Acute Effects of Complex Hand Proprioceptive Task on Low-Frequency Hand Rest Tremor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.3.1. Anthropometric Measurements
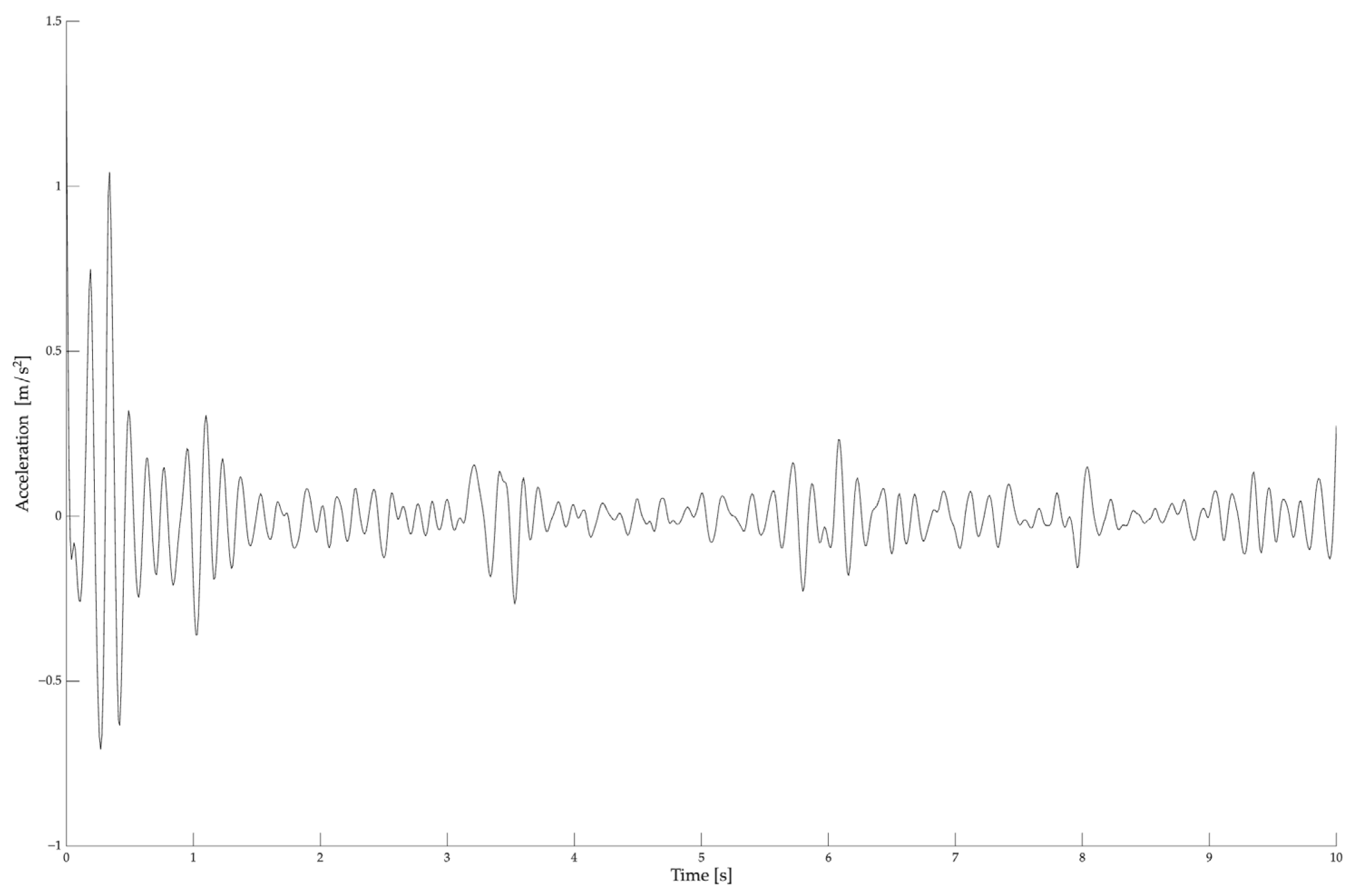
2.3.2. Hand Rest Tremor Measurement
2.3.3. Complex Hand Proprioceptive Task
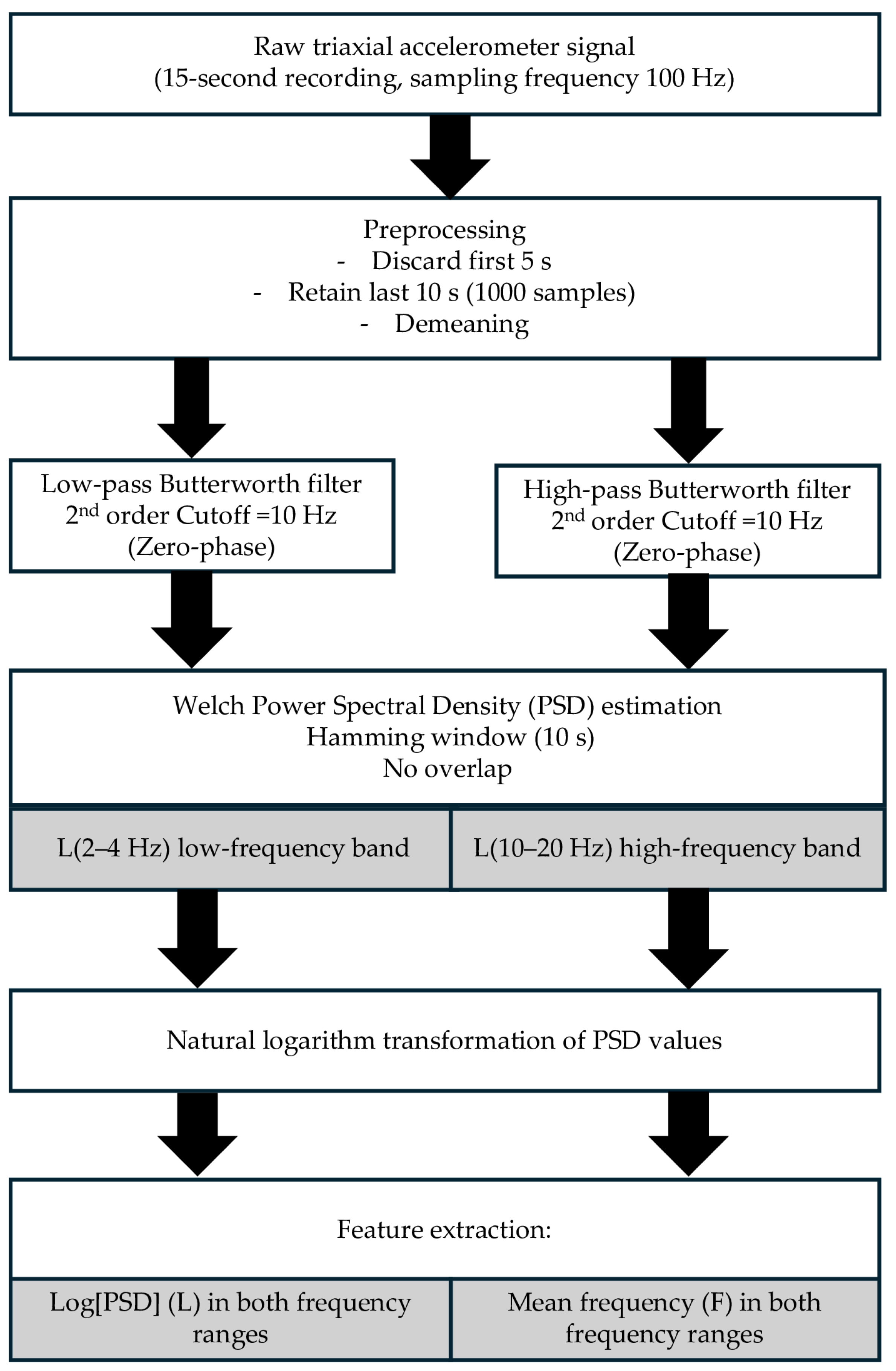
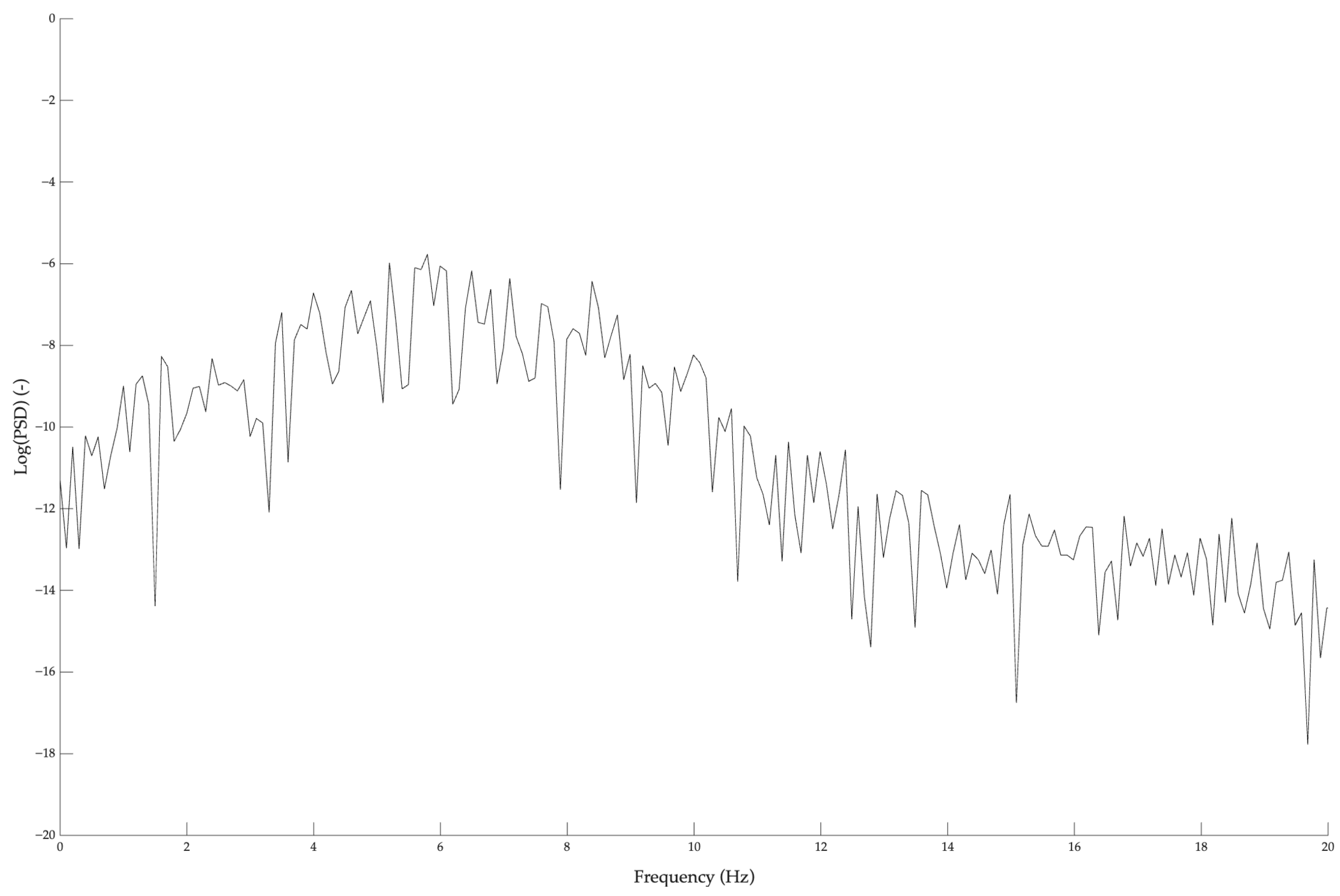
2.3.4. Frequency Analysis
- − The logarithmic amplitude indicator, L(f1, f2), was computed as the mean of the natural logarithm of the PSD values within each predefined frequency band of interest (2–4 Hz and 10–20 Hz), as described by Gajewski and colleagues [34]:
- − The mean frequency F(f1, f2) was calculated as the mean frequency weighted by the power components within each band:
2.3.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| PSD | Power Spectral Density |
| Log[PSD] | Log-transformed Spectral Density |
| WB | Wobble Board |
| IMUs | Inertial Measurement Units |
| PRE | Before |
| POST | After |
| BMI | Body Mass Index |
| HP | High-Pass Single-Pole IIR Filter |
| LP | Low-Pass Single-Pole IIR Filter |
| ANOVA | Analysis of Variance |
| FIR | Finite Impulse Response |
| EMG | Electromyography |
References
- Papale, O.; Rocco, F.D.; Festino, E.; Gammino, V.; Cortis, C.; Fusco, A. Do Hand Exercises Influence Physiological Hand Tremor? An Observational Cohort Study on Healthy Young Adults. Appl. Sci. Switz. 2024, 14, 4467. [Google Scholar] [CrossRef]
- Elble, R.J. Characteristics of Physiologic Tremor in Young and Elderly Adults. Clin. Neurophysiol. 2003, 114, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, A.; Gajewski, J.; Mazur–Różycka, J. Changes in Physiological Tremor Resulting from Sleep Deprivation under Conditions of Increasing Fatigue during Prolonged Military Training. Biol. Sport 2014, 31, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, J.M.; Uehli, K.; Rozitis, A.I. Muscle Fibre Recruitment Can Respond to the Mechanics of the Muscle Contraction. J. R. Soc. Interface 2006, 3, 533–544. [Google Scholar] [CrossRef]
- Kuliś, S.; Kłobuchowski, W.; Skorulski, M.; Pietraszewski, P.; Callegari, B. Upper Limb Tremor Variability in Elite Sport Dancers: The Influence of Competitive Simulation. Biomed. Hum. Kinet. 2025, 17, 219–228. [Google Scholar] [CrossRef]
- Heldman, D.A.; Jankovic, J.; Vaillancourt, D.E.; Prodoehl, J.; Elble, R.J.; Giuffrida, J.P. Essential Tremor Quantification during Activities of Daily Living. Park. Relat. Disord. 2011, 17, 537–542. [Google Scholar] [CrossRef]
- Vance, N.E.; Ulanowski, E.A.; Danzl, M.M. Yoga Led by a Physical Therapist for Individuals with Essential Tremor: An Explorative Pilot Study. Complement. Ther. Clin. Pract. 2019, 34, 17–22. [Google Scholar] [CrossRef]
- Norman, K.E.; Héroux, M.E. Measures of Fine Motor Skills in People with Tremor Disorders: Appraisal and Interpretation. Front. Neurol. 2013, 4, 50. [Google Scholar] [CrossRef]
- Verstynen, T.; Diedrichsen, J.; Albert, N.; Aparicio, P.; Ivry, R.B. Ipsilateral Motor Cortex Activity during Unimanual Hand Movements Relates to Task Complexity. J. Neurophysiol. 2005, 93, 1209–1222. [Google Scholar] [CrossRef]
- Fusco, A.; Giancotti, G.F.; Fuchs, P.X.; Wagner, H.; Varalda, C.; Cortis, C. Wobble Board Balance Assessment in Subjects with Chronic Ankle Instability. Gait Posture 2019, 68, 352–356. [Google Scholar] [CrossRef]
- Elphinston, J. Stability, Sport, and Performance Movement: Great Technique Without Injury; North atlantic books; Lotus Publishing Limited: Punjab, India, 2008; ISBN 1-55643-746-3. [Google Scholar]
- Weizman, Y.; Tirosh, O.; Fuss, F.K.; Tan, A.M.; Rutz, E. Recent State of Wearable IMU Sensors Use in People Living with Spasticity: A Systematic Review. Sensors 2022, 22, 1791. [Google Scholar] [CrossRef] [PubMed]
- Straczkiewicz, M.; James, P.; Onnela, J.-P. A Systematic Review of Smartphone-Based Human Activity Recognition Methods for Health Research. Npj Digit. Med. 2021, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- De Maio, M.; Castellani, L.; Cugusi, L.; Cortis, C.; Fusco, A. The Effect of a Combined Exercise Program on Postural Control and Fine Motor Skills in Parkinson’s Disease: Study Design. Int. J. Environ. Res. Public. Health 2022, 19, 15216. [Google Scholar] [CrossRef]
- De Maio, M.; Cortis, C.; Iannaccone, A.; Da Silva, R.A.; Fusco, A. Association between Anthropometric Variables, Sex, and Visual Biofeedback in Dynamic Postural Control Assessed on a Computerized Wobble Board. Appl. Sci. 2021, 11, 8370. [Google Scholar] [CrossRef]
- Morrison, S.; Sosnoff, J.J. The Impact of Localized Fatigue on Contralateral Tremor and Muscle Activity Is Exacerbated by Standing Posture. J. Electromyogr. Kinesiol. 2010, 20, 1211–1218. [Google Scholar] [CrossRef]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends Supporting the In-Field Use of Wearable Inertial Sensors for Sport Performance Evaluation: A Systematic Review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef]
- Sequeira, G.; Keogh, J.W.; Kavanagh, J.J. Resistance Training Can Improve Fine Manual Dexterity in Essential Tremor Patients: A Preliminary Study. Arch. Phys. Med. Rehabil. 2012, 93, 1466–1468. [Google Scholar] [CrossRef]
- Kadkhodaie, M.; Sharifnezhad, A.; Ebadi, S.; Marzban, S.; Habibi, S.A.; Ghaffari, A.; Forogh, B. Effect of Eccentric-Based Rehabilitation on Hand Tremor Intensity in Parkinson Disease. Neurol. Sci. 2020, 41, 637–643. [Google Scholar] [CrossRef]
- Pathare, S.D. A Comparative Study of Eye Hand Coordination among Games Players. Int. J. Phys. Educ. Sports Health IJPESH 2016, 3, 382–383. [Google Scholar]
- Szturm, T.; Peters, J.F.; Otto, C.; Kapadia, N.; Desai, A. Task-Specific Rehabilitation of Finger-Hand Function Using Interactive Computer Gaming. Arch. Phys. Med. Rehabil. 2008, 89, 2213–2217. [Google Scholar] [CrossRef]
- Polsley, S.; Powell, L.; Kim, H.-H.; Thomas, X.; Liew, J.; Hammond, T. Detecting Children’s Fine Motor Skill Development Using Machine Learning. Int. J. Artif. Intell. Educ. 2022, 32, 991–1024. [Google Scholar] [CrossRef]
- Caramia, S.; Gill, A.; Ohl, A.; Schelly, D. Fine Motor Activities in Elementary School Children: A Replication Study. Am. J. Occup. Ther. 2020, 74, 7402345010p1–7402345010p7. [Google Scholar] [CrossRef] [PubMed]
- Noritz, G.H.; Murphy, N.A.; Neuromotor Screening Expert Panel; Murphy, N.A.; Hagan, J.F.; Lipkin, P.H.; Macias, M.M.; Navsaria, D.; Noritz, G.H.; Peacock, G.; et al. Motor Delays: Early Identification and Evaluation. Pediatrics 2013, 131, e2016–e2027. [Google Scholar] [CrossRef] [PubMed]
- Lakie, M.; Walsh, E.G.; Arblaster, L.A.; Villagra, F.; Roberts, R.C. Limb Temperature and Human Tremors. J. Neurol. Neurosurg. Psychiatry 1994, 57, 35. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Wedderburn-Bisshop, J.; Keogh, J.W.L. Resistance Training Reduces Force Tremor and Improves Manual Dexterity in Older Individuals with Essential Tremor. J. Mot. Behav. 2016, 48, 20–30. [Google Scholar] [CrossRef]
- Amato, A.; Giustino, V.; Patti, A.; Proia, P.; Trivic, T.; Drid, P.; Obradovic, A.; Manojlovic, M.; Mondoni, M.; Paoli, A.; et al. Young Basketball Players Have Better Manual Dexterity Performance than Sportsmen and Non-Sportsmen of the Same Age: A Cross-Sectional Study. Sci. Rep. 2023, 13, 20953. [Google Scholar] [CrossRef]
- Pillitteri, G.; Thomas, E.; Battaglia, G.; Navarra, G.A.; Scardina, A.; Gammino, V.; Ricchiari, D.; Bellafiore, M. Validity and Reliability of an Inertial Sensor Device for Specific Running Patterns in Soccer. Sensors 2021, 21, 7255. [Google Scholar] [CrossRef]
- Fusco, A.; Giancotti, G.F.; Fuchs, P.X.; Wagner, H.; Varalda, C.; Capranica, L.; Cortis, C. Dynamic Balance Evaluation: Reliability and Validity of a Computerized Wobble Board. J. Strength Cond. Res. 2020, 34, 1709–1715. [Google Scholar] [CrossRef]
- Sutradhar, S.R.; Sayadat, N.; Rahman, A.; Munira, S.; Haque, A.K.M.F.; Sakib, S.N. IIR Based Digital Filter Design and Performance Analysis. In Proceedings of the 2017 2nd International Conference on Telecommunication and Networks (TEL-NET), Noida, India, 10–11 August 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Low-Pass Single-Pole IIR Filter|TomRoelandts.Com. Available online: https://tomroelandts.com/articles/low-pass-single-pole-iir-filter (accessed on 2 September 2025).
- Skogstad, S. Designing Digital IIR Low-Pass Differentiators with Multi-Objective Optimization. In Proceedings of the 2012 IEEE 11th International Conference on Signal Processing, Beijing, China, 21–25 October 2012. [Google Scholar] [CrossRef]
- Jwo, D.-J.; Chang, W.-Y.; Wu, I.-H. Windowing Techniques, the Welch Method for Improvement of Power Spectrum Estimation. Comput. Mater. Contin. 2021, 67, 3983–4003. [Google Scholar] [CrossRef]
- Gajewski, J.; Mazur-Różycka, J.; Łach, P.; Różycki, S.; Żmijewski, P.; Buśko, K.; Michalski, R. Changes of Physiological Tremor Following Maximum Intensity Exercise in Male and Female Young Swimmers. Hum. Mov. 2018, 16, 214–220. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kuliś, S.; Pietraszewski, P.; Callegari, B. Characteristics of Post-Exercise Lower Limb Muscle Tremor Among Speed Skaters. Sensors 2025, 25, 4301. [Google Scholar] [CrossRef]
- Lippold, O. The Tremor in Fatigue. In Ciba Foundation Symposium 82—Human Muscle Fatigue: Physiological Mechanisms; Novartis Foundation Symposia; Wiley: London, UK, 1981; pp. 234–248. ISBN 978-0-470-71542-0. [Google Scholar]
- Burne, J.A. Reflex Origin of Parkinsonian Tremor. Exp. Neurol. 1987, 97, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, C.; Berger, T.; Brandt, G.A.; Strelow, J.N.; Jergas, H.; Baldermann, J.C.; Visser-Vandewalle, V.; Fink, G.R.; Barbe, M.T.; Petry-Schmelzer, J.N.; et al. Accelerometric Classification of Resting and Postural Tremor Amplitude. Sensors 2023, 23, 8621. [Google Scholar] [CrossRef] [PubMed]
- Markose, N.R.; Moyya, P.D.; Asaithambi, M. Asaithambi Analysis of Tremors in Parkinson’s Disease Using Accelerometer. In Proceedings of the 2021 Seventh International conference on Bio Signals, Images, and Instrumentation (ICBSII), Chennai, India, 25 March 2021; pp. 1–5. [Google Scholar]
- Keogh, J.W.L.; O’Reilly, S.; O’Brien, E.; Morrison, S.; Kavanagh, J.J. Can Resistance Training Improve Upper Limb Postural Tremor, Force Steadiness and Dexterity in Older Adults? A Systematic Review. Sports Med. 2019, 49, 1199–1216. [Google Scholar] [CrossRef]
- Santos, P.S.A.; Santos, E.G.R.; Monteiro, L.C.P.; Santos-Lobato, B.L.; Pinto, G.H.L.; Belgamo, A.; Cabral, A.S.; De Athayde Costa E Silva, A.; Callegari, B.; Souza, G.S. The Hand Tremor Spectrum Is Modified by the Inertial Sensor Mass during Lightweight Wearable and Smartphone-Based Assessment in Healthy Young Subjects. Sci. Rep. 2022, 12, 16808. [Google Scholar] [CrossRef]
- Papale, O.; Festino, E.; Di Rocco, F.; De Maio, M.; Foster, C.; Cortis, C.; Fusco, A. Eating Right, Sleeping Tight? A Cross-Sectional Study on the Student-Athlete Paradox for Diet and Sleep Behaviors. Nutrients 2025, 17, 2946. [Google Scholar] [CrossRef]
- Mah, C.D.; Kezirian, E.J.; Marcello, B.M.; Dement, W.C. Poor Sleep Quality and Insufficient Sleep of a Collegiate Student-Athlete Population. Sleep Health 2018, 4, 251–257. [Google Scholar] [CrossRef]
| Male n = 29 | Female n = 21 | Total n = 50 | |
|---|---|---|---|
| Age (years) | 24.3 ± 1.1 | 26.1 ± 3.5 | 25.0 ± 2.5 |
| Height (m) | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| Weight (kg) | 74.3 ± 9.7 | 56.6 ± 8.9 | 66.6 ± 13.0 |
| BMI (kg/m2) | 24.5 ± 2.2 | 21.6 ± 2.5 | 23.2 ± 2.7 |
| Participants (n = 50) | PRE Dominant | POST Dominant | PRE Non-Dominant | POST Non-Dominant |
|---|---|---|---|---|
| L(2–4) | −8.63 ± 1.95 | −4.84 ± 1.46 * | −8.69 ± 0.77 | −5.03 ± 1.34 * |
| F(2–4) [Hz] | 3.18 ± 0.20 | 2.72 ± 0.20 * | 3.15 ± 0.17 | 2.77 ± 0.18 * |
| L(10–20) | −8.85 ± 1.64 | −7.84 ± 0.75 * | −9.10 ± 0.94 | −7.72 ± 0.85 * |
| F(10–20) [Hz] | 14.61 ± 1.21 | 14.16 ± 1.05 | 14.31 ± 1.39 | 14.09 ± 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rocco, F.; Festino, E.; Papale, O.; De Maio, M.; Cortis, C.; Fusco, A. Acute Effects of Complex Hand Proprioceptive Task on Low-Frequency Hand Rest Tremor. Sensors 2025, 25, 6502. https://doi.org/10.3390/s25206502
Di Rocco F, Festino E, Papale O, De Maio M, Cortis C, Fusco A. Acute Effects of Complex Hand Proprioceptive Task on Low-Frequency Hand Rest Tremor. Sensors. 2025; 25(20):6502. https://doi.org/10.3390/s25206502
Chicago/Turabian StyleDi Rocco, Francesca, Emanuel Festino, Olga Papale, Marianna De Maio, Cristina Cortis, and Andrea Fusco. 2025. "Acute Effects of Complex Hand Proprioceptive Task on Low-Frequency Hand Rest Tremor" Sensors 25, no. 20: 6502. https://doi.org/10.3390/s25206502
APA StyleDi Rocco, F., Festino, E., Papale, O., De Maio, M., Cortis, C., & Fusco, A. (2025). Acute Effects of Complex Hand Proprioceptive Task on Low-Frequency Hand Rest Tremor. Sensors, 25(20), 6502. https://doi.org/10.3390/s25206502








