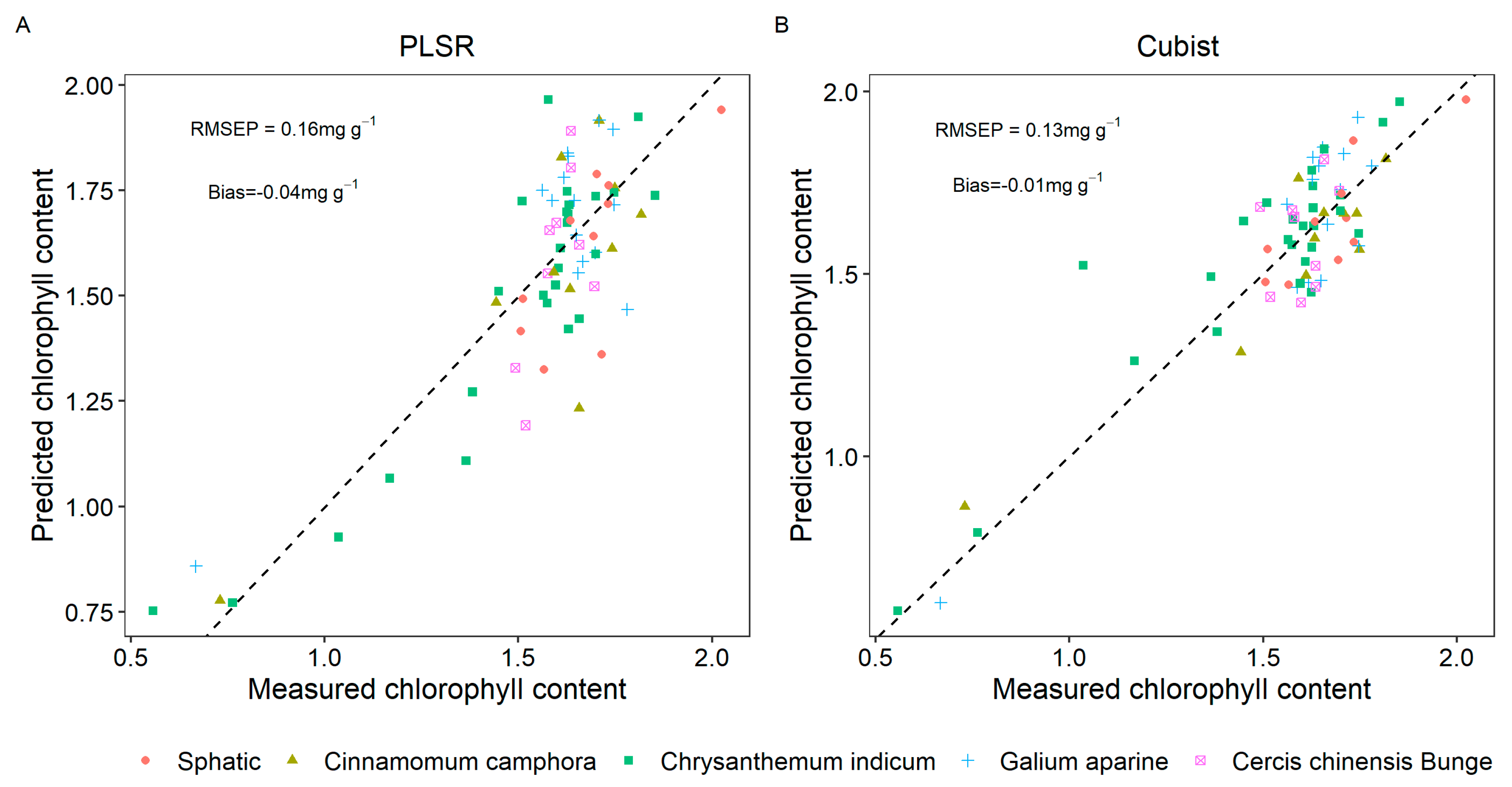
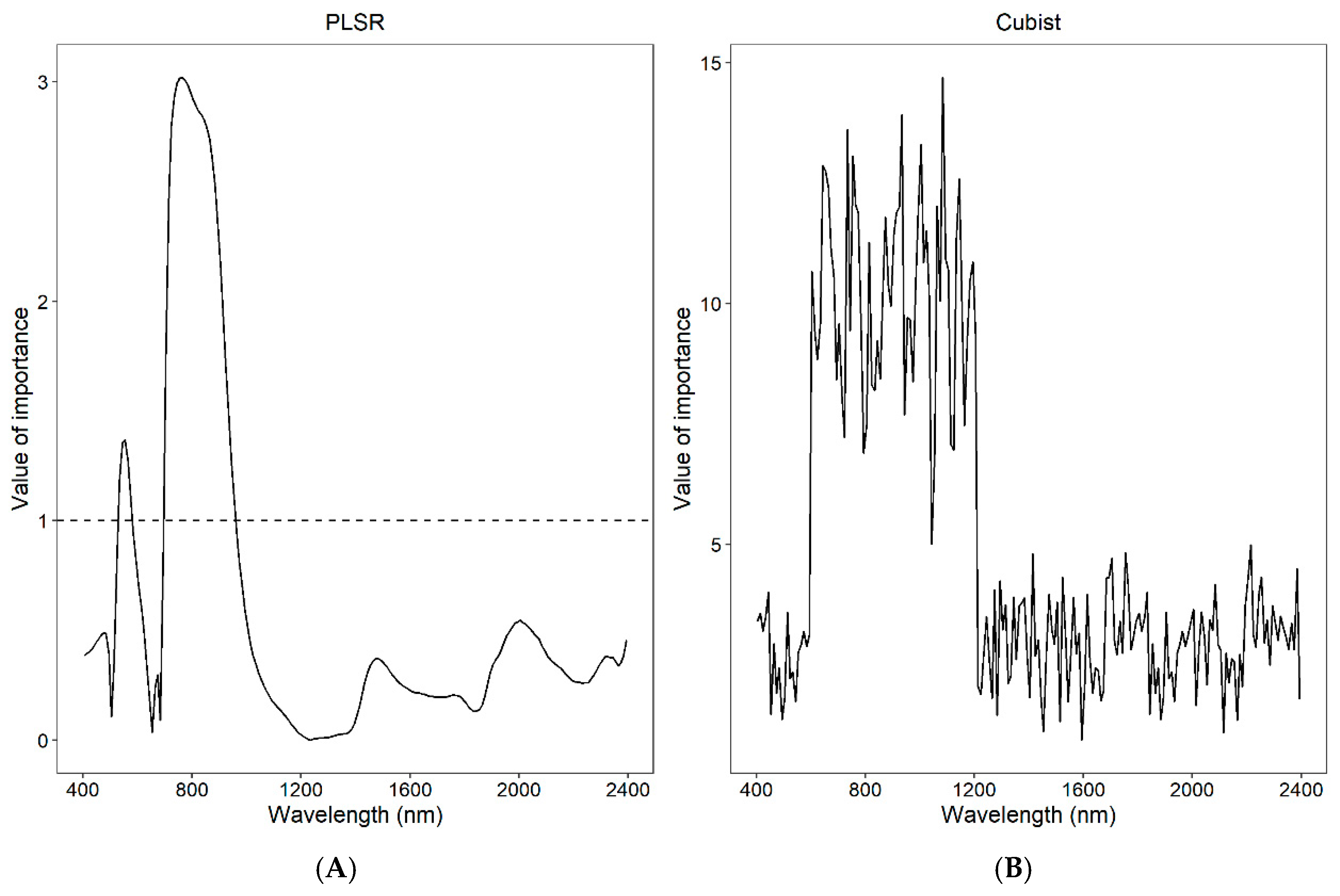
| Dataset | Max | Minimum | Mean | Stdev | Skew |
|---|---|---|---|---|---|
| Calibration (280) | 3.03 | 0.79 | 1.57 | 0.46 | 0.23 |
| Validation (70) | 2.78 | 0.96 | 1.68 | 0.32 | 0.33 |
| 60 | 3.03 | 0.89 | 1.63 | 0.34 | 0.37 |
| 100 | 2.98 | 0.81 | 1.66 | 0.35 | 0.31 |
| 140 | 2.91 | 0.79 | 1.70 | 0.38 | 0.34 |
| 180 | 3.03 | 0.88 | 1.69 | 0.41 | 0.23 |
| 220 | 3.01 | 0.81 | 1.60 | 0.44 | 0.24 |
| Galium aparine (70) | 2.11 | 0.96 | 1.47 | 0.32 | 0.33 |
| Chrysanthemum indicum (70) | 3.03 | 1.33 | 1.95 | 0.63 | 0.68 |
| Cercis chinensis Bunge (70) | 2.01 | 1.74 | 1.88 | 0.14 | −0.02 |
| Cinnamomum camphora (70) | 1.45 | 0.79 | 1.10 | 0.33 | 0.14 |
| Sphatic (70) | 1.84 | 1.67 | 1.77 | 0.09 | −0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Yang, M.; Ouyang, L.; Wang, Z.; Lin, J. Vis/NIR Spectroscopy and Chemometrics for Non-Destructive Estimation of Chlorophyll Content in Different Plant Leaves. Sensors 2025, 25, 1673. https://doi.org/10.3390/s25061673
Huang Q, Yang M, Ouyang L, Wang Z, Lin J. Vis/NIR Spectroscopy and Chemometrics for Non-Destructive Estimation of Chlorophyll Content in Different Plant Leaves. Sensors. 2025; 25(6):1673. https://doi.org/10.3390/s25061673
Chicago/Turabian StyleHuang, Qiang, Meihua Yang, Liao Ouyang, Zimiao Wang, and Jiayao Lin. 2025. "Vis/NIR Spectroscopy and Chemometrics for Non-Destructive Estimation of Chlorophyll Content in Different Plant Leaves" Sensors 25, no. 6: 1673. https://doi.org/10.3390/s25061673
APA StyleHuang, Q., Yang, M., Ouyang, L., Wang, Z., & Lin, J. (2025). Vis/NIR Spectroscopy and Chemometrics for Non-Destructive Estimation of Chlorophyll Content in Different Plant Leaves. Sensors, 25(6), 1673. https://doi.org/10.3390/s25061673





