Compact Quantum Cascade Laser-Based Noninvasive Glucose Sensor Upgraded with Direct Comb Data-Mining
Abstract
1. Introduction
2. Materials and Methods
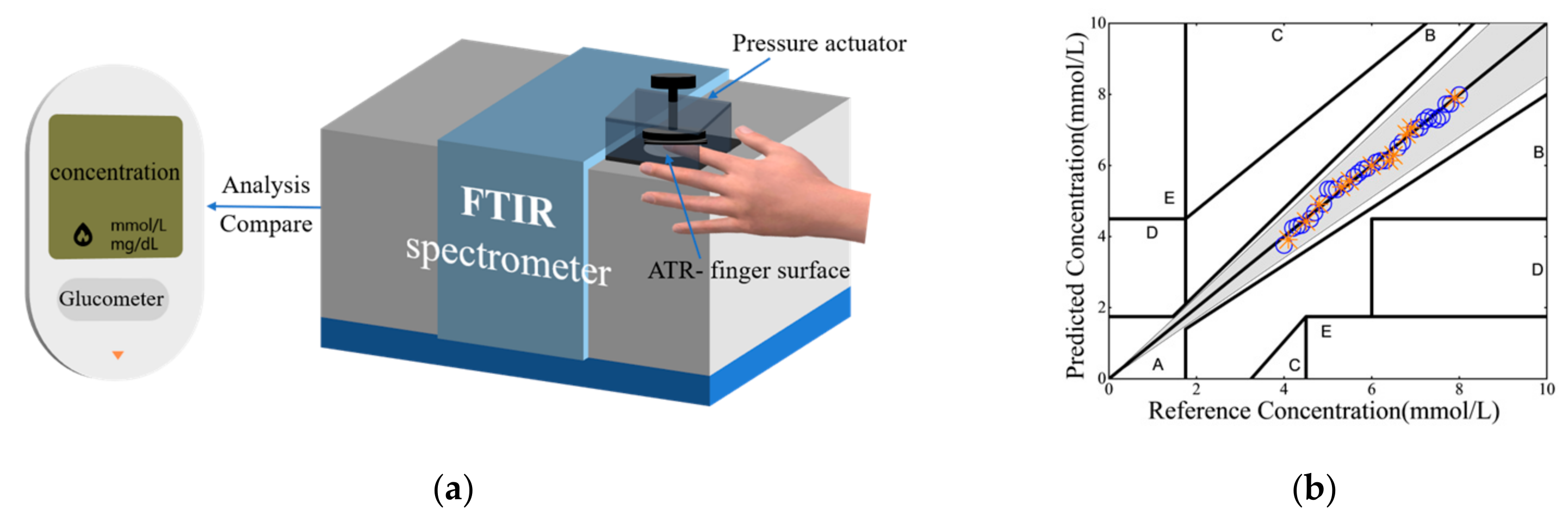
2.1. Hardware Designs
2.2. Acquisition Software and Data-Mining Designs
2.2.1. Translation of Comb-like Pulses in Time to Infrared Spectrum in Wavenumber
2.2.2. Direct Combs Data-Mining via Multifractal Detrended Fluctuation Analysis
2.2.3. Regression Analysis Method
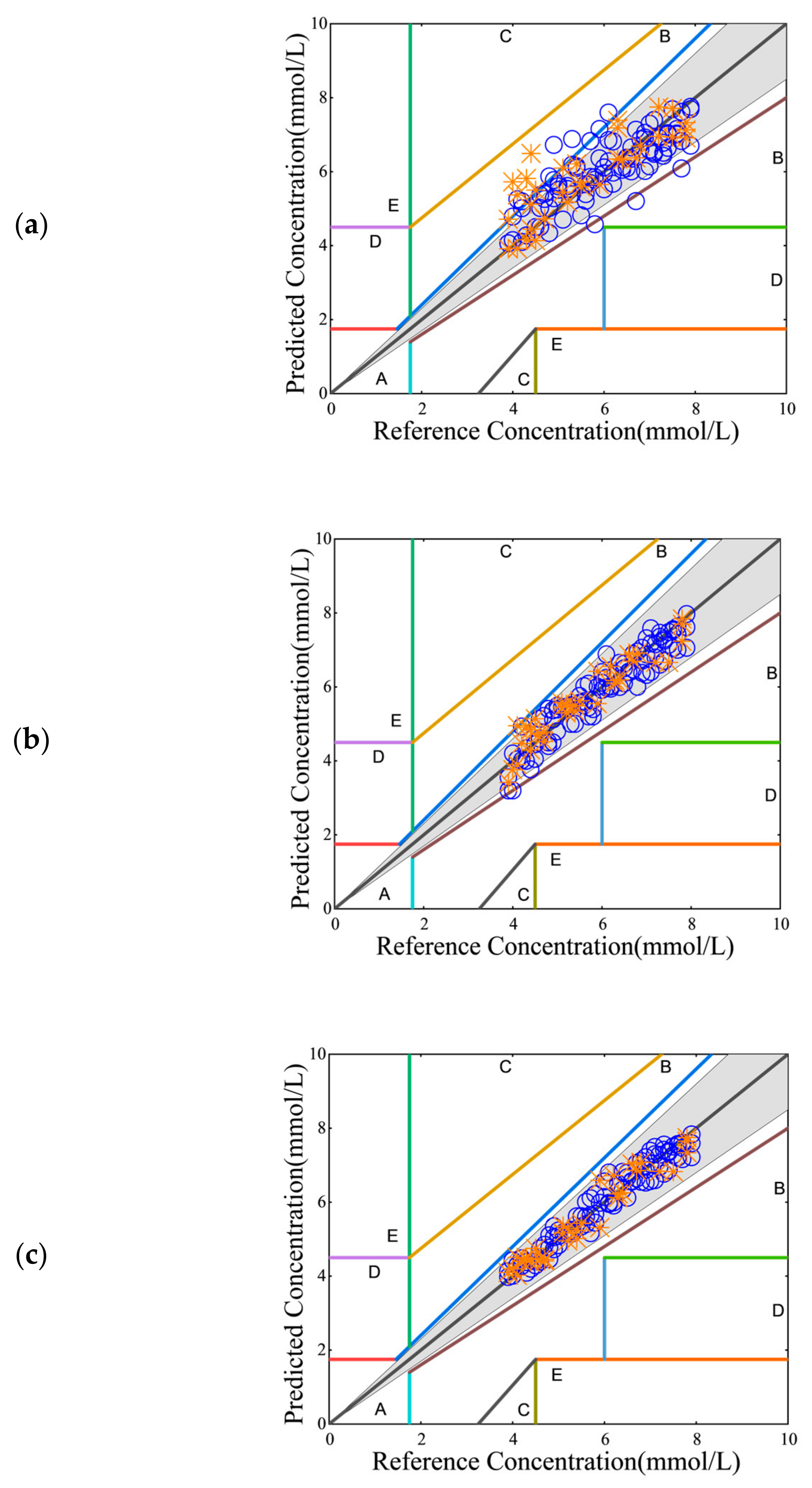
2.2.4. Clarke Error Grid Analysis
2.3. Experimental Procedure
- Specifically, 4 healthy and nondiabetic patient-subjects, 2 males and 2 females, conducting the tests are requested to fast for 10–12 h before 8 am every day. At the end of the fasting period, true blood glucose values are first detected and recorded with a standard glucometer. Then, noninvasive blood glucose measurements are conducted using Sensor-System #1 and Sensor-System #2 by taking 3 QCL scans from each of the four patient-subjects. The blood glucose concentrations thus measured are expected to near the normal “heathy” level of 3.9 mM.
- Afterwards, each of the four patient-subjects is requested to orally intake 75 g glucose and is then asked to accept a sequence of glucose tests to track the blood glucose variations. The sequence comprises a set of 12 tests in a total log time of 120 min, with a time duration of 10 min between two consecutive tests. The measured blood glucose concentrations exceeding 7.9 mM are discarded and nominally 41 concentrations ranging from 3.9 to 7.9 mM, with an increment of 0.1 mmol/L, are measured and recorded.
- For every set, each patient-subject is requested to take the quick sensor measurements 9 min after the finger-pricking step of the standard test with a glucometer. This time lag of 9 min is prescribed because the finger-pricking test measures glucose in the bloodstream, while the noninvasive sensors detect glucose in the interstitial fluid, and the diffusion of glucose from the bloodstream to the interstitial fluid is known to have an average time lag of about 8–10 min [34,35]. Here, it should be noted that tests employing Sensor-System #1 and Sensor-System #2 are completed at a rate of about one second per test; hence, the glucose variations in time are negligible for such a short time difference.
- Each noninvasive blood glucose test is conducted by requesting each patient-subject to press the subject’s hypothenar at ease against the MATR window of the sensor. A built-in pressure transducer records a pressure range of 1–5 N/cm2. To further enhance its accuracy, Sensor-System #1 is equipped with a pressure actuator to mechanically press the patient’s hypothenar against the MATR window at a firm pressure of 20 ± 0.4 N/cm2. Again, for each test, 3 QCL scans are taken.
3. Results and Discussion
Assessment and Validation of Sensor Accuracy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Noncommunicable Diseases. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 23 December 2024).
- So, H.C.; Wong, T.K.; Chung, J. Recent advances in noninvasive glucose monitoring. Med. Devices 2012, 2012, 45–52. [Google Scholar]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Thennadil, S.N.; Rennert, J.L.; Wenzel, B.J.; Hazen, K.H.; Ruchti, T.L.; Block, M.B. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol. Ther. 2004, 3, 357–365. [Google Scholar] [CrossRef]
- Steil, G.M.; Rebrin, K.; Hariri, F.; Jinagonda, S.; Tadros, S.; Darwin, C.; Saad, M.F. Interstitial fluid glucose dynamics during insulin-induced hypoglycaemia. Diabetologia 2005, 48, 1833–1840. [Google Scholar] [CrossRef]
- Kim, H.; Noh, I.; Yoon, G. Glucose Prediction in the Interstitial Fluid Based on Infrared Absorption Spectroscopy Using Multi-component Analysis. J. Environ. Biol. 2009, 13, 279–285. [Google Scholar] [CrossRef]
- Kino, S.; Omori, S.; Katagiri, T.; Matsuura, Y. Hollow optical-fiber based infrared spectroscopy for measurement of blood glucose level by using multi-reflection prism. Biomed. Opt. Express 2016, 7, 701–708. [Google Scholar] [CrossRef]
- Matsuura, Y.; Koyama, T.; Razeghi, M.; Lewis, J.S.; Khodaparast, G.A.; Tournié, E. Non-invasive blood glucose measurement using quantum cascade lasers. In Quantum Sensing and Nano Electronics and Photonics XVI; SPIE: San Francisco, CA, USA, 2019. [Google Scholar]
- Kong, C.R.; Barman, I.; Dingari, N.C.; Kang, J.W.; Galindo, L.; Dasari, R.R.; Feld, M.S. A novel non-imaging optics based Raman spectroscopy device for transdermal blood analyte measurement. Aip Adv. 2011, 1, 32175. [Google Scholar] [CrossRef]
- Shih, W.C.; Bechtel, K.L.; Rebec, M.V. Noninvasive glucose sensing by transcutaneous Raman spectroscopy. J. Biomed. Opt. 2015, 20, 051036. [Google Scholar] [CrossRef]
- Aloraynan, A.; Rassel, S.; Xu, C.; Ban, D. A Single Wavelength Mid-Infrared Photoacoustic Spectroscopy for Noninvasive Glucose Detection Using Machine Learning. Biosensors 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; Von Lilienfeld-Toal, H.; Mantele, W. In Vivo Noninvasive Monitoring of Glucose Concentration in Human Epidermis by Mid-Infrared Pulsed Photoacoustic Spectroscopy. Anal. Chem. 2013, 85, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, J.-P.; Zhu, J.; Liang, S.-L. An Accurate Noninvasive Blood Glucose Measurement System Using Portable Near-Infrared Spectrometer and Transfer Learning Framework. IEEE Sens. J. 2020, 21, 3506–3519. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tajima, T.; Seyama, M.; Waki, K. Differential Continuous Wave Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring. IEEE Sens. J. 2020, 20, 4453–4458. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhou, Q.; Xu, G.; Wang, R.T.; Tai, E.G.; Xie, L.; Zhang, Q.; Guan, Y.; Huang, X. Non-invasive blood glucose measurement of 95% certainty by pressure regulated Mid-IR. Talanta 2019, 197, 211–217. [Google Scholar] [CrossRef]
- Song, L.; Han, Z.; Lau, W.-M. Optimization of mid-infrared noninvasive blood-glucose prediction model by support vector regression coupled with different spectral features. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 15, 124738. [Google Scholar] [CrossRef]
- Song, L.; Han, Z.; Shum, P.-W.; Lau, W.-M. Enhancing the accuracy of blood-glucose tests by upgrading FTIR with multiple-reflections, quantum cascade laser, two-dimensional correlation spectroscopy and machine learning. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 15, 125400. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y. Fourier Transform Infrared Spectroscopic Analysis in Applied Cotton Fiber and Cottonseed Research: A Review. J. Cotton Sci. 2021, 25, 167–183. [Google Scholar] [CrossRef]
- Yao, Y.; Hoffman, A.J.; Gmachl, C.F. Mid-infrared quantum cascade lasers. Nat. Photonics 2012, 6, 432–439. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ding, K.K.; Wei, L.; Li, X.; Shi, J.C.; Li, Z.J.; Qu, Y.; Li, L.; Qiao, Z.L.; Liu, G.J.; et al. Research on Mid-Infrared External Cavity Quantum Cascade Lasers and Applications. Crystals 2022, 12, 1564. [Google Scholar] [CrossRef]
- Teuber, A.; Mizaikoff, B. Robust Attenuated Total Reflection Infrared Spectroscopic Sensors Based on Quantum Cascade Lasers for Harsh Environments. IEEE Sens. J. 2024, 24, 814–821. [Google Scholar] [CrossRef]
- Brandstetter, M.; Volgger, L.; Genner, A.; Jungbauer, C.; Lendl, B. Direct determination of glucose, lactate and triglycerides in blood serum by a tunable quantum cascade laser-based mid-IR sensor. Appl. Phys. B 2013, 110, 233–239. [Google Scholar] [CrossRef]
- Jernelv, I.L.; Strøm, K.; Hjelme, D.R.; Aksnes, A. Infrared Spectroscopy with a Fiber-Coupled Quantum Cascade Laser for Attenuated Total Reflection Measurements Towards Biomedical Applications. Sensors 2019, 19, 5130. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Shibata, N.; Kino, S.; Sugiyama, A.; Akikusa, N.; Matsuura, Y. A Compact Mid-Infrared Spectroscopy System for Healthcare Applications Based on a Wavelength-Swept, Pulsed Quantum Cascade Laser. Sensors 2020, 20, 3438. [Google Scholar] [CrossRef]
- Chen, J.; Furukawa, H. High-speed mid-infrared spectrometer based on wavelength-swept quantum cascade laser using asynchronous-signal method. Opt. Laser Technol. 2022, 149, 107800. [Google Scholar] [CrossRef]
- Kantelhardt, J.W.; Zschiegner, S.A.; Koscielny-Bunde, E.; Havlin, S.; Bunde, A.; Stanley, H.E. Multifractal detrended fluctuation analysis of nonstationary time series. Phys. A Stat. Mech. Appl. 2002, 316, 87–114. [Google Scholar] [CrossRef]
- Guo, J.; Li, A.; Zhang, R. Tool condition monitoring in milling process using multifractal detrended fluctuation analysis and support vector machine. Int. J. Adv. Manuf. Technol. 2020, 110, 1445–1456. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.; Liu, Y. Research on Risk Features and Prediction of China’s Crude Oil Futures Market Based on Machine Learning. Front. Energy Res. 2022, 10, 741018. [Google Scholar] [CrossRef]
- Vapnik, V.; Vapnik, V. The Natural of Statistical Learning Theory, 1st ed.; Vapnik, V.N., Ed.; Springer: New York, NY, USA, 1995; pp. 133–155. [Google Scholar]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef]
- ISO 15197:2013; In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. ISO: Geneva, Switzerland, 2013.
- Chuah, Z.-M.; Paramesran, R.; Thambiratnam, K.; Poh, S.-C. A two-level partial least squares system for non-invasive blood glucose concentration prediction. Chemom. Intell. Lab. Syst. 2010, 104, 347–351. [Google Scholar] [CrossRef]
- Yang, W.; Liao, N.; Cheng, H.; Li, Y.; Bai, X.; Deng, C. Determination of NIR informative wavebands for transmission non-invasive blood glucose measurement using a Fourier transform spectrometer. AIP Adv. 2018, 8, 035216. [Google Scholar] [CrossRef]







| Subjects | Sensors | w/wo Pressure Actuator | % of Measurements | |
|---|---|---|---|---|
| Error ± 15% | Error ± 20% | |||
| Subject 1 | Sensor-System #1 | without pressure actuator | 75.41 | 85.36 |
| with pressure actuator | 96.97 | 98.49 | ||
| Sensor-System #2 | without pressure actuator | 100.00 | 100.00 | |
| Subject 2 | Sensor-System #1 | without pressure actuator | 74.45 | 85.36 |
| with pressure actuator | 98.34 | 98.34 | ||
| Sensor-System #2 | without pressure actuator | 99.45 | 100.00 | |
| Subject 3 | Sensor-System #1 | without pressure actuator | 78.99 | 83.44 |
| with pressure actuator | 98.34 | 98.34 | ||
| Sensor-System #2 | without pressure actuator | 100.00 | 100.00 | |
| Subject 4 | Sensor-System #1 | without pressure actuator | 79.55 | 88.94 |
| with pressure actuator | 97.62 | 100.00 | ||
| Sensor-System #2 | without pressure actuator | 99.45 | 100.00 | |
| Average | Sensor-System #1 | without pressure actuator | 77.10 | 85.78 |
| with pressure actuator | 97.82 | 98.79 | ||
| Sensor-System #2 | without pressure actuator | 99.73 | 100.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Han, Z.; Nie, H.; Lau, W.-M. Compact Quantum Cascade Laser-Based Noninvasive Glucose Sensor Upgraded with Direct Comb Data-Mining. Sensors 2025, 25, 587. https://doi.org/10.3390/s25020587
Song L, Han Z, Nie H, Lau W-M. Compact Quantum Cascade Laser-Based Noninvasive Glucose Sensor Upgraded with Direct Comb Data-Mining. Sensors. 2025; 25(2):587. https://doi.org/10.3390/s25020587
Chicago/Turabian StyleSong, Liying, Zhiqiang Han, Hengyong Nie, and Woon-Ming Lau. 2025. "Compact Quantum Cascade Laser-Based Noninvasive Glucose Sensor Upgraded with Direct Comb Data-Mining" Sensors 25, no. 2: 587. https://doi.org/10.3390/s25020587
APA StyleSong, L., Han, Z., Nie, H., & Lau, W.-M. (2025). Compact Quantum Cascade Laser-Based Noninvasive Glucose Sensor Upgraded with Direct Comb Data-Mining. Sensors, 25(2), 587. https://doi.org/10.3390/s25020587






