A Highly Sensitive Silicon Nanowire Array Field Effect Transistor Biosensor for Detecting HBV-DNA and AFP
Abstract
1. Introduction
2. Materials and Methods
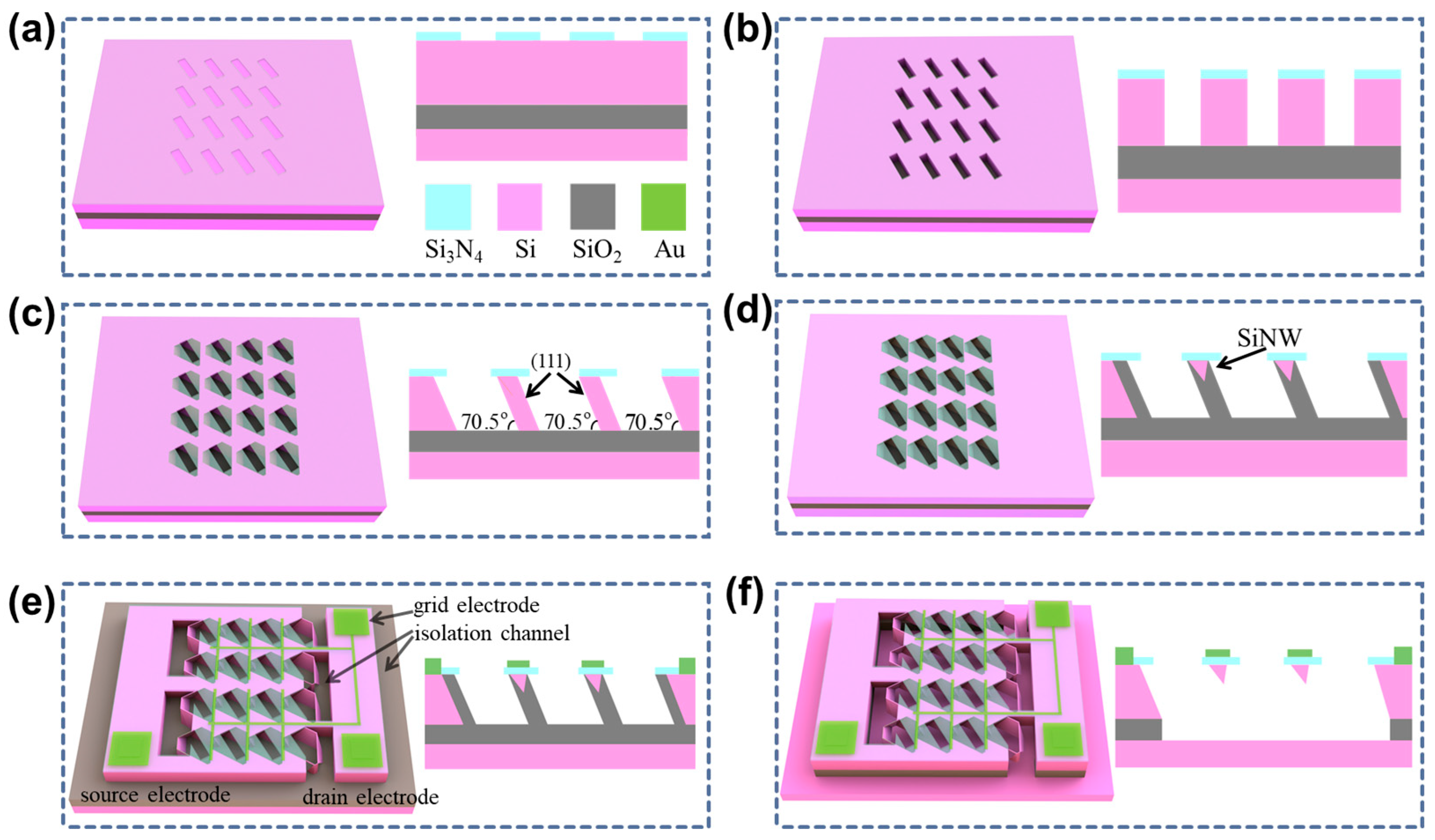
2.1. The Fabrication Process of SiNW-Array FET
2.2. Materials and Reagents
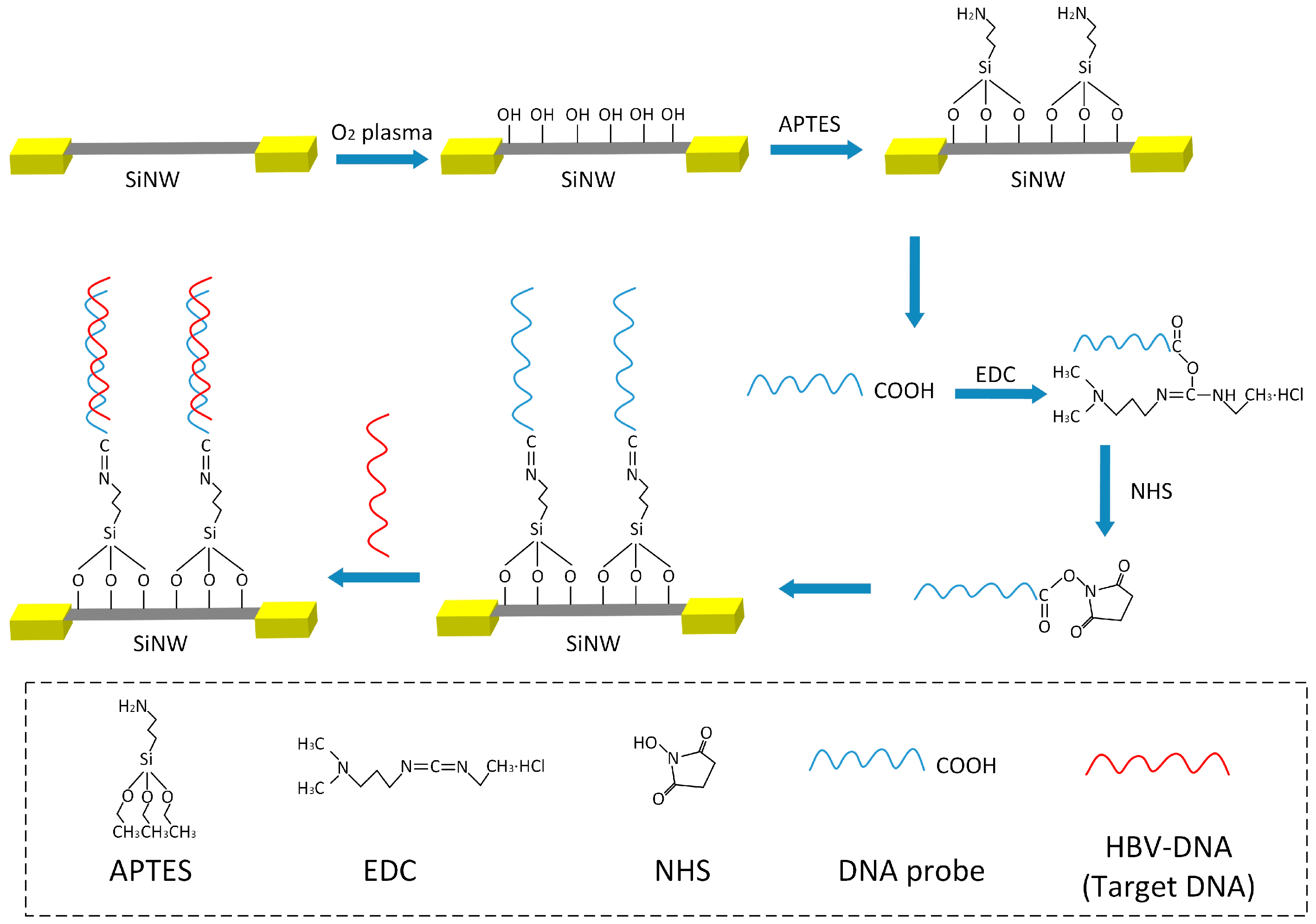
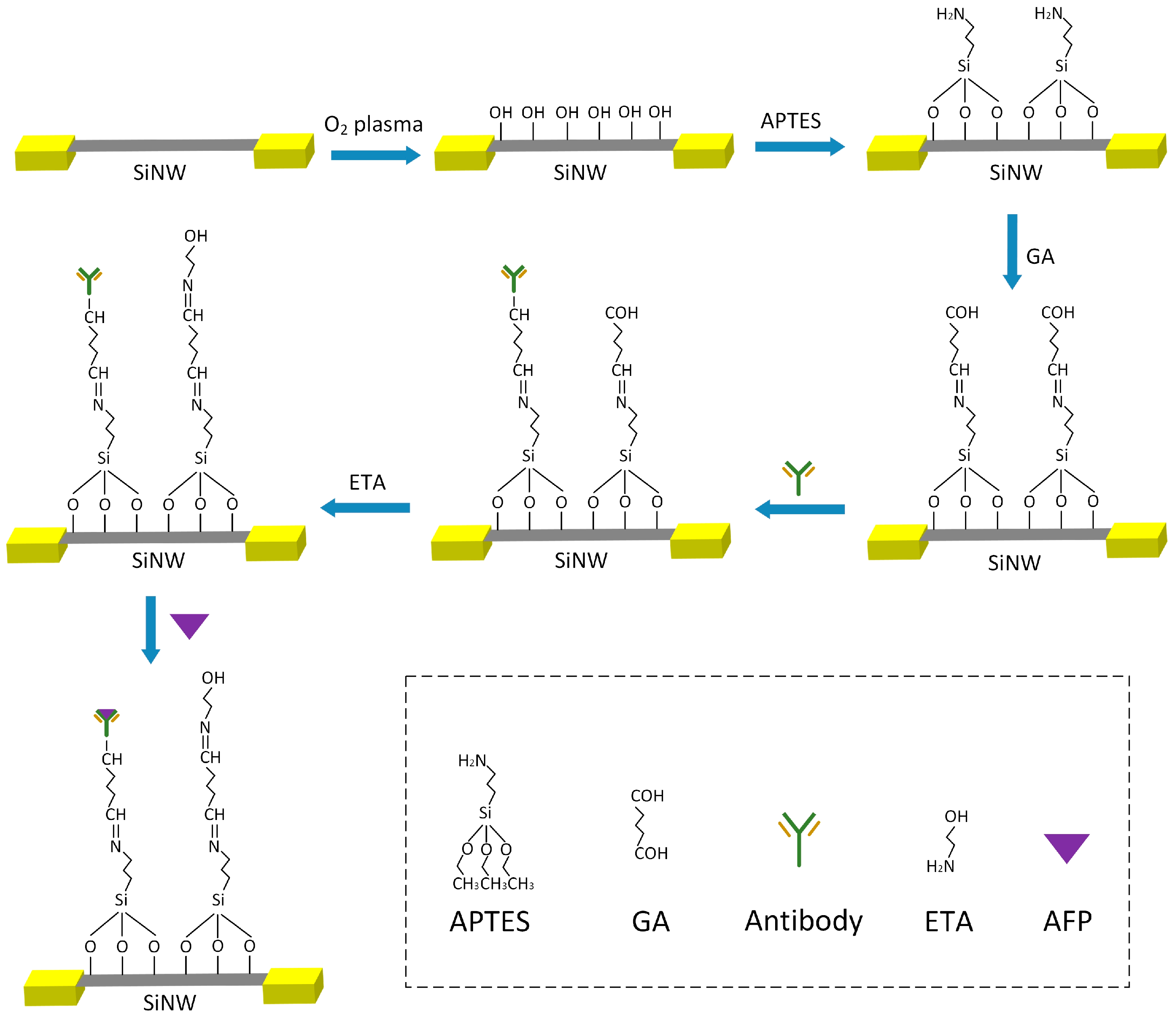
2.3. Surface Modification Process of the SiNW-Array FET
2.4. Experimental Conditions
3. Results and Discussion
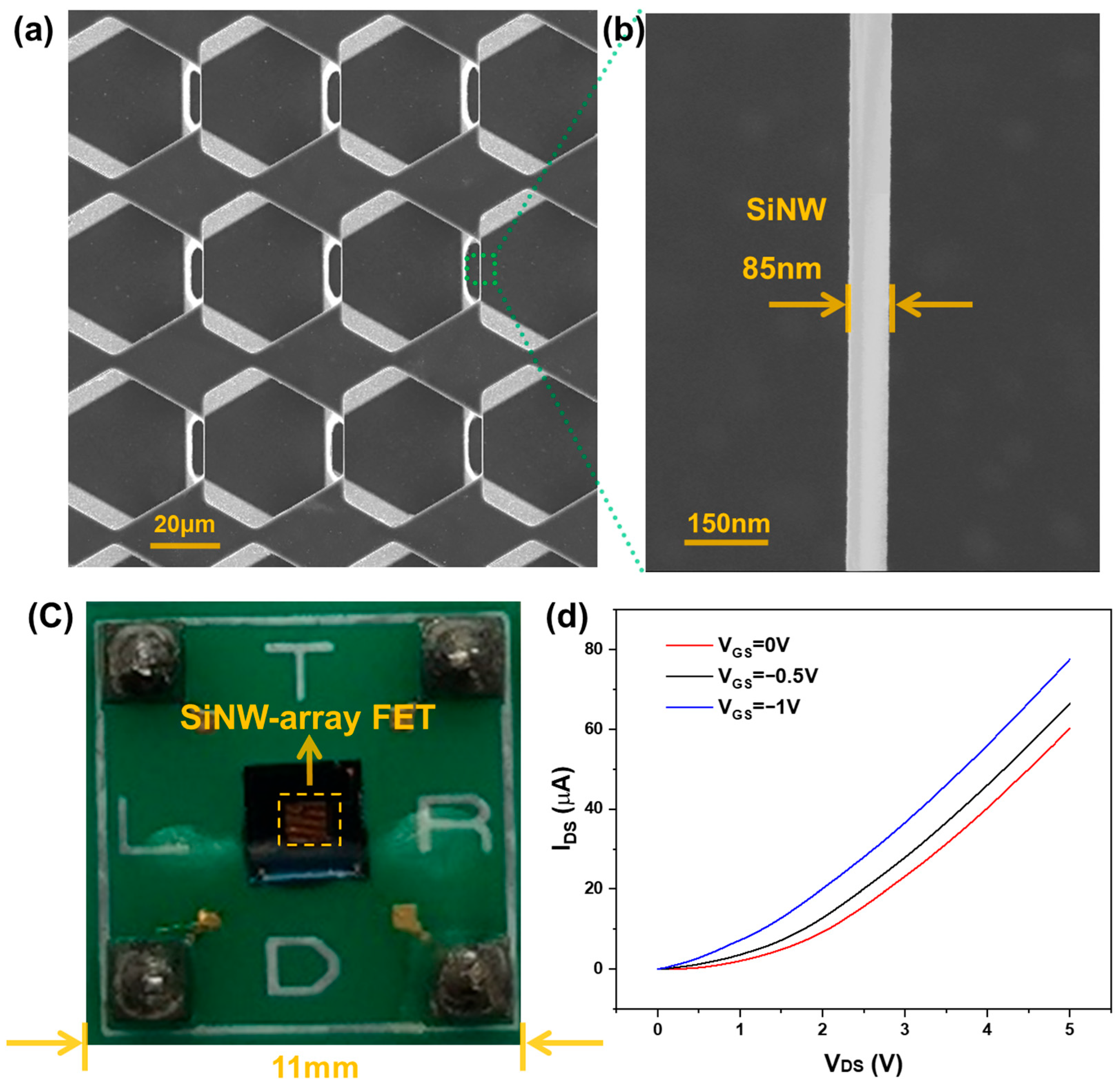
3.1. Morphology Characterization and Electrical Properties of SiNW-Array FET
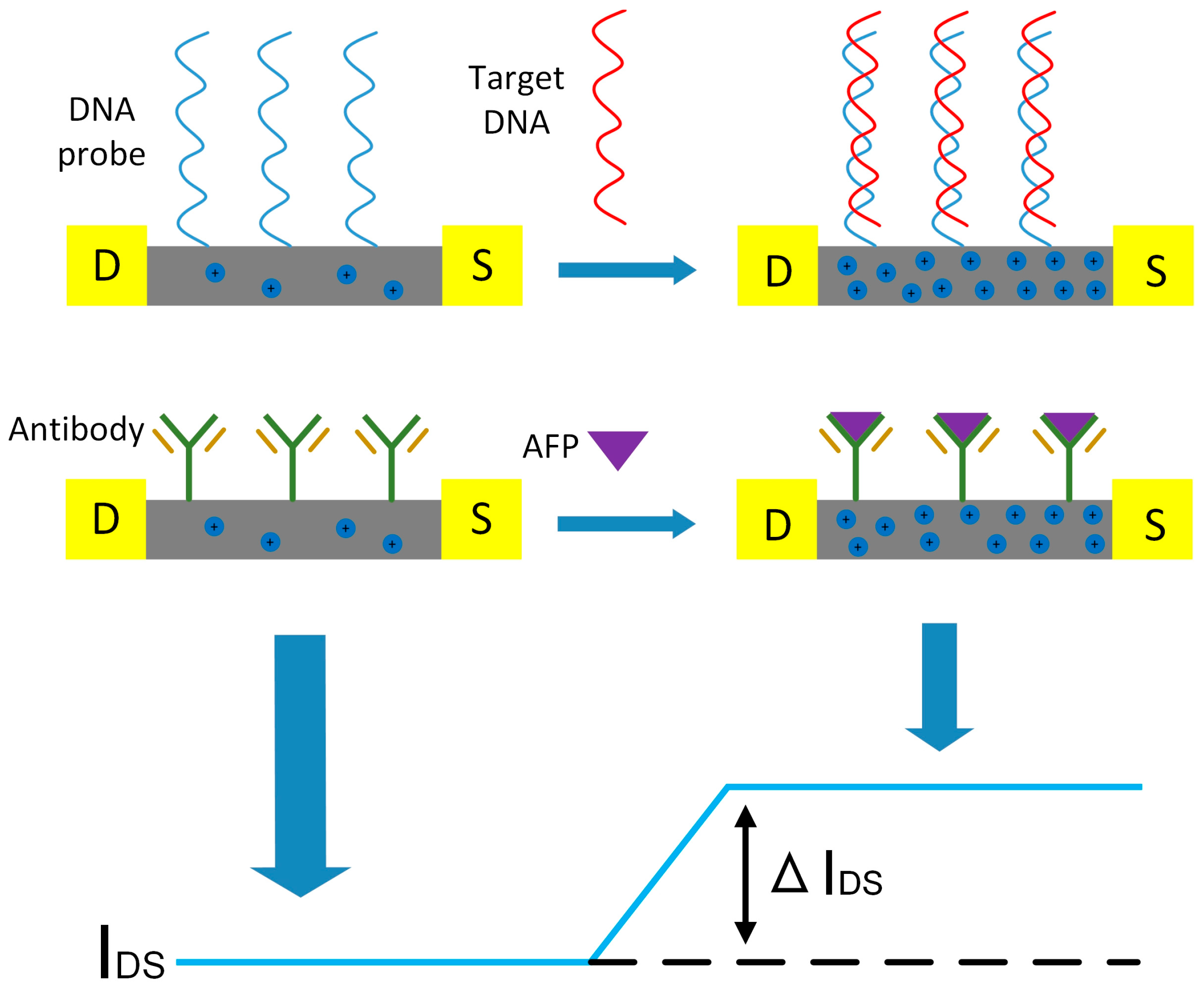
3.2. The Sensing Mechanism of the SiNW-Array FET
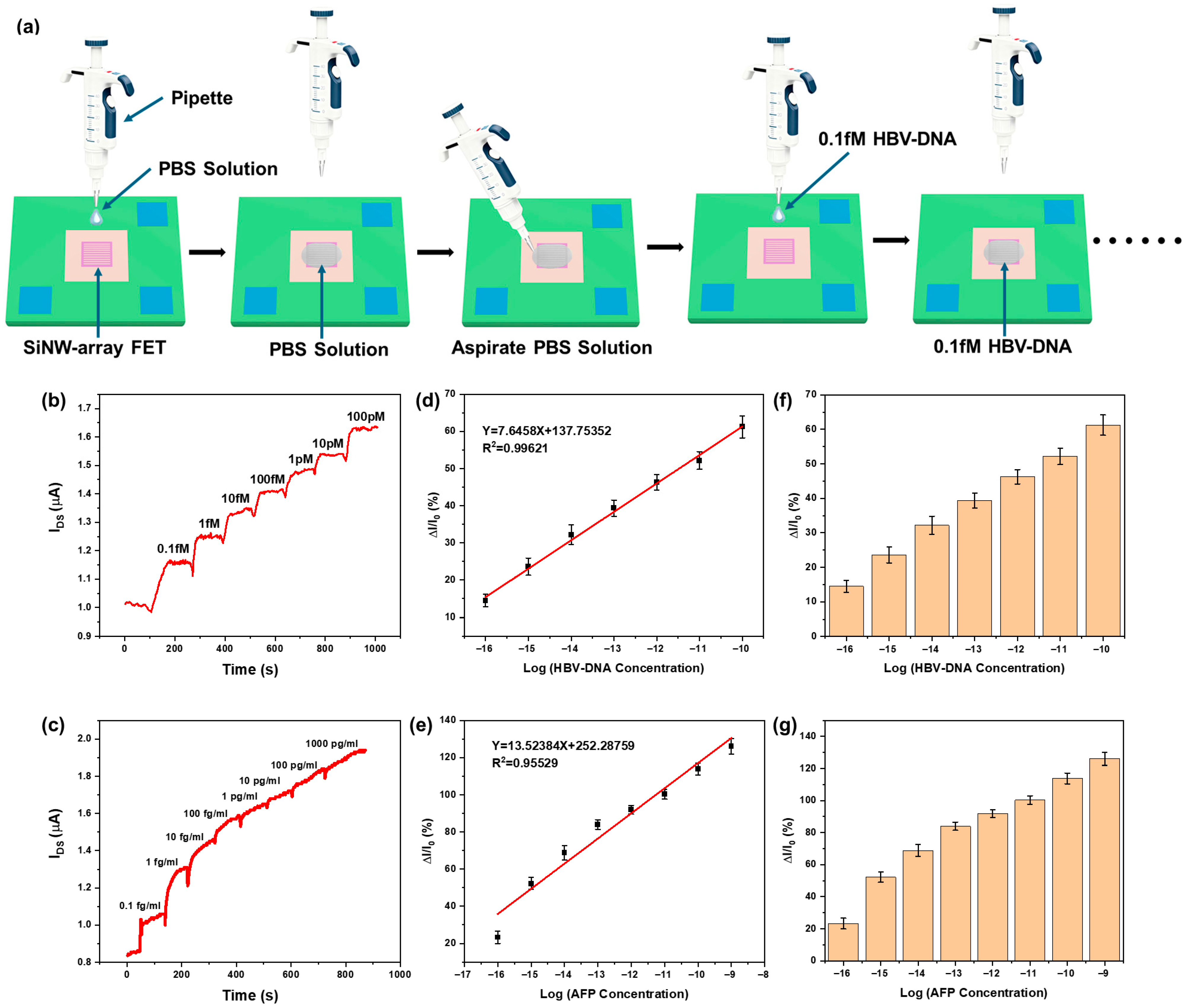
3.3. Sensitivity of the SiNW-Array FET
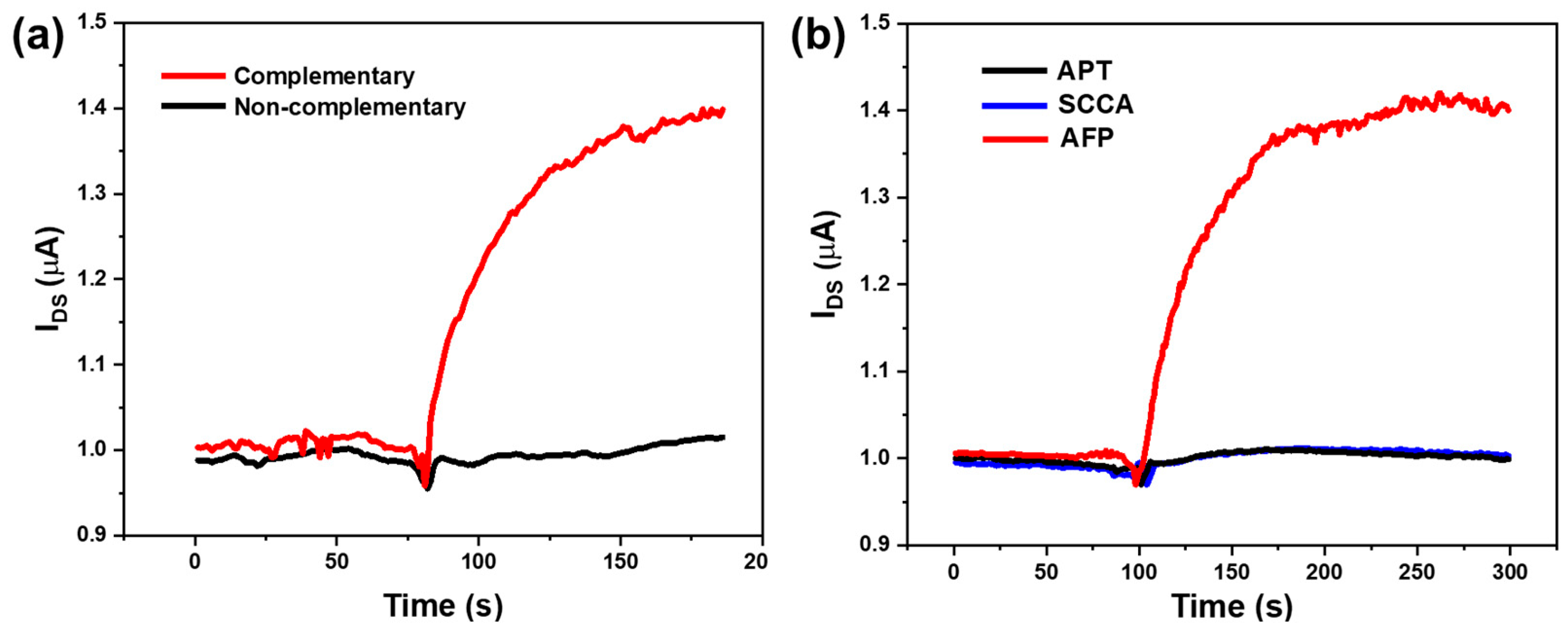
3.4. Detection Specificity of the SiNW-Array FET
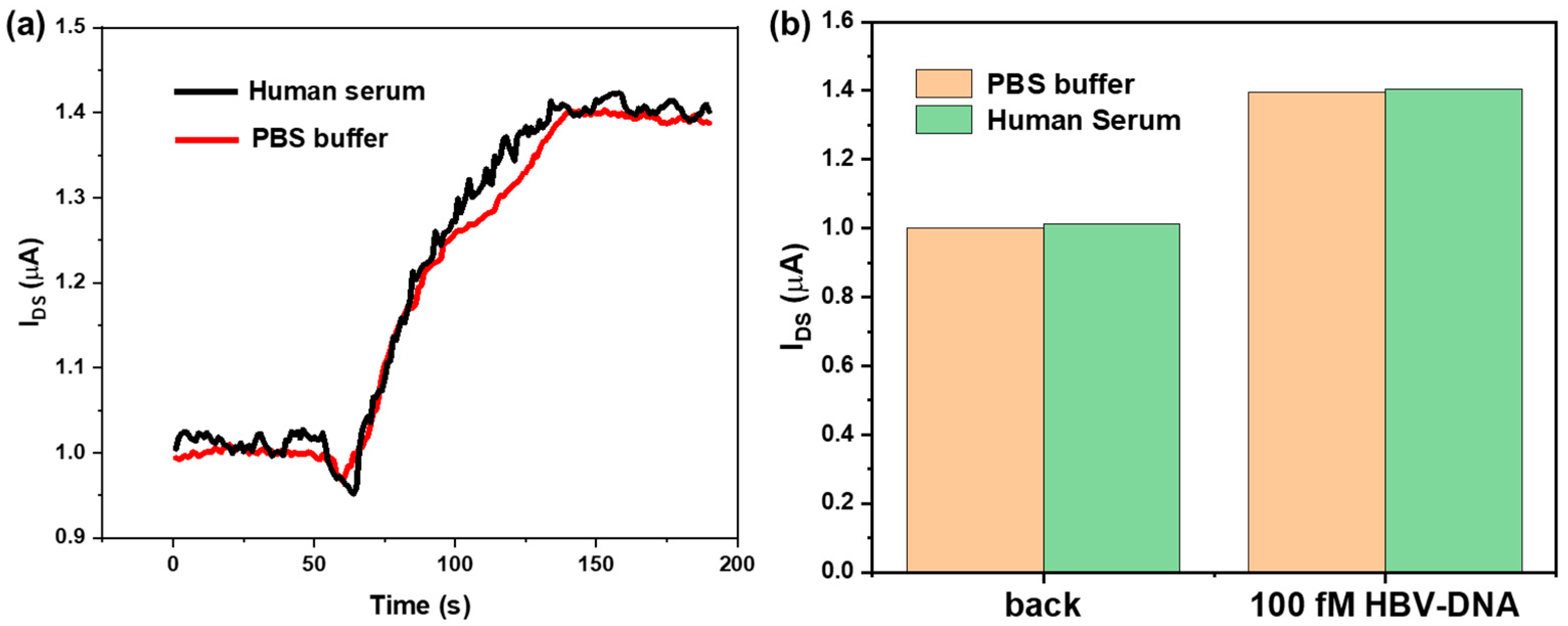
3.5. Simulation Analysis of Human Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeng, W.J.; Papatheodoridis, G.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039–1052. [Google Scholar] [CrossRef]
- Ott, J.J.; Stevens, G.A.; Groeger, J.; Wiersma, S.T. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012, 30, 2212–2219. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, D.S. Global control of hepatitis B virus infection. Lancet Infect. Dis. 2002, 2, 395–403. [Google Scholar] [CrossRef]
- Locarnini, S.; Hatzakis, A.; Chen, D.S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef]
- Zheng, G.F.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Hu, J.W.; Li, Y.L.; Zhang, X.F.; Wang, Y.R.; Zhang, J.; Yan, J.; Li, J.J.; Zhang, Z.H.; Yin, H.X.; Wei, Q.H.; et al. Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C. Biosensors 2023, 13, 645. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Li, X.S.; Cheng, C.; Shen, X.P.; Wang, T. Dual-Channel Detection of Breast Cancer Biomarkers CA15-3 and CEA in Human Serum Using Dialysis-Silicon Nanowire Field Effect Transistor. Int. J. Nanomed. 2022, 17, 6289–6299. [Google Scholar] [CrossRef]
- Lee, Q.; Yu, X.; Yu, W. The value of PIVKA-II versus AFP for the diagnosis and detection of postoperative changes in hepatocellular carcinoma. J. Interv. Med. 2021, 4, 77–81. [Google Scholar] [CrossRef]
- Yang, S.H.; Zhang, F.F.; Wang, Z.H.; Liang, Q.L. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef]
- Abd El Gawad, I.A.; Mossallam, G.I.; Radwan, N.H.; Elzawahry, H.M.; Elhifnawy, N.M. Comparing prothrombin induced by vitamin K absence-II (PIVKA-II) with the oncofetal proteins glypican-3, Alpha feto protein and carcinoembryonic antigen in diagnosing hepatocellular carcinoma among Egyptian patients. J. Egypt. Natl. Cancer Inst. 2014, 26, 79–85. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, F.; Liu, Y.; Cai, C.Q. Simply and sensitively simultaneous detection hepatocellular carcinoma markers AFP and miRNA-122 by a label-free resonance light scattering sensor. Talanta 2018, 186, 473–480. [Google Scholar] [CrossRef]
- Fan, K.; Li, D.J.; Liu, H.Y.; Wu, J. An Automated Immunoassay Based on Magnetic Microspheres for the Determination of Clenbuterol in Food. Instrum. Sci. Technol. 2015, 43, 524–535. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.P.; Li, D.J.; Li, Y.B. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef]
- Sahoo, P.; Suresh, S.; Dhara, S.; Saini, G.; Rangarajan, S.; Tyagi, A.K. Direct label free ultrasensitive impedimetric DNA biosensor using dendrimer functionalized GaN nanowires. Biosens. Bioelectron. 2013, 44, 164–170. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Sim, S.J. Nanoplasmonic biosensor: Detection and amplification of dual bio-signatures of circulating tumor DNA. Biosens. Bioelectron. 2015, 67, 443–449. [Google Scholar] [CrossRef]
- Kang, Q.; Henry, N.L.; Paoletti, C.; Jiang, H.; Vats, P.; Chinnaiyan, A.M.; Hayes, D.F.; Merajver, S.D.; Rae, J.M.; Tewari, M. Comparative analysis of circulating tumor DNA stability In K3EDTA, Streck, and CellSave blood collection tubes. Clin. Biochem. 2016, 49, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.Y.; Li, Z.J.; Ying, X.T.; Lin, J.M. Development of high-performance magnetic chemiluminescence enzyme immunoassay for α-fetoprotein (AFP) in human serum. Clin. Chim. Acta 2008, 393, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, X.Q.; Chen, H.; Bai, L.J.; Xu, H.; Wang, W.X.; Yang, H.W.; Wei, D.L.; Yang, L.X. Fabrication of novel electrochemical immunosensor by mussel-inspired chemistry and surface-initiated PET-ATRP for the simultaneous detection of CEA and AFP. React. Funct. Polym. 2020, 154, 104632. [Google Scholar] [CrossRef]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent Advances in Silicon Nanowire Biosensors: Synthesis Methods, Properties, and Applications. Nanoscale Res. Lett. 2016, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, W.; Kang, W.Q.; Ye, Y.T.; Pan, Q.Q.; Zhang, X.Q.; Ke, Y.Z.; Wang, C.; Qiu, Z.Q.; Tang, Y. A review on silicon nanowire-based anodes for next-generation high-performance lithium-ion batteries from a material-based perspective. Sustain. Energy Fuels 2020, 4, 1577–1594. [Google Scholar] [CrossRef]
- Chen, D.Q.; Xu, T.; Dou, Y.Z.; Li, T. A Calibration Strategy for Silicon Nanowire Field-Effect Transistor Biosensors and Its Application in Ultra-Sensitive, Label-Free Biosensing. Acs Nano 2024, 18, 21873–21885. [Google Scholar] [CrossRef]
- Jin, Z.Y.; Fang, Z.X.; Wang, Z.; Wu, W.; Yan, D.; Yang, X.; Gao, Y.; Yang, W.; Linxi, D.; Wang, G.; et al. A high-performance NO2 gas Sensor Based on Silicon Nanowire Array. Phys. Scr. 2025, 100, 095404. [Google Scholar] [CrossRef]
- Azmi, M.A.M.; Tehrani, Z.; Lewis, R.P.; Walker, K.A.D.; Jones, D.R.; Daniels, D.R.; Doak, S.H.; Guy, O.J. Highly sensitive covalently functionalised integrated silicon nanowire biosensor devices for detection of cancer risk biomarker. Biosens. Bioelectron. 2014, 52, 216–224. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, M.; Su, R.G.; Kong, T.; Zhang, D.; Zhou, C.W.; Cheng, G.S. Silicon nanowire FET biosensor and its application in acute myocardial infarction. Nanotechnology 2024, 35, 112001. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Geary, S.M.; Salem, A.K. Silicon Nanowires and Their Impact on Cancer Detection and Monitoring. Acs Appl. Nano Mater. 2020, 3, 8522–8536. [Google Scholar] [CrossRef]
- Shehada, N.; Brönstrup, G.; Funka, K.; Christiansen, S.; Leja, M.; Haick, H. Ultrasensitive Silicon Nanowire for Real-World Gas Sensing: Noninvasive Diagnosis of Cancer from Breath Volatolome. Nano Lett. 2015, 15, 1288–1295. [Google Scholar] [CrossRef]
- Jia, Y.H.; Wang, J.Y.; Yosinski, S.; Xu, Y.H.; Reed, M.A. A Fast and Label-Free Potentiometric Method for Direct Detection of Glutamine with Silicon Nanowire Biosensors. Biosensors 2022, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.P.; Sudhölter, E.J.R.; de Smet, L. Silicon Nanowire-Based Devices for Gas-Phase Sensing. Sensors 2014, 14, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.D.; Qiao, Z.X.; Han, X.G.; Wang, X.Z.; Shakoor, A.; Liu, C.; Lu, D.; Yang, P.; Zhao, L.; Wang, Y.; et al. A MEMS Sensors Based on A Laterally Movable Gate Field-Effect Transistor (LMGFET) with a Novel Decoupling Sandwich Structure. Engineering 2023, 21, 61–74. [Google Scholar] [CrossRef]
- Li, Q.; Lu, N.; Wang, L.H.; Fan, C.H. Advances in Nanowire Transistor-Based Biosensors. Small Methods 2018, 2, 1700263. [Google Scholar] [CrossRef]
- Kim, S.; Lee, R.; Kwon, D.; Kim, T.H.; Park, T.J.; Choi, S.J.; Mo, H.S.; Kim, D.H.; Park, B.G. Multiplexed Silicon Nanowire Tunnel FET-Based Biosensors with Optimized Multi-Sensing Currents. IEEE Sens. J. 2021, 21, 8839–8846. [Google Scholar] [CrossRef]
- Gao, A.R.; Wang, Y.; Zhang, D.W.; He, Y.Q.; Zhang, L.; Liu, Y.X.; Wang, Y.L.; Song, H.F.; Li, T. Highly sensitive and selective detection of human-derived volatile organic compounds based on odorant binding proteins functionalized silicon nanowire array. Sens. Actuators B-Chem. 2020, 309, 127762. [Google Scholar] [CrossRef]
- Li, Y.L.; Wei, S.H.; Xiong, E.Y.; Hu, J.W.; Zhang, X.F.; Wang, Y.R.; Zhang, J.; Yan, J.; Zhang, Z.H.; Yin, H.X.; et al. Ultrasensitive 3D Stacked Silicon Nanosheet Field-Effect Transistor Biosensor with Overcoming Debye Shielding Effect for Detection of DNA. Biosensors 2024, 14, 144. [Google Scholar] [CrossRef]
- Wasfi, A.; Alnaji, N.; Mustafa, A.; Awwad, F. Detection of DNA nucleotides via silicon nanowire field-effect transistor sensors with a nanogap: Semi-empirical modeling. Aeu-Int. J. Electron. Commun. 2024, 173, 154983. [Google Scholar] [CrossRef]
- Qin, M.Y.; Hu, J.W.; Li, X.; Liu, J.L.; Jiang, R.; Shi, Y.M.; Wang, Z.Z.; Zhang, L.Q.; Zhao, Y.; Gao, H.; et al. Exosomal membrane proteins analysis using a silicon nanowire field effect transistor biosensor. Talanta 2024, 278, 126534. [Google Scholar] [CrossRef]
- Nuzaihan, M.N.M.; Hashim, U.; Arshad, M.K.M.; Kasjoo, S.R.; Rahman, S.F.A.; Ruslinda, A.R.; Fathil, M.F.M.; Adzhri, R.; Shahimin, M.M. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Biosens. Bioelectron. 2016, 83, 106–114. [Google Scholar] [CrossRef]
- Li, J.; He, G.; Ueno, H.; Jia, C.C.; Noji, H.; Qi, C.M.; Guo, X.F. Direct real-time detection of single proteins using silicon nanowire-based electrical circuits. Nanoscale 2016, 8, 16172–16176. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Li, Z.Y.; Pisano, A.P.; Williams, R.S. Selective surface functionalization of silicon nanowires via nanoscale Joule heating. Nano Lett. 2007, 7, 3106–3111. [Google Scholar] [CrossRef]
- Lee, K.N.; Jung, S.W.; Shin, K.S.; Kim, W.H.; Lee, M.H.; Seong, W.K. Fabrication of suspended silicon nanowire arrays. Small 2008, 4, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, A.R.; Wang, Y.L.; Li, T. Wafer-level and highly controllable fabricated silicon nanowire transistor arrays on (111) silicon-on-insulator (SOI) wafers for highly sensitive detection in liquid and gaseous environments. Nano Res. 2018, 11, 1520–1529. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.X.; Chen, H.L.; Yan, Q.H. Development of a sandwich-type electrochemical DNA sensor based on CeO2/AuPt nanoprobes for highly sensitive detection of hepatitis B virus DNA. Bioelectrochemistry 2025, 163, 108901. [Google Scholar] [CrossRef]
- Li, Z.B.; Zou, S.Y.; Wu, S.J.; Miao, X.M.; Ma, D.L. Polymerase chain reaction-based ultrasensitive detection of HBV DNA via G-quadruplex selective iridium(III) complex luminescent probe. Talanta 2021, 221, 121661. [Google Scholar] [CrossRef]
- Liu, X.; Shuai, H.L.; Liu, Y.J.; Huang, K.J. An electrochemical biosensor for DNA detection based on tungsten disulfide/multi-walled carbon nanotube composites and hybridization chain reaction amplification. Sens. Actuators B-Chem. 2016, 235, 603–613. [Google Scholar] [CrossRef]
- Derakhshan, M.; Molaakbari, E.; Shamspur, T.; Mostafavi, A. Novel hybridization indicator for DNA sequences related to the hepatitis B virus: Electrochemical and in-silico approach. Microchem. J. 2023, 193, 109118. [Google Scholar] [CrossRef]
- Moustakim, H.; Amine, A.; Mohammadi, H. Affordable infectious pathogen detection using a dual-mode biosensor integrating exonuclease III-assisted target recycling amplification with high-throughput 96-well microplate format. Enzym. Microb. Technol. 2025, 183, 110549. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Jiang, Y.; Chen, X.; Chen, L.; Zhang, X.H.; Cui, D.X.; Li, Y.Y.; Liu, Z.X.; Zhao, Q.; Diao, A.P. Development of active affibody aggregates induced by a self-assembling peptide for high sensitive detection of alpha-fetoprotein. Chem. Eng. J. 2022, 436, 135208. [Google Scholar] [CrossRef]
- Liu, J.J.; Cui, D.X.; Jiang, Y.; Li, Y.Y.; Liu, Z.X.; Tao, L.; Zhao, Q.; Diao, A.P. Selection and characterization of a novel affibody peptide and its application in a two-site ELISA for the detection of cancer biomarker alpha-fetoprotein. Int. J. Biol. Macromol. 2021, 166, 884–892. [Google Scholar] [CrossRef]
- Li, W.Z.; Chen, M.; Liang, J.T.; Lu, C.T.; Zhang, M.; Hu, F.R.; Zhou, Z.D.; Li, G.Y. Electrochemical aptasensor for analyzing alpha-fetoprotein using RGO-CS-Fc nanocomposites integrated with gold-platinum nanoparticles. Anal. Methods 2020, 12, 4956–4966. [Google Scholar] [CrossRef]
- Lu, L.C.; Yu, J.L.; Liu, X.X.; Yang, X.S.; Zhou, Z.H.; Jin, Q.; Xiao, R.; Wang, C.W. Rapid, quantitative and ultra-sensitive detection of cancer biomarker by a SERRS-based lateral flow immunoassay using bovine serum albumin coated Au nanorods. RSC Adv. 2020, 10, 271–281. [Google Scholar] [CrossRef]
- Tan, M.S.; Zhang, C.Y.; Li, Y.Y.; Xu, Z.; Wang, S.J.; Liu, Q.; Li, Y.Y. An efficient electrochemical immunosensor for Alpha-Fetoprotein detection based on the CoFe prussian blue analog combined PdAg hybrid nanodendrites. Bioelectrochemistry 2022, 145, 108080. [Google Scholar] [CrossRef]
| DNA | Sequences |
|---|---|
| HBV-DNA probe | 5′-COOH-CCCCCCTATGCCTCATCTTCTTGTTGGTTCTT-3′ |
| complementary DNA (HBV-DNA) | 5′-AAGAACCAACAAGAAGATGAGGCATA-3′ |
| non-complementary DNA | 5′-CCACTACGCTTCCGCTTACCTCTCAT-3′ |
| Classification | Linear Range | LOD | Ref. |
|---|---|---|---|
| Electrochemical DNA sensor | 1 fM to 10 nM | 0.36 fM | [41] |
| Label-free fluorescent sensor | 3 fM to 800 pM | 1.6 fM | [42] |
| Electrochemical biosensor | 10 fM to 100 pM | 2.5 fM | [43] |
| DNA hybridization biosensor | 10 nM to 3 μM | 3.8 nM | [44] |
| Dual-mode biosensor | 100 fM to 100 nM | 5.62 fM | [45] |
| SiNW-array FET in this work | 0.1 fM to 100 pM | 0.1 fM | This work |
| Classification | Linear Range | LOD | Ref. |
|---|---|---|---|
| Two-size enzyme-linked immunosensor | 20 to 600 ng/mL | 9.7 ng/mL | [46] |
| Two-site ELISA sensor | 6 to 100 ng/mL | 2 ng/mL | [47] |
| Electrochemical aptasensor | 1 ng/mL to 10 μg/mL | 0.3013 ng/mL | [48] |
| SERRS-based lateral flow immunoassay | 10 pg/mL to 500 ng/mL | 9.2 pg/mL | [49] |
| Electrochemical immunosensor | 100 fg/mL to 200 ng/mL | 18.6 fg/mL | [50] |
| SiNW-array FET in this work | 0.1 fg/mL to 1000 pg/mL | 0.1 fg/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Liu, M.; Zhang, Y.; Liu, C.; Yang, X. A Highly Sensitive Silicon Nanowire Array Field Effect Transistor Biosensor for Detecting HBV-DNA and AFP. Sensors 2025, 25, 6385. https://doi.org/10.3390/s25206385
Sun P, Liu M, Zhang Y, Liu C, Yang X. A Highly Sensitive Silicon Nanowire Array Field Effect Transistor Biosensor for Detecting HBV-DNA and AFP. Sensors. 2025; 25(20):6385. https://doi.org/10.3390/s25206385
Chicago/Turabian StyleSun, Peng, Mingbin Liu, Yongxin Zhang, Chaoran Liu, and Xun Yang. 2025. "A Highly Sensitive Silicon Nanowire Array Field Effect Transistor Biosensor for Detecting HBV-DNA and AFP" Sensors 25, no. 20: 6385. https://doi.org/10.3390/s25206385
APA StyleSun, P., Liu, M., Zhang, Y., Liu, C., & Yang, X. (2025). A Highly Sensitive Silicon Nanowire Array Field Effect Transistor Biosensor for Detecting HBV-DNA and AFP. Sensors, 25(20), 6385. https://doi.org/10.3390/s25206385





