Novel Wearable-Based Real-Time Temperature Monitoring in Hospitals for Febrile Adverse Events in Patients with Cancer: A Prospective Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Sample Size
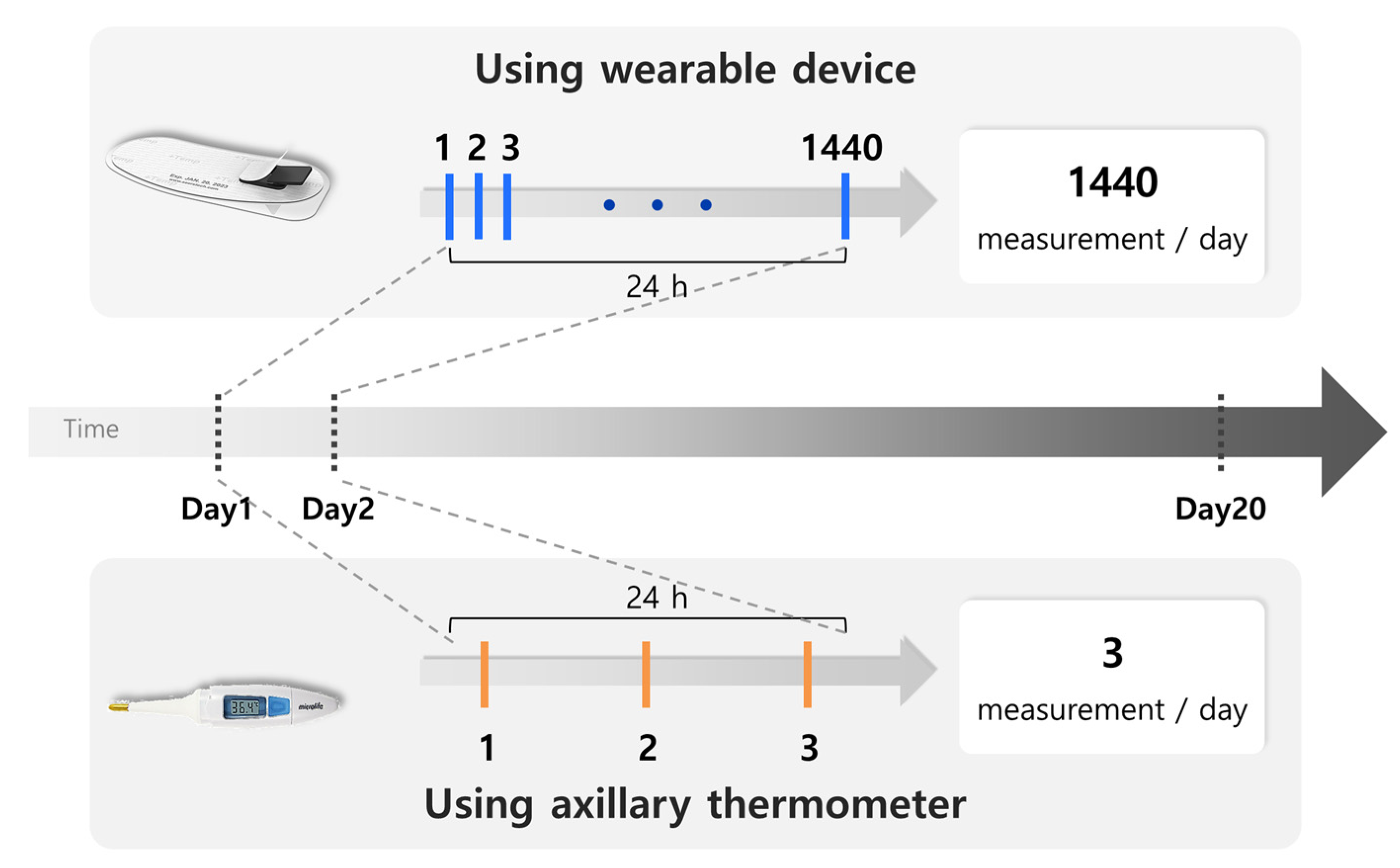
2.3. Data Preparation
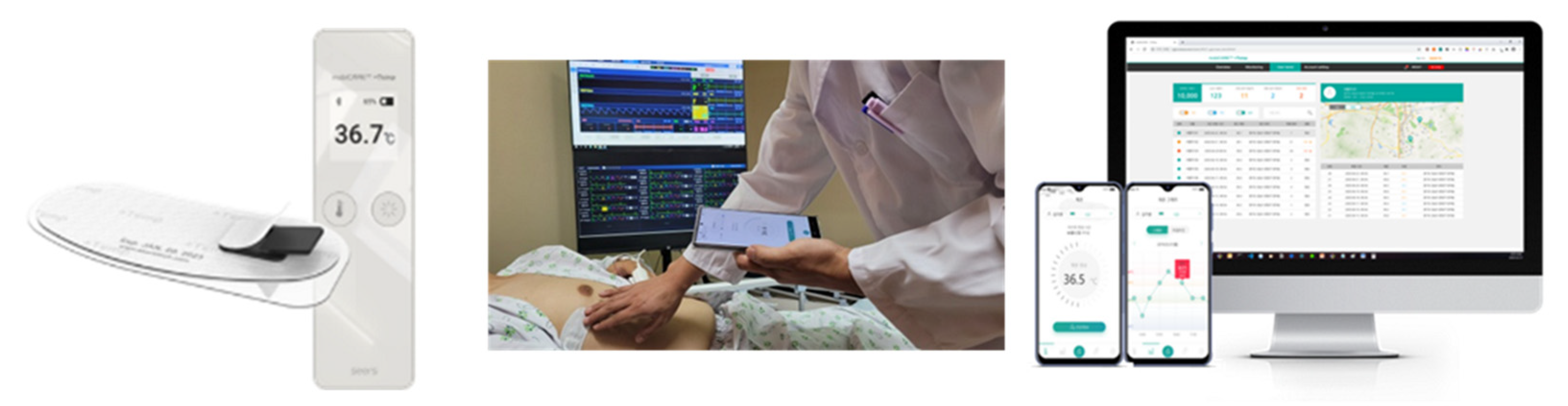
2.4. RBT Monitoring System
2.5. Axillary Thermometer
2.6. Evaluation
2.7. Statistical Analysis
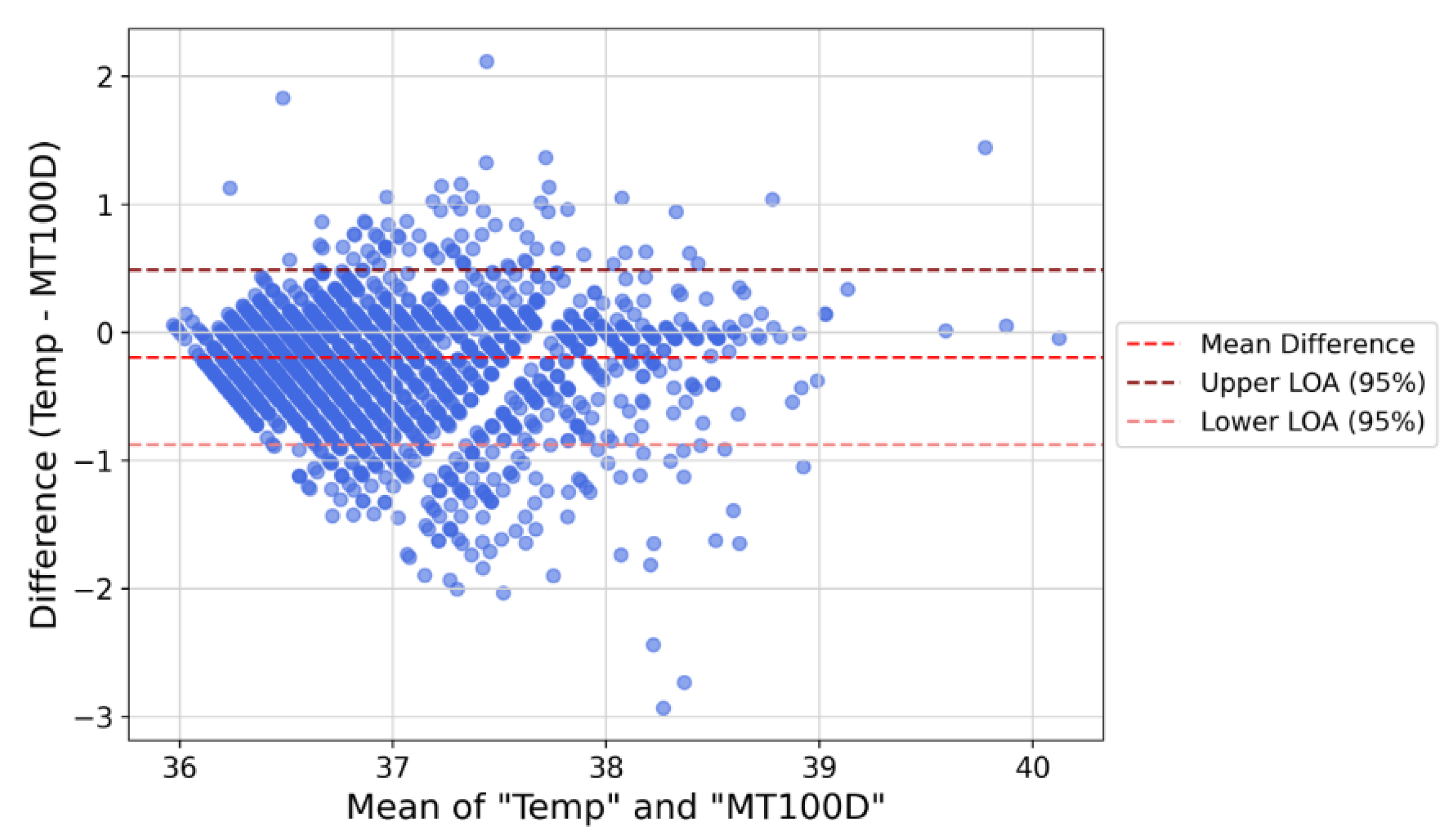
2.8. Agreement Evaluation
2.9. Fever Detection
2.10. Assessment of Satisfaction and Usability
3. Results
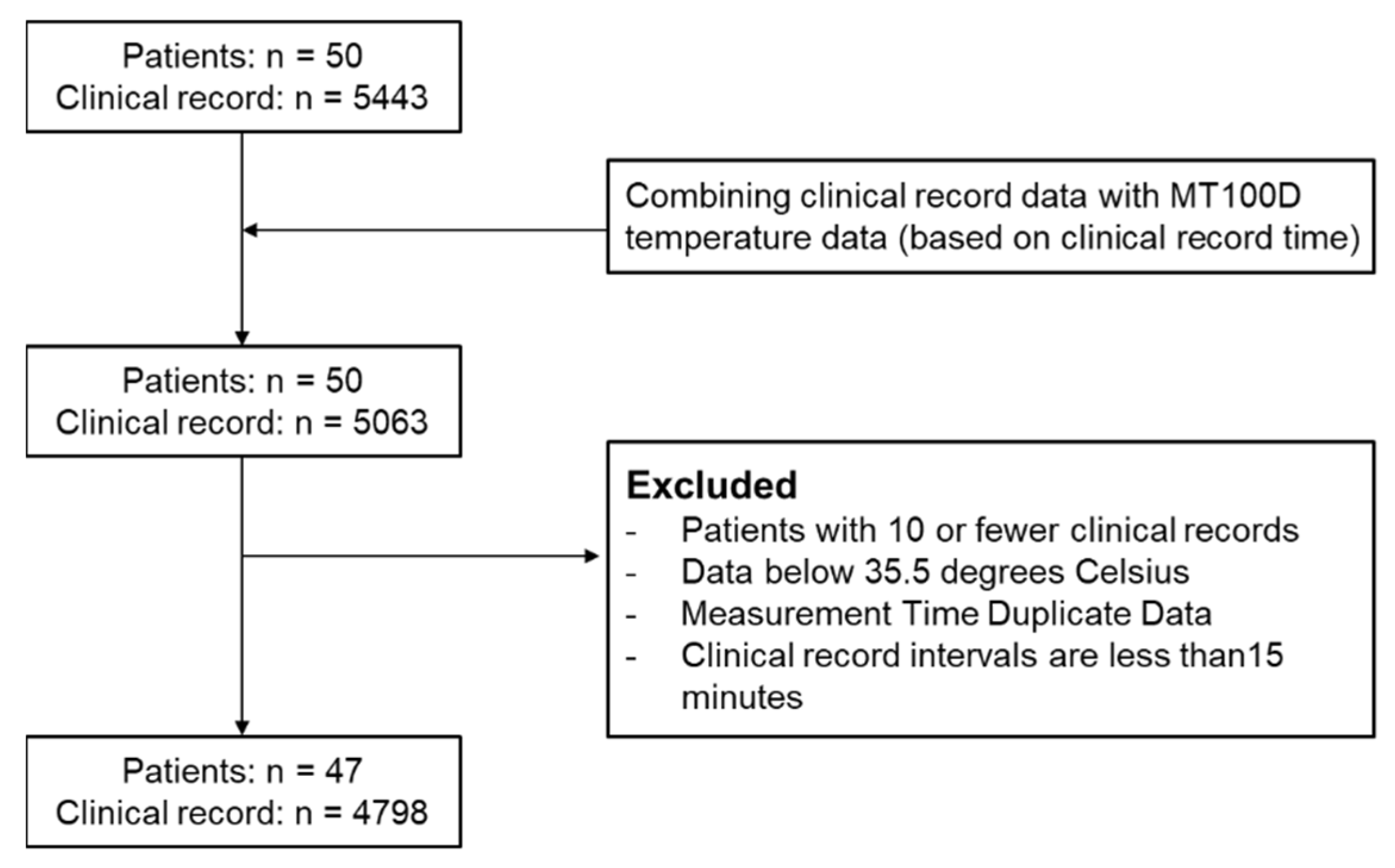
3.1. Patient Enrollment and Data Inclusion
3.2. Patient Characteristics
3.3. Body Temperature Agreement Performance
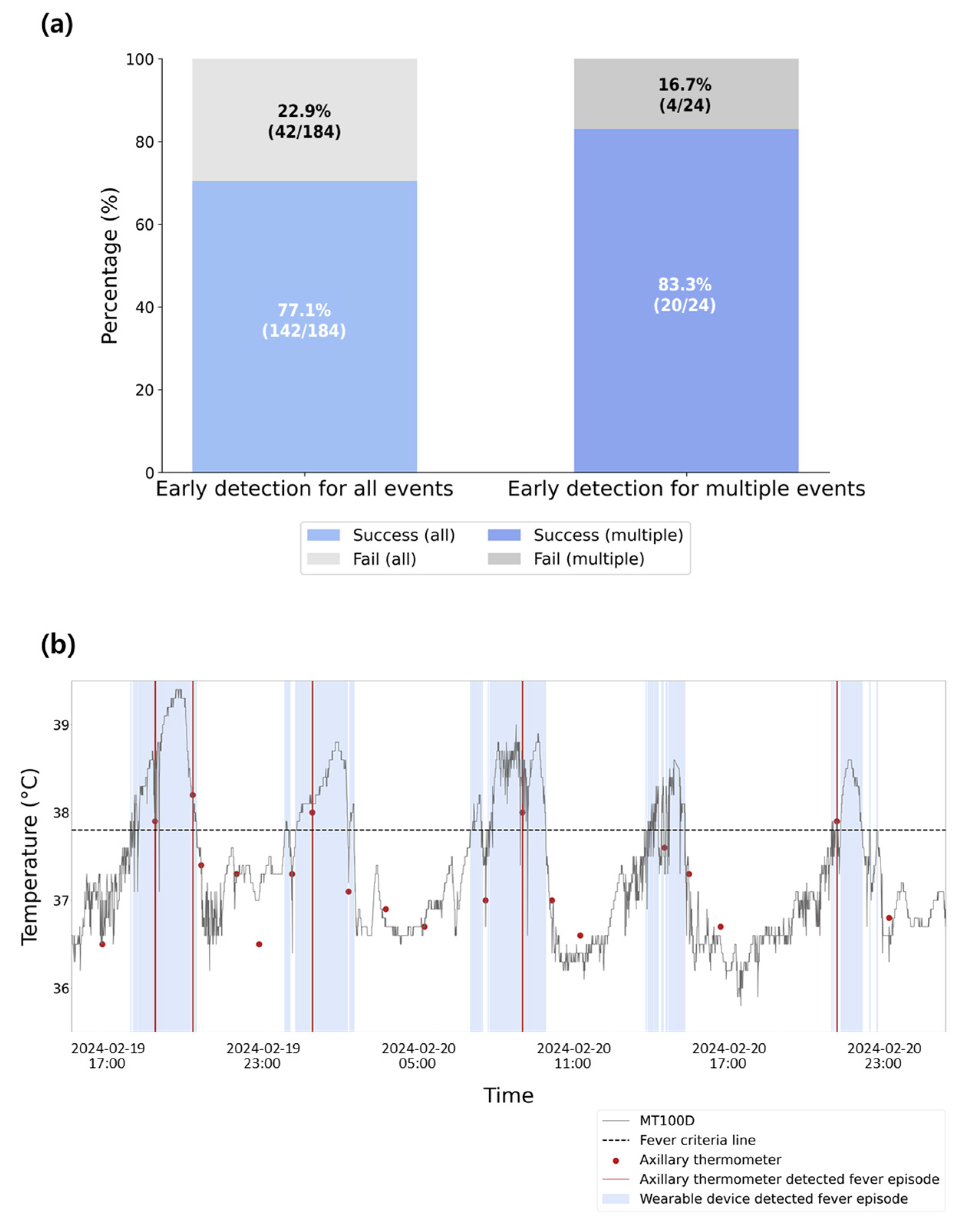
3.4. Fever Detection Performance
3.5. Adverse Event Analysis
3.6. Satisfaction and Usability Results
4. Discussion
4.1. Clinical Significance
4.2. Strengths
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| ASCT | Autologous stem cell transplantation |
| AUROC | Area under the receiver operating characteristic curve |
| CAR-T | Chimeric antigen receptor T-cell |
| CI | Confidence interval |
| CTCAE | Common terminology criteria for adverse events |
| EMR | Electronic medical record |
| FN | Febrile neutropenia |
| HCT | Hematopoietic stem cell transplant |
| ICC | Intraclass correlation coefficient |
| LoA | Limits of agreement |
| MDS | Myelodysplastic syndrome |
| RBT | Real-time body temperature |
| SD | Standard deviation |
| STARD | Standards for reporting of diagnostic accuracy studies |
| WD | Wearable device |
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Bodey, G.P.; Buckley, M.; Sathe, Y.S.; Freireich, E.J. Fever and infection in leukemic patients: A study of 494 consecutive patients. Cancer 1978, 41, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Michels, S.L.; Reynolds, M.W.; Barron, R.L.; Tomic, K.S.; Yu, J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 2010, 116, 5555–5563. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Du, X.; Bishnoi, R.; Bian, J. Risk of mortality in adult cancer febrile neutropenia patients with a machine learning approach. J. Clin. Oncol. 2018, 36, e13562. [Google Scholar] [CrossRef]
- Tai, E.; Guy, G.P., Jr.; Dunbar, A.; Richardson, L.C. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J. Oncol. Pract. 2017, 13, e552–e561. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Swaminathan, S.; Angarone, M.; Blouin, G.; Camins, B.C.; Casper, C.; Cooper, B.; Dubberke, E.R.; Engemann, A.M.; Freifeld, A.G.; et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 882–913. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.M.; Santolaya, M.; et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Phillips, R.S. Updated systematic review and meta-analysis of the predictive value of serum biomarkers in the assessment and management of fever during neutropenia in children with cancer. Pediatr. Blood Cancer 2019, 66, e27887. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.L.; Croop, J.; Carroll, A.E. Fever and neutropenia hospital discharges in children with cancer: A 2012 update. Pediatr. Hematol. Oncol. 2016, 33, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Chang, M.L.; Hanauer, D.A.; Shaffer-Hartman, J.; Teitelbaum, D.; Lewis, I.; Blackwood, A.; Akcasu, N.; Steel, J.; Christensen, J.; et al. Rapid reduction of central line infections in hospitalized pediatric oncology patients through simple quality improvement methods. Pediatr. Blood Cancer 2013, 60, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Choi, E.K.; Lee, S.R.; Oh, S.; Song, H.S.; Lee, Y.S.; Han, S.J.; Lim, H.E. Comparison of novel telemonitoring system using the single-lead electrocardiogram patch with conventional telemetry system. Korean Circ. J. 2024, 54, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Kuang, R.; Wang, Z.; Ma, L.; Wang, H.; Chen, Q.; Junior, A.L.; Kumar, S.; Li, X.; Marques, C.; Min, R. Smart photonic wristband for pulse wave monitoring. Opto-Electron. Sci. 2024, 3, 240009. [Google Scholar] [CrossRef]
- Verma, N.; Haji-Abolhassani, I.; Ganesh, S.; Vera-Aguilera, J.; Paludo, J.; Heitz, R.; Markovic, S.N.; Kulig, K.; Ghoreyshi, A. A novel wearable device for continuous temperature monitoring and fever detection. IEEE J. Transl. Eng. Health Med. 2021, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flora, C.; Tyler, J.; Mayer, C.; Warner, D.E.; Khan, S.N.; Gupta, V.; Lindstrom, R.; Mazzoli, A.; Rozwadowski, M.; Braun, T.M.; et al. High-frequency temperature monitoring for early detection of febrile adverse events in patients with cancer. Cancer Cell 2021, 39, 1167–1168. [Google Scholar] [CrossRef] [PubMed]
- Nessle, C.N.; Flora, C.; Sandford, E.; Choi, S.W.; Tewari, M. High-frequency temperature monitoring at home using a wearable device: A case series of early fever detection and antibiotic administration for febrile neutropenia with bacteremia. Pediatr. Blood Cancer 2022, 69, e29835. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Smarr, B.L.; Aschbacher, K.; Fisher, S.M.; Chowdhary, A.; Dilchert, S.; Puldon, K.; Rao, A.; Hecht, F.M.; Mason, A.E. Feasibility of continuous fever monitoring using wearable devices. Sci. Rep. 2020, 10, 21640. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, P.; Varthya, S.B. ISO 10993: Biological evaluation of medical devices. In Medical Device Guidelines and Regulations Handbook; Springer International Publishing: Cham, Switzerland, 2022; pp. 163–187. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bush, L.M. Fever. Merck Manual Professional Edition. 2024. Available online: https://www.merckmanuals.com/professional/infectious-diseases/biology-of-infectious-disease/fever (accessed on 1 November 2024).
- De Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients (Na = 47) |
|---|---|
| Age, median (range) | 52 (6–88) |
| Male sex, n (%) | 23 (46) |
| Duration of hospitalization, days (range) | 14 (6–24) |
| Number of patients with febrile episodes, n (%) | 29 (58) |
| Disease, n (%) | |
| Acute myeloid leukemia | 36 (76.6) |
| Acute lymphoblastic leukemia | 6 (12.8) |
| Myelodysplastic syndrome | 1 (2.1) |
| Severe aplastic anemia | 1 (2.1) |
| Brain tumor | 3 (6.4) |
| Category | Performance (%) |
|---|---|
| Fever incidence rates among patients | |
| Fever incidence among hospitalized patients | 53.33 (24/45) |
| Incidence of fever requiring medication | 82.07 (151/184) |
| Static fever detection | |
| Sensitivity | 81.5 (95% CI b: 76.8–85.6) |
| Specificity | 96.3 (95% CI: 95.7–96.8) |
| AUROC a | 96.6 (95% CI: 95.6–97.4) |
| Question | Mean | Standard Deviation | 95% CI a |
|---|---|---|---|
| (1) Applying the patch is more convenient than taking a traditional temperature measurement. | 4.74 | 0.65 | ±0.19 |
| (2) If I ever need to take the body temperature in the future, I would use this patch again. | 4.65 | 0.82 | ±0.24 |
| (3) I felt a foreign body sensation while applying the patch. | 4.63 | 0.90 | ±0.26 |
| (4) The area where the patch was applied was itchy and had skin problems. | 4.76 | 0.77 | ±0.22 |
| (5) I felt like I wanted to take the patch off while I was putting it on. | 4.57 | 1.05 | ±0.30 |
| (6) It is easy to remove the patch from the skin. | 4.65 | 0.71 | ±0.20 |
| (7) No skin problems occurred after removing the patch. | 4.80 | 0.75 | ±0.22 |
| Question | Average | Standard Deviation | 95% CI a |
|---|---|---|---|
| (1) It is easy to confirm the precautions and warnings regarding the use of the product. | 3.58 | 0.77 | ±0.35 |
| (2) The instructions for the thermometer are easy to understand. | 3.74 | 0.87 | ±0.39 |
| (3) The product is easy to use overall. | 3.74 | 0.87 | ±0.39 |
| (4) It is easy to combine the core module with the product. | 3.84 | 1.01 | ±0.46 |
| (5) It is easy to remove the product from the skin. | 4.11 | 0.94 | ±0.42 |
| (6) It is easy to separate the product from the core module. | 4.00 | 0.94 | ±0.42 |
| (7) I frequently checked the monitoring webpage. | 3.74 | 0.93 | ±0.42 |
| (8) It is easy to check measurement values on the monitoring webpage. | 2.89 | 1.05 | ±0.47 |
| (9) It is easy to check the fever alarm on the monitoring webpage. | 3.16 | 0.90 | ±0.40 |
| (10) I intend to continue using the MT100D for body temperature measurement even after the study is over. | 3.68 | 0.89 | ±0.40 |
| (11) I think MT100D is useful for measuring body temperature. | 3.53 | 1.02 | ±0.46 |
| (12) I expect that the MT100D will allow me to obtain body temperature information quickly. | 3.68 | 0.95 | ±0.43 |
| (13) Learning how to use the MT100D is easy. | 3.68 | 0.82 | ±0.37 |
| (14) I can use the MT100D skillfully. | 3.63 | 1.12 | ±0.50 |
| (15) Using the MT100D is easy and simple. | 3.68 | 1.11 | ±0.50 |
| (16) I am satisfied with the MT100D overall. | 3.47 | 1.07 | ±0.48 |
| (17) I would recommend the MT100D to people around me. | 3.53 | 1.02 | ±0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.K.; Ahn, S.-Y.; Lee, S.J.; Song, C.-B.; Hong, Y.; Kim, R.; Baek, H.J.; Kook, H.; Kim, M.; Song, G.-Y.; et al. Novel Wearable-Based Real-Time Temperature Monitoring in Hospitals for Febrile Adverse Events in Patients with Cancer: A Prospective Feasibility Study. Sensors 2025, 25, 7111. https://doi.org/10.3390/s25237111
Kim YK, Ahn S-Y, Lee SJ, Song C-B, Hong Y, Kim R, Baek HJ, Kook H, Kim M, Song G-Y, et al. Novel Wearable-Based Real-Time Temperature Monitoring in Hospitals for Febrile Adverse Events in Patients with Cancer: A Prospective Feasibility Study. Sensors. 2025; 25(23):7111. https://doi.org/10.3390/s25237111
Chicago/Turabian StyleKim, Yun Kwan, Seo-Yeon Ahn, Sun Jung Lee, Chae-Bin Song, YeWon Hong, Ray Kim, Hee Jo Baek, Hoon Kook, Mihee Kim, Ga-Young Song, and et al. 2025. "Novel Wearable-Based Real-Time Temperature Monitoring in Hospitals for Febrile Adverse Events in Patients with Cancer: A Prospective Feasibility Study" Sensors 25, no. 23: 7111. https://doi.org/10.3390/s25237111
APA StyleKim, Y. K., Ahn, S.-Y., Lee, S. J., Song, C.-B., Hong, Y., Kim, R., Baek, H. J., Kook, H., Kim, M., Song, G.-Y., Jang, H. C., Jung, S.-H., Yang, D.-H., Lee, J.-J., Kim, H.-J., Kim, G. C., Kim, H. H., Lee, Y.-S., & Ahn, J.-S. (2025). Novel Wearable-Based Real-Time Temperature Monitoring in Hospitals for Febrile Adverse Events in Patients with Cancer: A Prospective Feasibility Study. Sensors, 25(23), 7111. https://doi.org/10.3390/s25237111






