Assessment of the Mechanical Properties of Soft Tissue Phantoms Using Impact Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
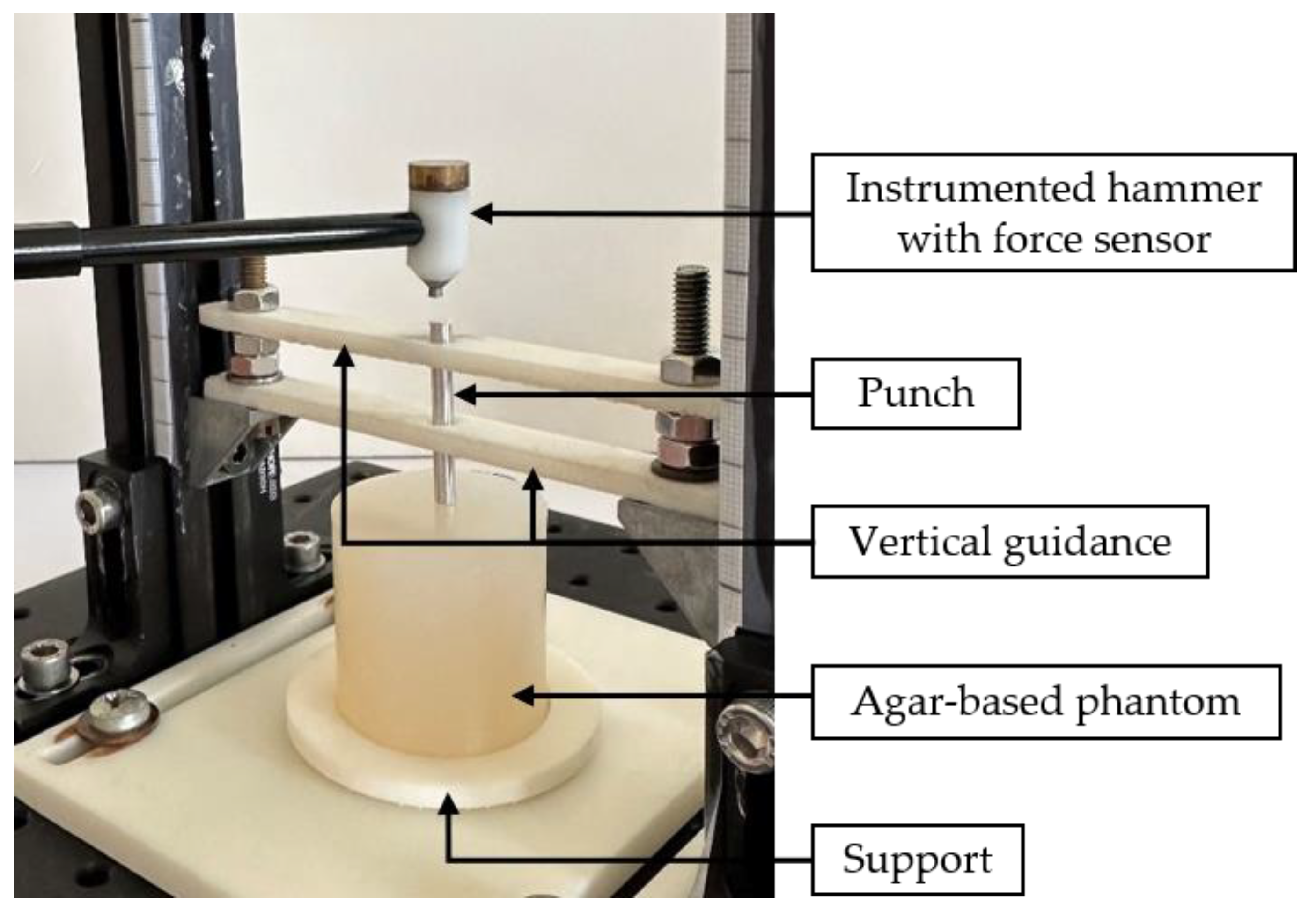
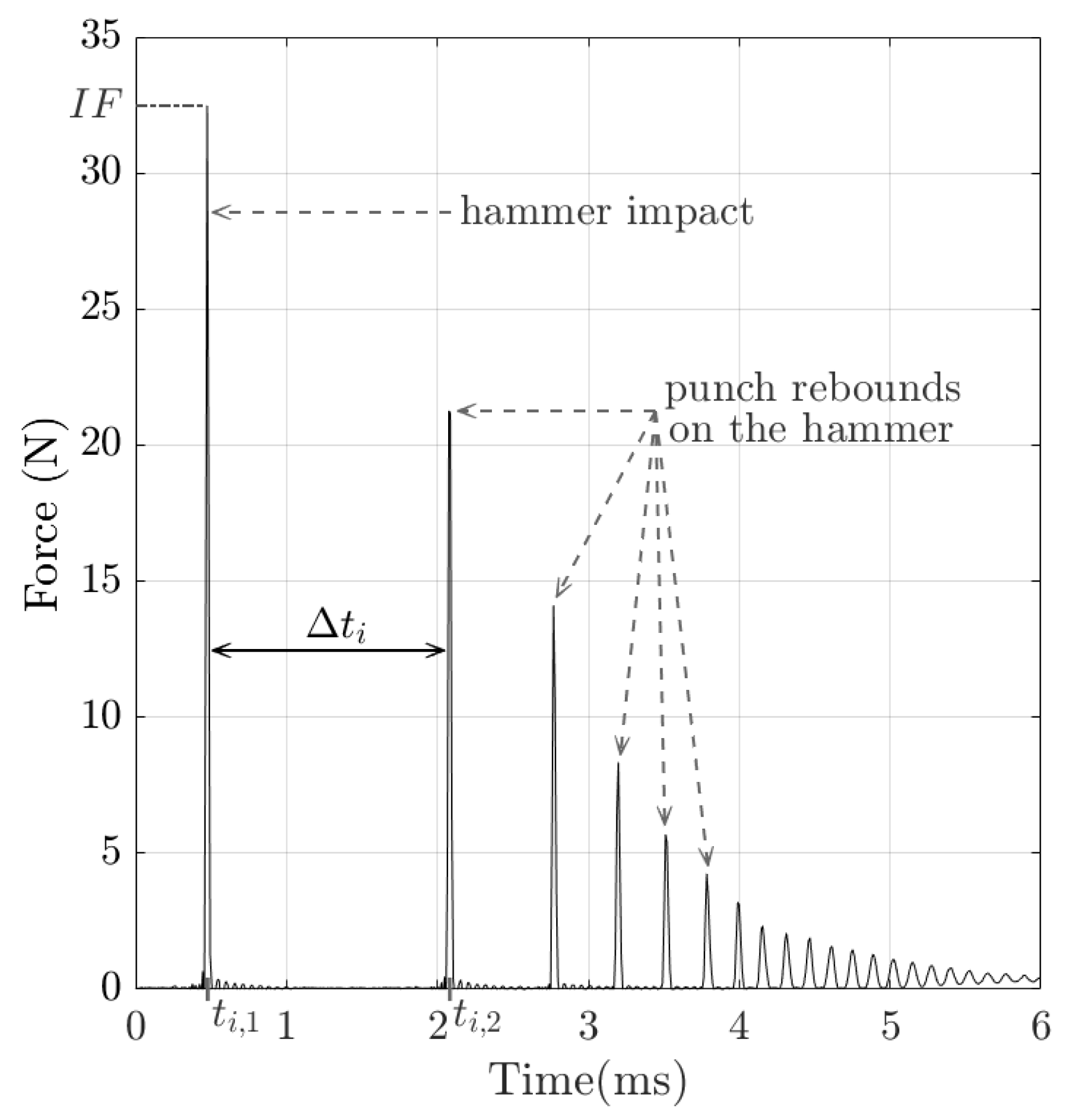
2.2. Impact-Based Analysis Method (IBAM)
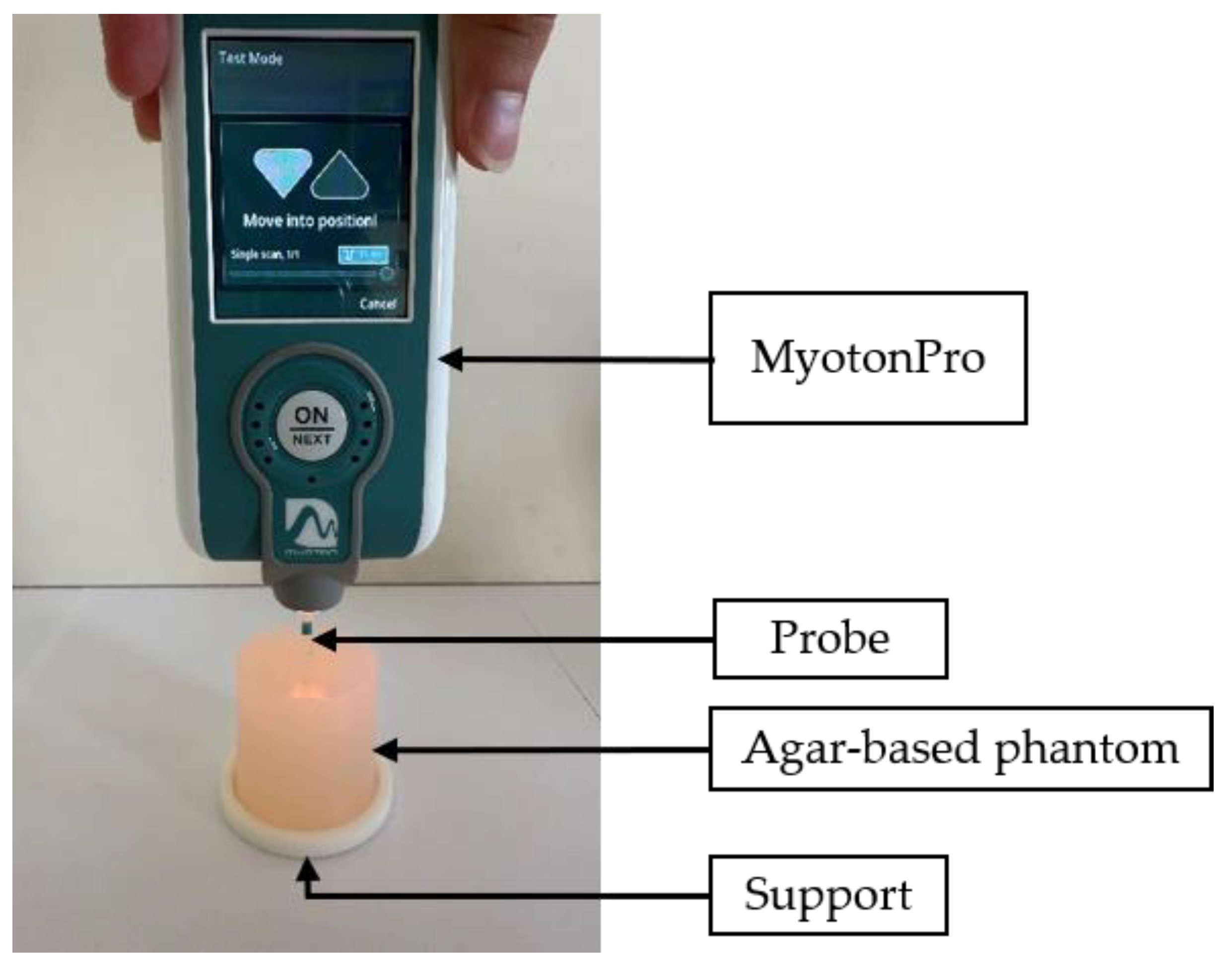
2.3. MyotonPro®
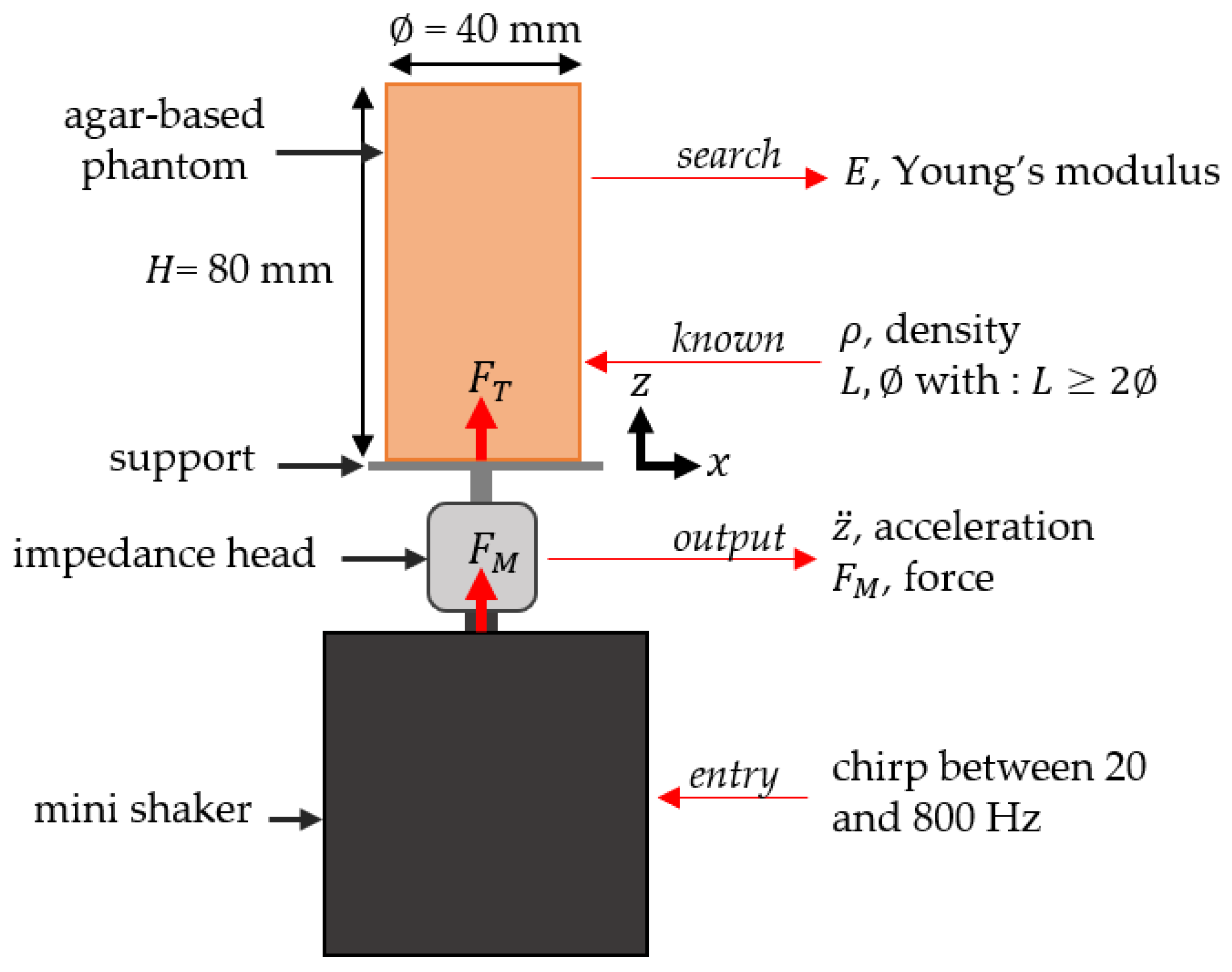
2.4. Dynamic Mechanical Analysis
2.5. Characterization Protocols
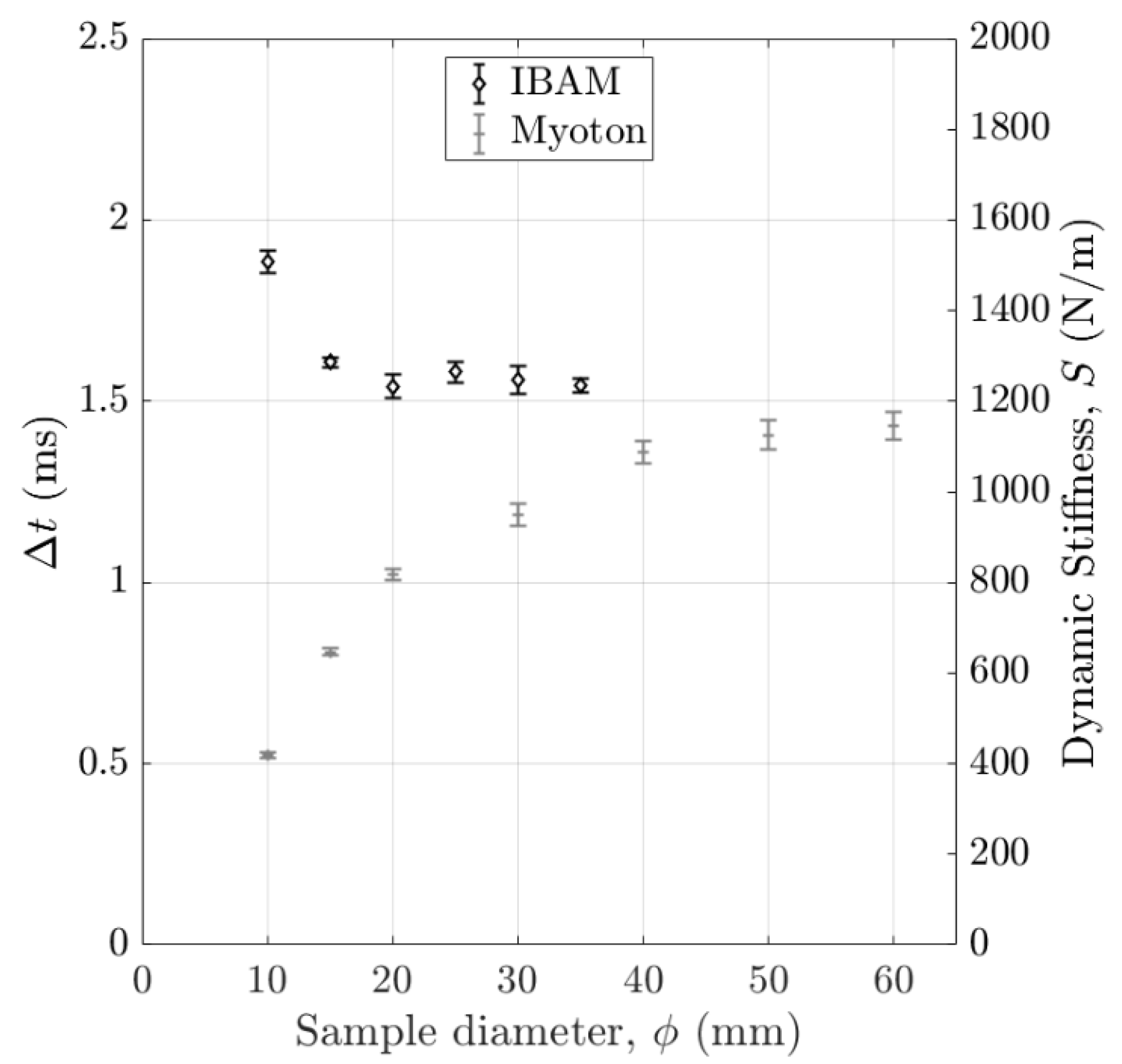
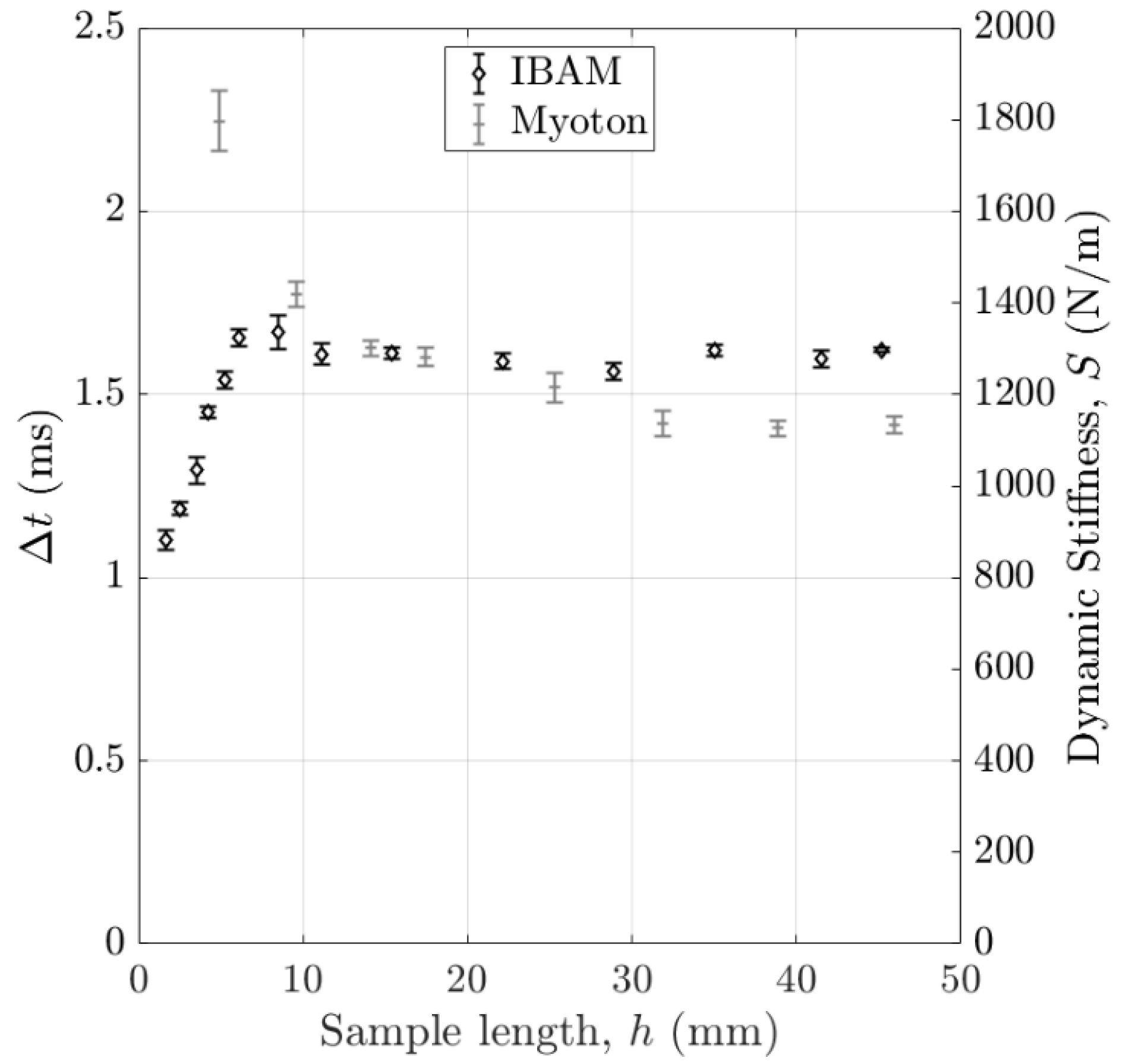
2.5.1. Estimation of the Measured Volume of Interest (VOI)
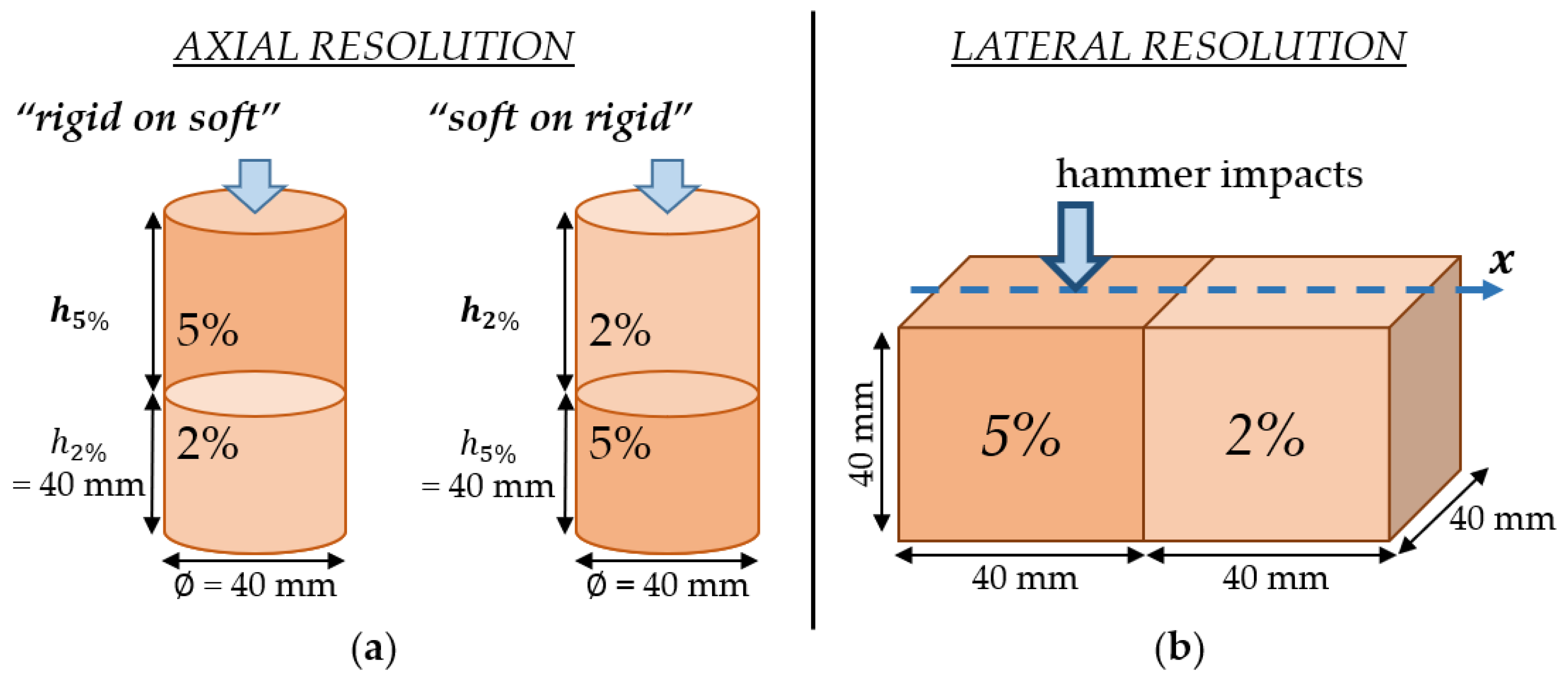
2.5.2. Axial and Lateral Resolutions Using Bilayer Configurations
2.5.3. Stiffness Discrimination
3. Results
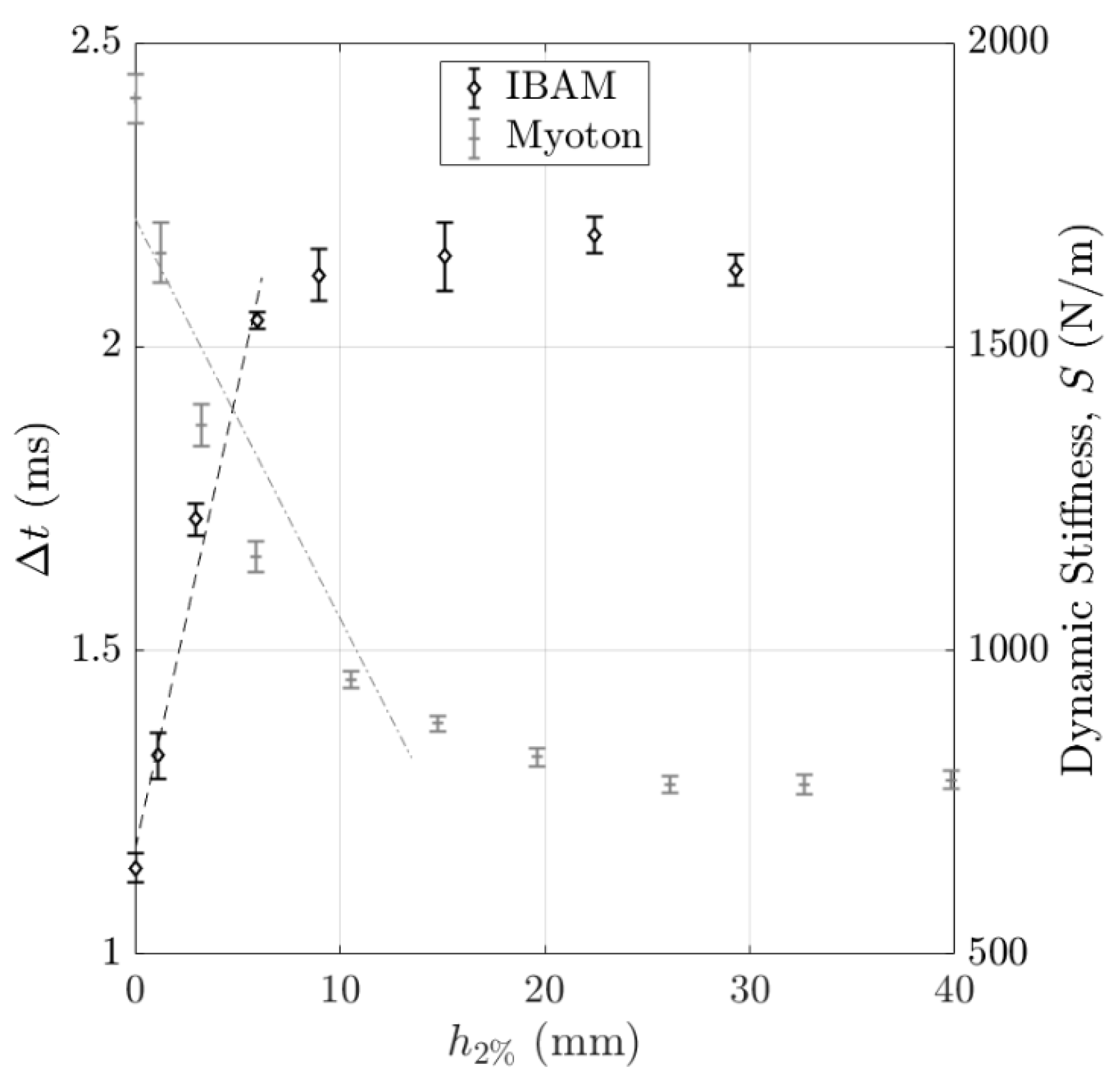
3.1. Volume of Interest (VOI) of the Measurement
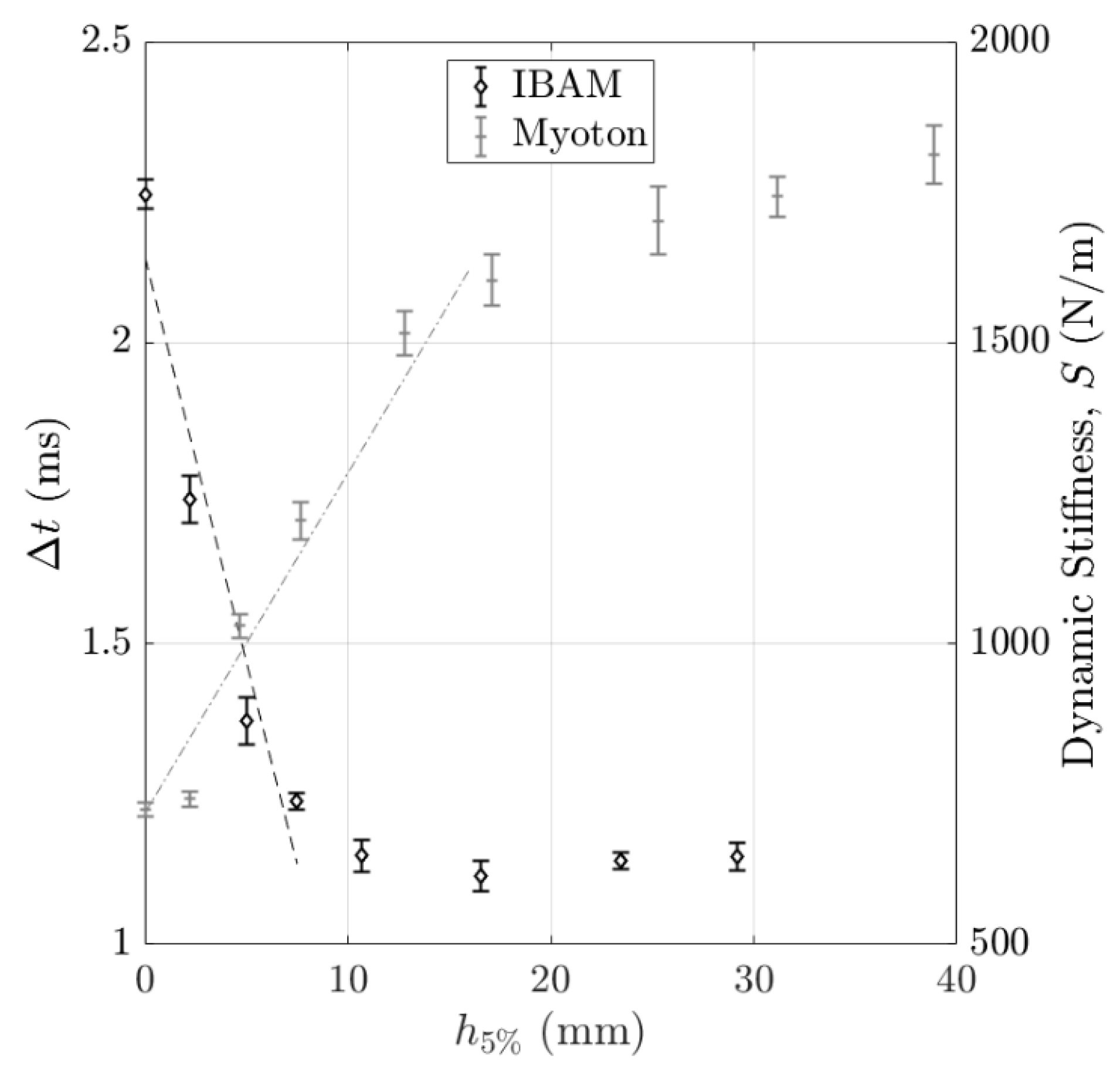
3.2. Axial Resolution
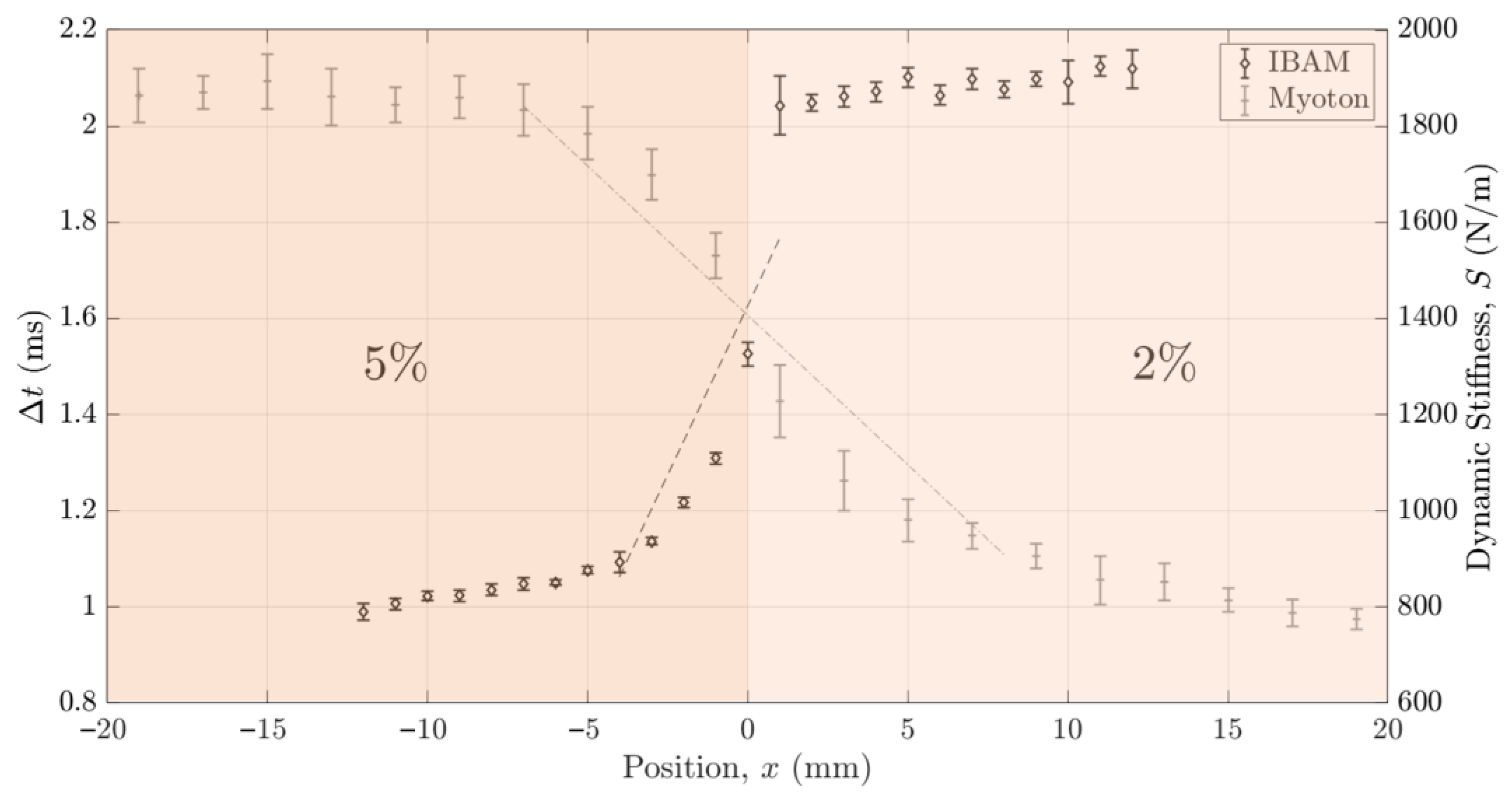
3.3. Lateral Resolution
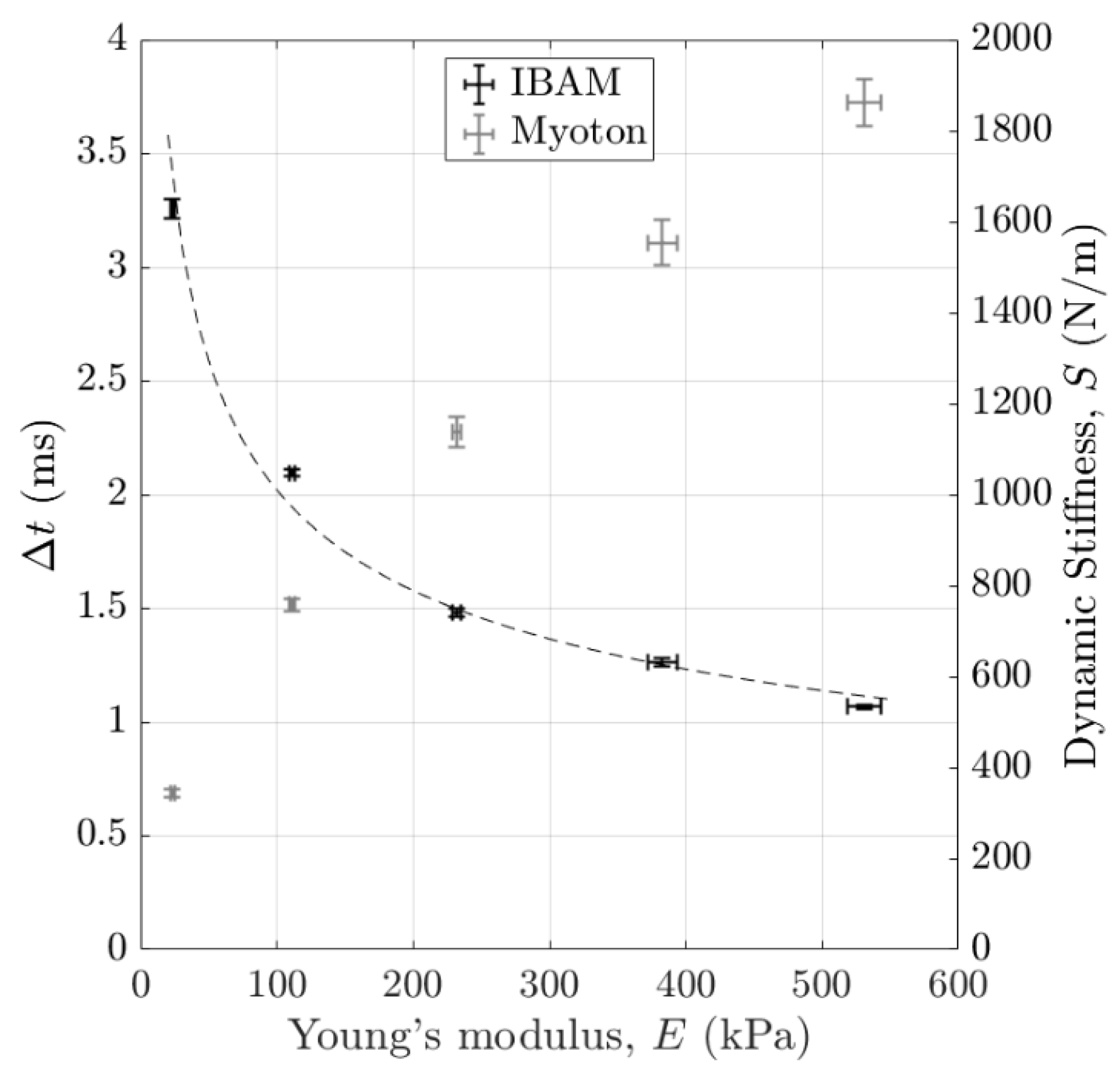
3.4. Stiffness Discrimination
4. Discussion
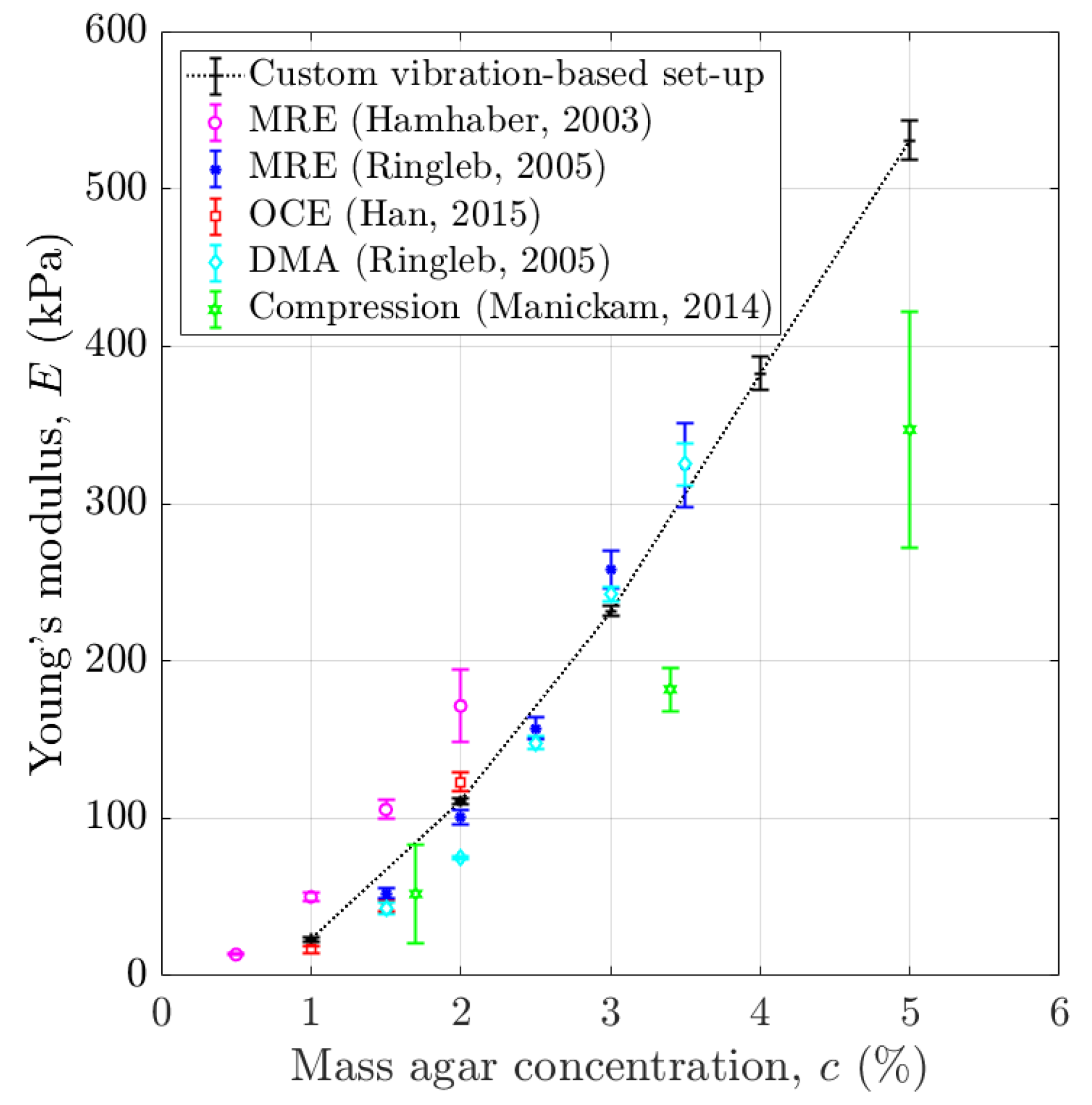
4.1. Young’s Modulus of Agar-Based Phantoms Mimicking Soft Tissues
4.2. Physical Interpretation of and
4.2.1. Effect of the Impact Force
4.2.2. Effect of the Sample Young’s Modulus
4.2.3. Effect of the Volume of Interest (VOI)
4.2.4. Axial and Lateral Resolutions
4.2.5. Stiffness Sensitivity Comparison
4.3. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBAM | impact-based analysis method |
| DMA | dynamic mechanical analysis |
| MRE | magnetic resonance imaging |
| OCE | optical coherence tomography |
| VOI | volume of interest |
References
- Krouskop, T.A.; Wheeler, T.M.; Kallel, F.; Garra, B.S.; Hall, T. Elastic moduli of breast and prostate tissues under compression. Ultrason. Imaging 1998, 20, 260–274. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.-H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Skovoroda, A.R.; Klishko, A.; Gusakyan, D.A.; Mayevskii, Y.I.; Yermilova, V.D.; Oranskaya, G.A.; Sarvazyan, A. Quantitative analysis of the mechanical characteristics of pathologically changed soft biological tissues. Biophysics 1995, 40, 1359–1364. [Google Scholar]
- Dobrev, H.P. In vivo study of skin mechanical properties in patients with systemic sclerosis. J. Am. Acad. Dermatol. 1999, 40, 436–442. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Baldeweck, T.; Tanter, M.; Catheline, S.; Fink, M.; Sandrin, L.; Cornillon, C.; Querleux, B. Assessment of elastic parameters of human skin using dynamic elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, H. Application of Cutometer area parameters for the study of human skin fatigue. Skin Res. Technol. 2005, 11, 120–122. [Google Scholar] [CrossRef]
- Stroumza, N.; Bosc, R.; Hersant, B.; Hermeziu, O.; Meningaud, J.-P. Intérêt du cutomètre pour l’évaluation de l’efficacité des traitements cutanés en chirurgie plastique et maxillo-faciale. Rev. Stomatol. Chir. Maxillo Faciale Et Chir. Orale 2015, 116, 77–81. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Non-invasive objective devices for monitoring the inflammatory, proliferative and remodelling phases of cutaneous wound healing and skin scarring. Exp. Dermatol. 2016, 25, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Catheline, S.; Gennisson, J.-L.; Fink, M. Measurement of elastic nonlinearity of soft solid with transient elastography. J. Acoust. Soc. Am. 2003, 114, 3087–3091. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Dellalana, L.E.; Chen, F.; Vain, A.; Gandelman, J.S.; Põldemaa, M.; Chen, H.; Tkaczyk, E.R. Reproducibility of the durometer and myoton devices for skin stiffness measurement in healthy subjects. Skin Res. Technol. 2019, 25, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Lee, H. The Measurement of Stiffness for Major Muscles with Shear Wave Elastography and Myoton: A Quantitative Analysis Study. Diagnostics 2021, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Poudrel, A.-S.; Bouffandeau, A.; Demeet, O.L.; Rosi, G.; Nguyen, V.-H.; Haiat, G. Characterization of the concentration of agar-based soft tissue mimicking phantoms by impact analysis. J. Mech. Behav. Biomed. Mater. 2024, 152, 106465. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Bosc, R.; Vayron, R.; Haiat, G. In vitro evaluation of the acetabular cup primary stability by impact analysis. J. Biomech. Eng. 2015, 137, 031011. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Bosc, R.; Meningaud, J.-P.; Hernigou, P.; Haiat, G. Assessing the Acetabular Cup Implant Primary Stability by Impact Analyses: A Cadaveric Study. PLoS ONE 2016, 11, e0166778. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Nguyen, V.-H.; Bosc, R.; Vayron, R.; Hernigou, P.; Naili, S.; Haiat, G. Finite element model of the impaction of a press-fitted acetabular cup. Med. Biol. Eng. Comput. 2017, 55, 781–791. [Google Scholar] [CrossRef]
- Albini Lomami, H.; Damour, C.; Rosi, G.; Poudrel, A.-S.; Dubory, A.; Flouzat-Lachaniette, C.-H.; Haiat, G. Ex vivo estimation of cementless femoral stem stability using an instrumented hammer. Clin. Biomech. 2020, 76, 105006. [Google Scholar] [CrossRef]
- Dubory, A.; Rosi, G.; Tijou, A.; Lomami, H.A.; Flouzat-Lachaniette, C.-H.; Haïat, G. A cadaveric validation of a method based on impact analysis to monitor the femoral stem insertion. J. Mech. Behav. Biomed. Mater. 2020, 103, 103535. [Google Scholar] [CrossRef] [PubMed]
- Tijou, A.; Rosi, G.; Vayron, R.; Lomami, H.A.; Hernigou, P.; Flouzat-Lachaniette, C.-H.; Haïat, G. Monitoring cementless femoral stem insertion by impact analyses: An in vitro study. J. Mech. Behav. Biomed. Mater. 2018, 88, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Rosi, G.; Bosc, R.; Haiat, G. Using an Impact Hammer to Estimate Elastic Modulus and Thickness of a Sample During an Osteotomy. J. Biomech. Eng. 2020, 142, 071009. [Google Scholar] [CrossRef]
- Bas Dit Nugues, M.; Rosi, G.; Hériveaux, Y.; Haïat, G. Using an Instrumented Hammer to Predict the Rupture of Bone Samples Subject to an Osteotomy. Sensors 2023, 23, 2304. [Google Scholar] [CrossRef] [PubMed]
- Lamassoure, L.; Giunta, J.; Rosi, G.; Poudrel, A.-S.; Meningaud, J.-P.; Bosc, R.; Haïat, G. Anatomical subject validation of an instrumented hammer using machine learning for the classification of osteotomy fracture in rhinoplasty. Med. Eng. Phys. 2021, 95, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lamassoure, L.; Giunta, J.; Rosi, G.; Poudrel, A.-S.; Bosc, R.; Haïat, G. Using an impact hammer to perform biomechanical measurements during osteotomies: Study of an animal model. Proc. Inst. Mech. Eng. H. 2021, 235, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Manickam, K.; Machireddy, R.R.; Seshadri, S. Characterization of biomechanical properties of agar based tissue mimicking phantoms for ultrasound stiffness imaging techniques. J. Mech. Behav. Biomed. Mater. 2014, 35, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Manickam, K.; Machireddy, R.R.; Seshadri, S. Study of ultrasound stiffness imaging methods using tissue mimicking phantoms. Ultrasonics 2014, 54, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Pretty, C.G.; Signal, M.K.; Haggers, M.; Zhou, C.; Chase, J.G. Mechanical behaviour of tissue mimicking breast phantom materials. Biomed. Phys. Eng. Express 2017, 3, 045010. [Google Scholar] [CrossRef]
- Hall, T.J.; Bilgen, M.; Insana, M.F.; Krouskop, T.A. Phantom materials for elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1997, 44, 1355–1365. [Google Scholar] [CrossRef]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Nayar, V.T.; Weiland, J.D.; Nelson, C.S.; Hodge, A.M. Elastic and viscoelastic characterization of agar. J. Mech. Behav. Biomed. Mater. 2012, 7, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of soft hydrated materials for application to vascular tissues. J. Biomed. Mater. Res. A 2004, 69, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, M.; Mannion, A.F. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef] [PubMed]
- John, A.J.U.K.; Galdo, F.D.; Gush, R.; Worsley, P.R. An evaluation of mechanical and biophysical skin parameters at different body locations. Skin Res. Technol. 2023, 29, e13292. [Google Scholar] [CrossRef] [PubMed]
- MyotonPro Digital Palpation Device User Manual. Available online: www.myoton.com (accessed on 30 November 2022).
- Menard, K.P.; Noah, M. Dynamic Mechanical Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Ewins, D.J. Modal Testing: Theory, Practice and Application, 2nd ed.; Research Studies Press Ltd.: Baldock, UK, 2000. [Google Scholar]
- Silva, J.M.M.; Maia, N.M.M.; Ribeiro, A.M.R. Cancellation of Mass-Loading Effects of Transducers and Evaluation of Unmeasured Frequency Response Functions. J. Sound Vib. 2000, 236, 761–779. [Google Scholar] [CrossRef]
- Cakar, O.; Sanliturk, K.Y. Elimination of transducer mass loading effects from frequency response functions. Mech. Syst. Signal Process. 2005, 19, 87–104. [Google Scholar] [CrossRef]
- Keswick, P.R.; Norton, M.P. A comparison of modal density measurement techniques. Appl. Acoust. 1987, 20, 137–153. [Google Scholar] [CrossRef]
- Rao, S.S. Vibration of Continuous Systems, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hamhaber, U.; Grieshaber, F.A.; Nagel, J.H.; Klose, U. Comparison of quantitative shear wave MR-elastography with mechanical compression tests. Magn. Reson. Med. 2003, 49, 71–77. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Singh, M.; Wu, C.; Liu, C.; Wang, S.; Idugboe, R.; Raghunathan, R.; Sudheendran, N.; Aglyamov, S.R.; et al. Quantitative methods for reconstructing tissue biomechanical properties in optical coherence elastography: A comparison study. Phys. Med. Biol. 2015, 60, 3531–3547. [Google Scholar] [CrossRef] [PubMed]
- Ringleb, S.I.; Chen, Q.; Lake, D.S.; Manduca, A.; Ehman, R.L.; An, K.-N. Quantitative shear wave magnetic resonance elastography: Comparison to a dynamic shear material test. Magn. Reson. Med. 2005, 53, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.; Schaefer, E.; Nair, K.; Kelly, J.; Masi, A. Repeatability, Reproducibility, and Calibration of the MyotonPro® on Tissue Mimicking Phantoms. American Society of Mechanical Engineers Digital Collection. In Proceedings of the ASME 2013 Summer Bioengineering Conference, Sunriver, OR, USA, 26–29 June 2013. [Google Scholar]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of tissue stiffness of the infraspinatus, erector spinae, and gastrocnemius muscle using ultrasound shear wave elastography and superficial mechanical deformation. J. Electromyogr. Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef] [PubMed]
- Rosicka, K.; Mierzejewska-Krzyżowska, B.; Mrówczyński, W. Comparison of different MyotonPRO probes for skin stiffness evaluation in young women. Skin Res. Technol. 2021, 27, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Junker, H.J.; Thumm, B.; Halvachizadeh, S.; Mazza, E. A quantitative comparison of devices for in vivo biomechanical characterization of human Skin. Mech. Soft Mater. 2023, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Mathieu, V.; Michel, A.; Haïat, G. Assessment of in vitro dental implant primary stability using an ultrasonic method. Ultrasound Med. Biol. 2014, 40, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Nguyen, V.-H.; Lecuelle, B.; Haiat, G. Evaluation of dental implant stability in bone phantoms: Comparison between a quantitative ultrasound technique and resonance frequency analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Klingler, W.; Schleip, R. Assessing reliability and validity of different stiffness measurement tools on a multi-layered phantom tissue model. Sci. Rep. 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Kissin, E.Y.; Schiller, A.M.; Gelbard, R.B.; Anderson, J.J.; Falanga, V.; Simms, R.W.; Korn, J.H.; Merkel, P.A. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006, 55, 603–609. [Google Scholar] [CrossRef]
- Koch, V.; Wilke, J. Reliability of a New Indentometer Device for Measuring Myofascial Tissue Stiffness. J. Clin. Med. 2022, 11, 5194. [Google Scholar] [CrossRef] [PubMed]
| Interface Distance Estimation Error (mm) | Axial Resolution | Lateral Resolution | ||
|---|---|---|---|---|
| “Rigid on Soft” | “Soft on Rigid” | |||
| Method | ||||
| , impact-based analysis method (IBAM) | (ms/mm) | −0.13 | 0.15 | 0.14 |
(ms) | 0.03 | 0.03 | 0.02 | |
(mm) | 0.22 | 0.17 | 0.15 | |
| , MyotonPro | (N/m × mm) | 56.51 | −65.77 | −62.17 |
(N/m) | 25.33 | 29.39 | 49.21 | |
(mm) | 0.45 | 0.45 | 0.79 | |
| Experimental Data 1 | Agar Mass Concentration | ||||
|---|---|---|---|---|---|
| ms/kPa | ms | kPa | |||
| , impact-based analysis method (IBAM) | 1% to 2% | −0.013 | 0.03 | 2.22 | |
| 1% to 5% | −0.003 | 0.02 | 5.42 | ||
| N/m × kPa | N/m | kPa | |||
| , MyotonPro | 1% to 2% | 4.73 | 10.86 | 2.29 | |
| 1% to 5% | 2.94 | 31.28 | 10.65 | ||
| - | kPa | kPa | |||
| Custom vibration-based set-up | 1% to 2% | 1 | 1.70 | 1.70 | |
| 1% to 5% | 1 | 5.97 | 5.97 | ||
| Literature data 2 | agar mass concentration | ||||
| - | kPa | kPa | |||
| Elastography | DMA [44] | 1% to 5% | 1 | 5.30 | 5.30 |
| MRE [44] | 1% to 5% | 0.95 | 10.74 | 11.27 | |
| MRE [42] | 1% to 2% | 0.92 | 7.98 | 8.71 | |
| OCE [43] | 1% to 2% | 0.78 | 3.86 | 4.94 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouffandeau, A.; Poudrel, A.-S.; Brossier, C.; Rosi, G.; Nguyen, V.-H.; Flouzat-Lachaniette, C.-H.; Meningaud, J.-P.; Haïat, G. Assessment of the Mechanical Properties of Soft Tissue Phantoms Using Impact Analysis. Sensors 2025, 25, 1344. https://doi.org/10.3390/s25051344
Bouffandeau A, Poudrel A-S, Brossier C, Rosi G, Nguyen V-H, Flouzat-Lachaniette C-H, Meningaud J-P, Haïat G. Assessment of the Mechanical Properties of Soft Tissue Phantoms Using Impact Analysis. Sensors. 2025; 25(5):1344. https://doi.org/10.3390/s25051344
Chicago/Turabian StyleBouffandeau, Arthur, Anne-Sophie Poudrel, Chloé Brossier, Giuseppe Rosi, Vu-Hieu Nguyen, Charles-Henri Flouzat-Lachaniette, Jean-Paul Meningaud, and Guillaume Haïat. 2025. "Assessment of the Mechanical Properties of Soft Tissue Phantoms Using Impact Analysis" Sensors 25, no. 5: 1344. https://doi.org/10.3390/s25051344
APA StyleBouffandeau, A., Poudrel, A.-S., Brossier, C., Rosi, G., Nguyen, V.-H., Flouzat-Lachaniette, C.-H., Meningaud, J.-P., & Haïat, G. (2025). Assessment of the Mechanical Properties of Soft Tissue Phantoms Using Impact Analysis. Sensors, 25(5), 1344. https://doi.org/10.3390/s25051344









