A Novel Whole-Body Wearable Technology for Motor Assessment in Multiple Sclerosis: Feasibility and Usability Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
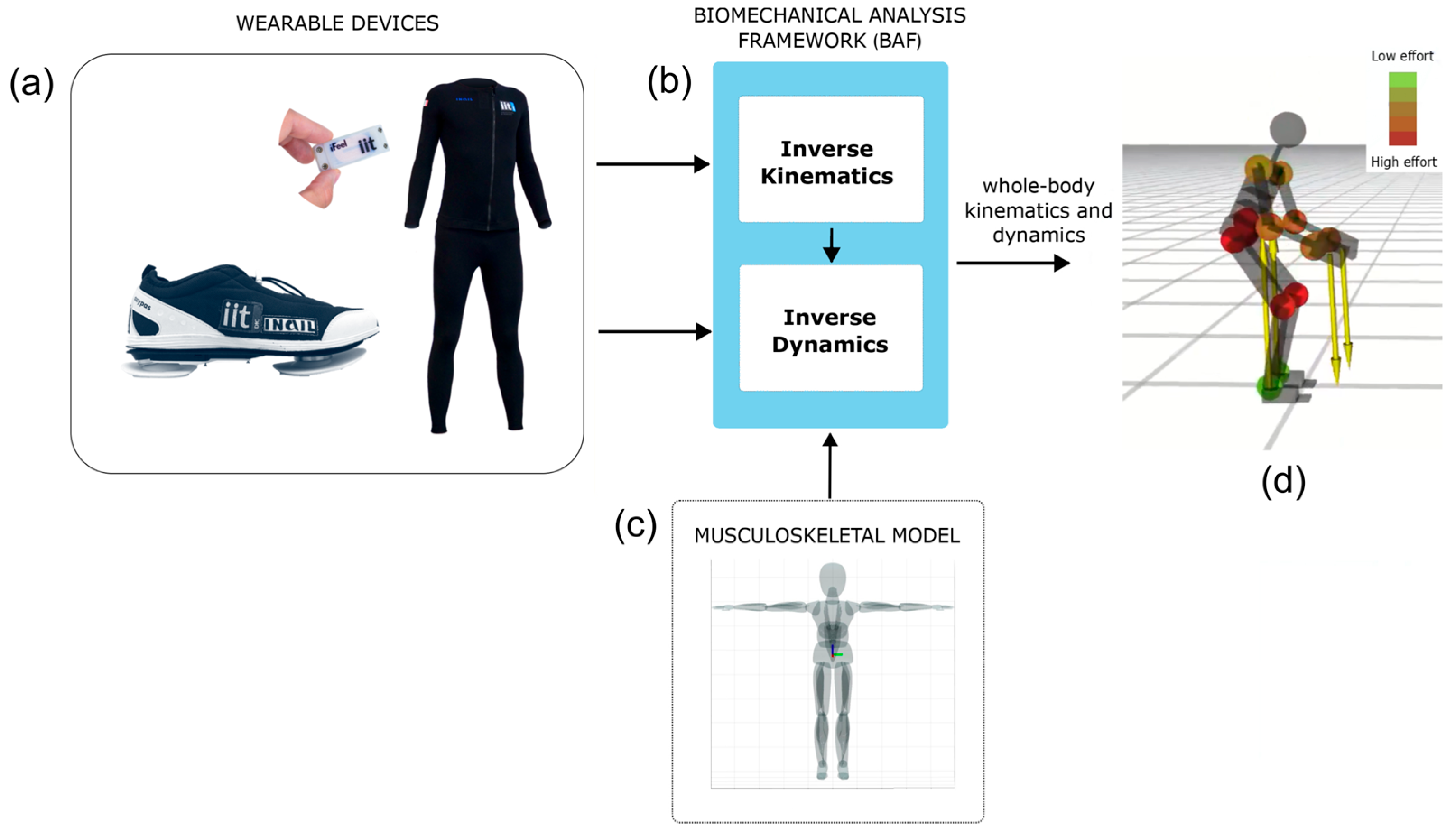
2.2. The Whole-Body Technology: iFeel
- A distributed network of wearable sensing devices;
- A pair of sensorized shoes equipped with force/torque sensors;
- An AI-driven algorithm for real-time estimation.
- A microcontroller-based electronics core with integrated power management and radio communication;
- An Inertial Measurement Unit (IMU) that provides acceleration data at 1 kHz and fused motion data at 100 Hz;
- A medical-grade infrared (IR) temperature sensor for high-resolution skin temperature monitoring;
- A haptic feedback transducer to simulate tactile sensations and enhance user interaction.

2.3. Procedure
- The impact of MS on an individual’s walking ability (MSWS-12) [5];
- The individual perception on dual-task impact on common daily activities (Daily living Activities Questionnaire—DIDA-Q), divided into cognition (DIDA-Q_COG) and balance and mobility (DIDA-Q_BAL & MOB) [15];
- The perceived fatigue in terms of physical (MFIS_P), cognitive (MFIS_C), and psychosocial functioning (MFIS_PS) (Modified Fatigue Impact Scale—MFIS) [16].
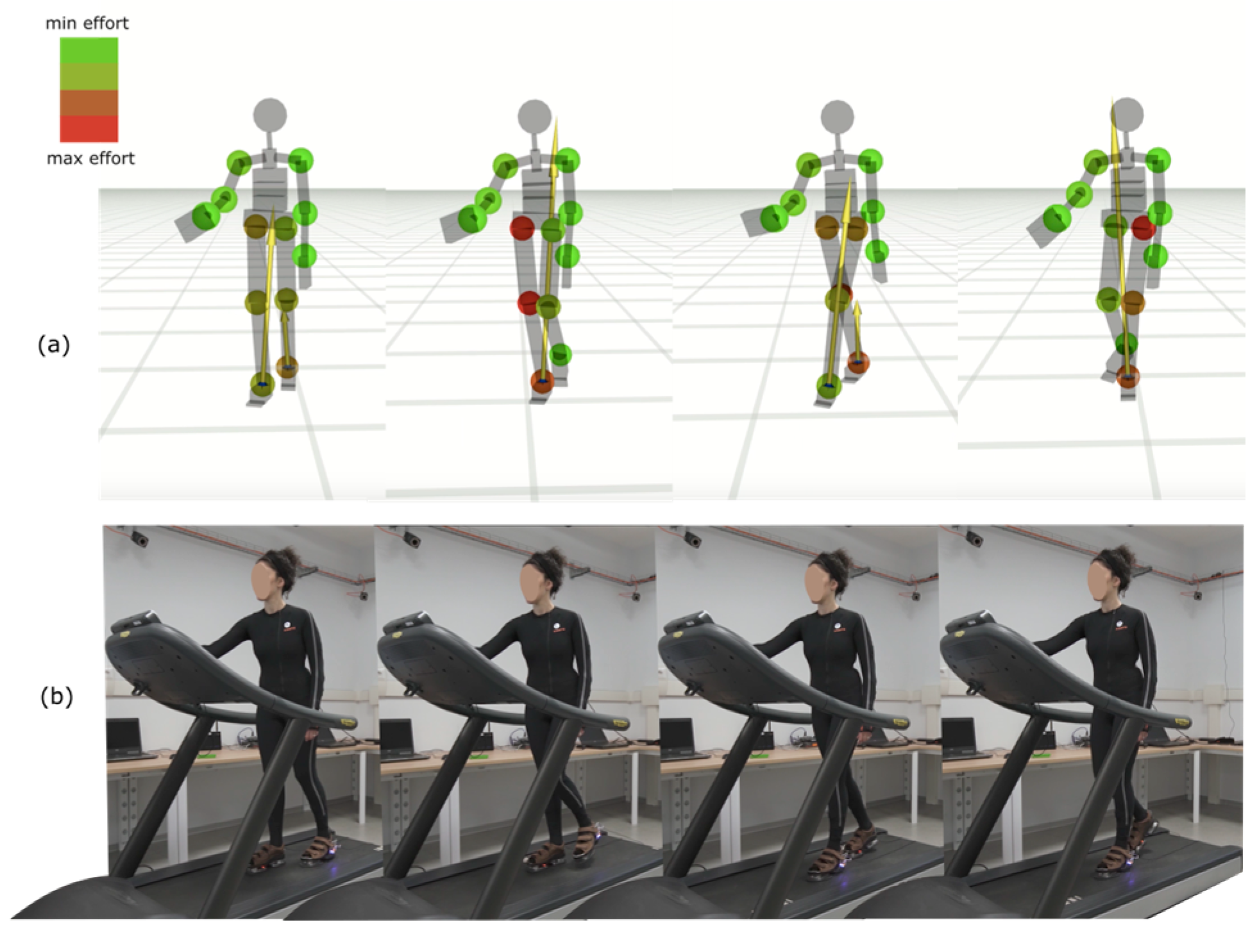
2.4. Key Performance Indicators (KPsI)
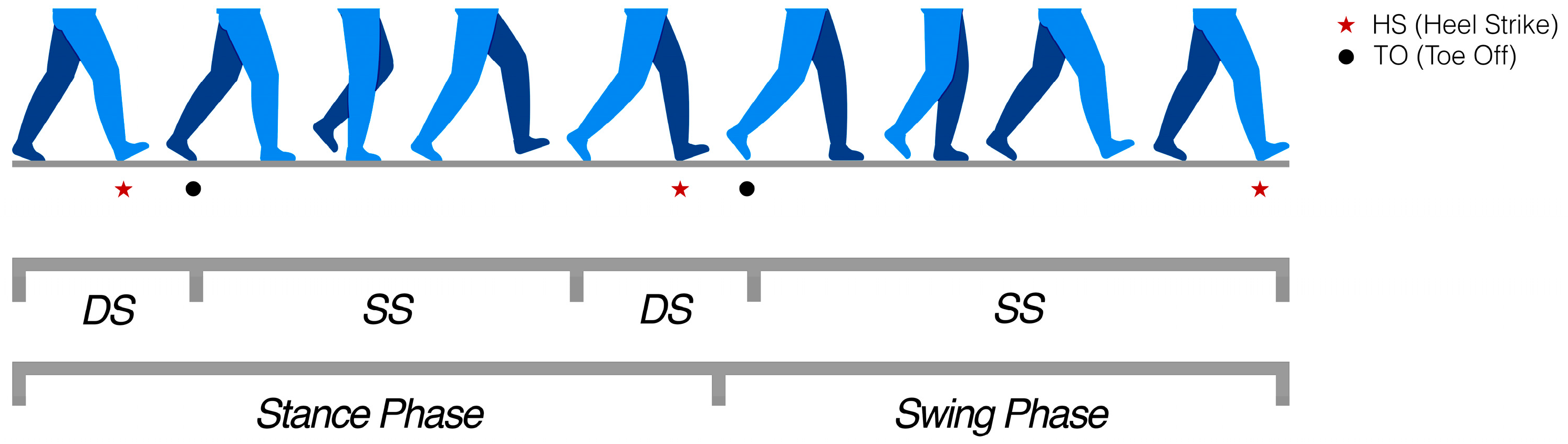
- Cycle duration (s): the elapsed time between two consecutive HSs of the same foot.
- Cadence (steps/min): a measure of walking rhythm, representing how quickly a person takes steps. It is derived from the total number of heel strikes during steady walking and expressed as steps per minute.
- Stride length (m): the distance covered between two consecutive HSs of the same foot, estimated from the foot trajectory reconstructed via kinematic data.
- Walking speed (m/s): the average forward velocity, obtained by dividing stride length by cycle duration.
- Double support (% of cycle): the percentage of the gait cycle during which both feet are simultaneously in contact with the ground. This corresponds to the sum of the initial and terminal double support phases.
- Stance phase (% of cycle): the proportion of the gait cycle in which a foot is in contact with the ground, from HS until the subsequent TO of the same foot.
- Swing phase (% of cycle): the complementary portion of the cycle, from TO until the following HS of the same foot, when the foot moves through the air to prepare for the next step.
- Maximum angular velocity (deg/s): the peak angular velocity of the foot during swing, obtained from the inertial and kinematic measurements of the shoes.
- Swing width (% of stride length): the medio-lateral deviation of the foot trajectory during swing, normalized by stride length, providing an indicator of gait stability.
- Path length (% of stride length): the actual length of the trajectory traced by the foot during swing, expressed relative to stride length. Values above 100% indicate less direct and more irregular trajectories.
2.5. Statistical Analysis
3. Results
3.1. Sample Demographic and Clinical Characteristics
3.2. Assessing the Agreement Between Clinician and Machine with iFeel and Exploring the Effect of Sensorization on Test Performance
3.3. iFeel Technology Usability
4. Discussion
- IMU data loss due to occasional 2.45 GHz wireless communication dropouts. Across all sessions, packet loss remained limited (<20% of the acquired stream at 60 Hz) and did not compromise the computation of gait cycles, as sufficient steady-state data were available for KPI extraction. Moreover, the pipeline was designed to log synchronized data across nodes, allowing corrupted packets to be identified and excluded. Ongoing developments focus on the implementation of a fully wired sensing suit with centralized Wi-Fi connectivity, which is expected to substantially minimize packet loss by eliminating interference from the 2.45 GHz channel. In parallel, post-processing gap-filling algorithms are being developed to reconstruct short missing segments (time windows <200 ms) through interpolation and model-based estimation, thereby maintaining the continuity of biomechanical signals.
- Modeling inaccuracies in human link dimensions within the biomechanical model, which may affect joint trajectory reconstruction. This issue is being addressed by the integration of an enhanced URDF-based anthropometric model.
- Sensor displacement relative to anatomical landmarks during data acquisition, occasionally reducing accuracy. This limitation underscores the importance of improved ergonomics and fixation supports, which are currently under refinement.
- Inverse kinematics resolution, which may reduce the detail in reconstructed joint trajectories, though they are still adequate for detecting clinically meaningful differences.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIDA-Q_BAL & MOB | Balance and mobility subscale of the Daily living Activities Questionnaire |
| DIDA-Q_COG | Cognition subscale of the Daily living Activities Questionnaire |
| DIDA-Q_TOT | Total score of the Daily living Activities Questionnaire |
| EDSS | Expanded Disability Status Scale |
| ICC | Intraclass correlation coefficient |
| M | Mean |
| MFIS_C | Cognitive subscale of the Modified Fatigue Impact Scale |
| MFIS_P | Physical subscale of the Modified Fatigue Impact Scale |
| MFIS_PS | Psychosocial subscale of the Modified Fatigue Impact Scale |
| MFIS_TOT | Total score of the Modified Fatigue Impact Scale |
| MS | Multiple Sclerosis |
| MSWS-12 | 12-item Multiple Sclerosis Walking Scale |
| PPMS | Primary progressive Multiple Sclerosis |
| PwMS | People with Multiple Sclerosis |
| RRMS | Relapsing–remitting Multiple Sclerosis |
| SD | Standard deviation |
| SE | Standard error |
| SPMS | Secondary progressive Multiple Sclerosis |
| SUS | System Usability Scale |
| T25FW | Timed-25 Foot Walk Test |
| TUG | Timed-Up and Go Test |
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple Sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, F.; Gaßner, H.; Hannik, J.; Lee, D.H.; Flachenecker, P.; Winkler, J.; Eskofier, B.; Linker, R.A.; Klucken, J. Objective Sensor-Based Gait Measures Reflect Motor Impairment in Multiple Sclerosis Patients: Reliability and Clinical Validation of a Wearable Sensor Device. Mult. Scler. Relat. Disord. 2020, 39, 101903. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Cohen, J.A.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium. Validity of the Timed 25-Foot Walk as an Ambulatory Performance Outcome Measure for Multiple Sclerosis. Mult. Scler. J. 2017, 23, 704–710. [Google Scholar] [CrossRef]
- Cattaneo, D.; Regola, A.; Meotti, M. Validity of Six Balance Disorders Scales in Persons with Multiple Sclerosis. Disabil. Rehabil. 2006, 28, 789–795. [Google Scholar] [CrossRef]
- Motl, R.W.; Snook, E.M. Confirmation and Extension of the Validity of the Multiple Sclerosis Walking Scale-12 (MSWS-12). J. Neurol. Sci. 2008, 268, 69–73. [Google Scholar] [CrossRef]
- Nilsagård, Y.; Carling, A.; Forsberg, A. Activities-Specific Balance Confidence in People with Multiple Sclerosis. Mult. Scler. Int. 2012, 2012, 613925. [Google Scholar] [CrossRef]
- Brichetto, G.; Pedullà, L.; Podda, J.; Tacchino, A. Beyond Center-Based Testing: Understanding and Improving Functioning with Wearable Technology in MS. Mult. Scler. J. 2019, 25, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Dini, M.; Comi, G.; Leocani, L. Digital Remote Monitoring of People with Multiple Sclerosis. Front. Immunol. 2025, 16, 1514813. [Google Scholar] [CrossRef]
- Berg-Hansen, P.; Moen, S.M.; Austeng, A.; Gonzales, V.; Klyve, T.D.; Negård, H.; Seeberg, T.M.; Celius, E.G.; Meyer, F. Sensor-Based Gait Analyses of the Six-Minute Walk Test Identify Qualitative Improvement in Gait Parameters of People with Multiple Sclerosis after Rehabilitation. J. Neurol. 2022, 269, 3723–3734. [Google Scholar] [CrossRef]
- Tulipani, L.J.; Meyer, B.; Fox, S.; Solomon, A.J.; McGinnis, R.S. The Sit-to-Stand Transition as a Biomarker for Impairment: Comparison of Instrumented 30-Second Chair Stand Test and Daily Life Transitions in Multiple Sclerosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1213–1222. [Google Scholar] [CrossRef]
- Salis, F.; Bertuletti, S.; Bonci, T.; Caruso, M.; Scott, K.; Alcock, L.; Buckley, E.; Gazit, E.; Hansen, C.; Schwickert, L.; et al. A Multi-Sensor Wearable System for the Assessment of Diseased Gait in Real-World Conditions. Front. Bioeng. Biotechnol. 2023, 11, 1143248. [Google Scholar] [CrossRef]
- Chitnis, T.; Glanz, B.I.; Gonzalez, C.; Healy, B.C.; Saraceno, T.J.; Sattarnezhad, N.; Diaz-cruz, C.; Polgar-turcsanyi, M.; Tummala, S.; Bakshi, R.; et al. Quantifying Neurologic Disease Using Biosensor Measurements In-Clinic and in Free-Living Settings in Multiple Sclerosis. Npj Digit. Med. 2019, 2, 123. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Pedullà, L.; Tacchino, A.; Podda, J.; Bragadin, M.M.; Bonzano, L.; Battaglia, M.A.; Bove, M.; Brichetto, G.; Ponzio, M. The Patients’ Perspective on the Perceived Difficulties of Dual-Tasking: Development and Validation of the Dual-Task Impact on Daily-Living Activities Questionnaire (DIDA-Q). Mult. Scler. Relat. Disord. 2020, 46, 102601. [Google Scholar] [CrossRef]
- Kos, D.; Kerckhofs, E.; Carrea, I.; Verza, R.; Ramos, M.; Jansa, J. Evaluation of the Modified Fatigue Impact Scale in Four Different European Countries. Mult. Scler. J. 2005, 11, 76–80. [Google Scholar] [CrossRef]
- Brooke, J. Sus: A ‘quick and Dirty’usability. Usability Eval. Ind. 1996, 189. [Google Scholar]
- Venkatesh, V.; Tech, V.; Thong, J.Y.L.; Xu, X. Consumer Acceptance and Use of Information Technology: Extending the Unified Theory of Acceptance and Use of Technology. MIS Q. 2012, 36, 157–178. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.; Zo, H.; Choi, M. User Acceptance of Wearable Devices: An Extended Perspective of Perceived Value. Telemat. Inform. 2016, 33, 256–269. [Google Scholar] [CrossRef]
- Nam, C.; Lee, Y.-A. Validation of the Wearable Acceptability Range Scale for Smart Apparel. Fash. Text. 2020, 7, 13. [Google Scholar] [CrossRef]
- Weng, M. Digitization for Fun or Reward?: A Study of Acceptance of Wearable Devices for Personal Healthcare Digitization for Fun or Reward? A Study of Acceptance of Wearable Devices for Personal Healthcare. In Proceedings of the AcademicMindtrek ‘17: Proceedings of the 21st International Academic Mindtrek Conference, Tampere, Finland, 20–21 September 2017; pp. 154–163. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Rea, L.M.; Parker, R.A. Designing and Conducting Survey Research: A Comprehensive Guide; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 1118767039. [Google Scholar]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Rovaris, M.; Solaro, C.; Ferrarin, M.; et al. Instrumentally Assessed Gait Quality Is More Relevant than Gait Endurance and Velocity to Explain Patient-Reported Walking Ability in Early-Stage Multiple Sclerosis. Eur. J. Neurol. 2021, 28, 2259–2268. [Google Scholar] [CrossRef]
- Cohen, J. Set Correlation and Contingency Tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Müller, R.; Hamacher, D.; Hansen, S.; Oschmann, P.; Keune, P.M. Wearable Inertial Sensors Are Highly Sensitive in the Detection of Gait Disturbances and Fatigue at Early Stages of Multiple Sclerosis. BMC Neurol. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, P.G.; VanNostrand, M.; Takla, T.N.; Fritz, N.E. Predicting Real-World Physical Activity in Multiple Sclerosis: An Integrated Approach Using Clinical, Sensor-Based, and Self-Reported Measures. Sensors 2025, 25, 1780. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Caggiari, S.; Mura, A.; Corona, F.; Leban, B.; Coghe, G.; Lorefice, L.; Marrosu, M.G.; Cocco, E. Clinical Assessment of Gait in Individuals with Multiple Sclerosis Using Wearable Inertial Sensors: Comparison with Patient-Based Measure. Mult. Scler. Relat. Disord. 2016, 10, 187–191. [Google Scholar] [CrossRef]
- Kalron, A. Association between Perceived Fatigue and Gait Parameters Measured by an Instrumented Treadmill in People with Multiple Sclerosis: A Cross-Sectional Study. J. Neuroeng. Rehabil. 2015, 12, 34. [Google Scholar] [CrossRef]
- Brabrand, M.; Kellett, J.; Opio, M.; Cooksley, T.; Nickel, C.H. Should Impaired Mobility on Presentation Be a Vital Sign? Acta Anaesthesiol. Scand. 2018, 62, 945–952. [Google Scholar] [CrossRef]
- Keogh, A.; Argent, R.; Anderson, A.; Caulfield, B.; Johnston, W. Assessing the Usability of Wearable Devices to Measure Gait and Physical Activity in Chronic Conditions: A Systematic Review. J. Neuroeng. Rehabil. 2021, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Attig, C.; Franke, T. Abandonment of Personal Quantification: A Review and Empirical Study Investigating Reasons for Wearable Activity Tracking Attrition. Comput. Hum. Behav. 2020, 102, 223–237. [Google Scholar] [CrossRef]
| Characteristic | Value | |
|---|---|---|
| Age (in years), mean (SD) | 55.2 (8.0) | |
| Years of education, mean (SD) | 13.2 (3.5) | |
| Disease duration, mean (SD) | 12.6 (8.7) | |
| MS course, n (%) | RRMS | 12 (75%) |
| SPMS | 3 (19%) | |
| PPMS | 1 (6%) | |
| EDSS, mean (SD) score | 3.3 (1.3) | |
| Medications, n (%) | Siponimod | 2 (12.5%) |
| Gilenya | 2 (12.5%) | |
| Tecfidera | 1 (6%) | |
| Aubagio | 1 (6%) | |
| Natalizumab | 1 (6%) | |
| Copaxone | 1 (6%) | |
| Ocrelizumab | 1 (6%) | |
| Avonex | 1 (6%) | |
| No treatment | 6 (38%) | |
| MFIS_P, mean (SD) score | 15.7 (9.5) | |
| MFIS_C, mean (SD) score | 11.7 (7.9) | |
| MFIS_PS, mean (SD) score | 2.2 (1.8) | |
| MFIS_TOT, mean (SD) score | 29.7 (17.0) | |
| MSWS-12, mean (SD) score | 27.7 (11.6) | |
| DIDA-Q_COG, mean (SD) score | 5.9 (5.4) | |
| DIDA-Q_BAL & MOB, mean (SD) score | 12.7 (2.2) | |
| DIDA-Q_TOT, mean (SD) score | 18.6 (13.6) | |
| Test | N | M | SE | p | M Diff | Mean SE | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Min | Max | |||||||
| Clinician-scored T25FW (s) | 16 | 8.2 | 0.5 | 0.383 | −0.238 | 0.265 | −0.80 | 0.32 |
| System-scored T25FW (s) | 16 | 8.4 | 0.5 | |||||
| Clinician-scored TUG (s) | 16 | 9.9 | 0.5 | 0.447 | −0.284 | 0.364 | −1.06 | 0.49 |
| System-scored TUG (s) | 16 | 10.26 | 0.6 | |||||
| Test | N | M | SE | p | M Diff | Mean SE | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Min | Max | |||||||
| Traditional T25FW | 16 | 6.9 | 0.3 | 0.001 | −1.339 | 0.298 | −1.97 | −0.70 |
| Sensorized T25FW | 16 | 8.2 | 0.5 | |||||
| Traditional TUG | 16 | 9.9 | 0.5 | <0.001 | −2.33 | 0.343 | −3.06 | −1.60 |
| Sensorized TUG | 16 | 12.2 | 0.8 | |||||
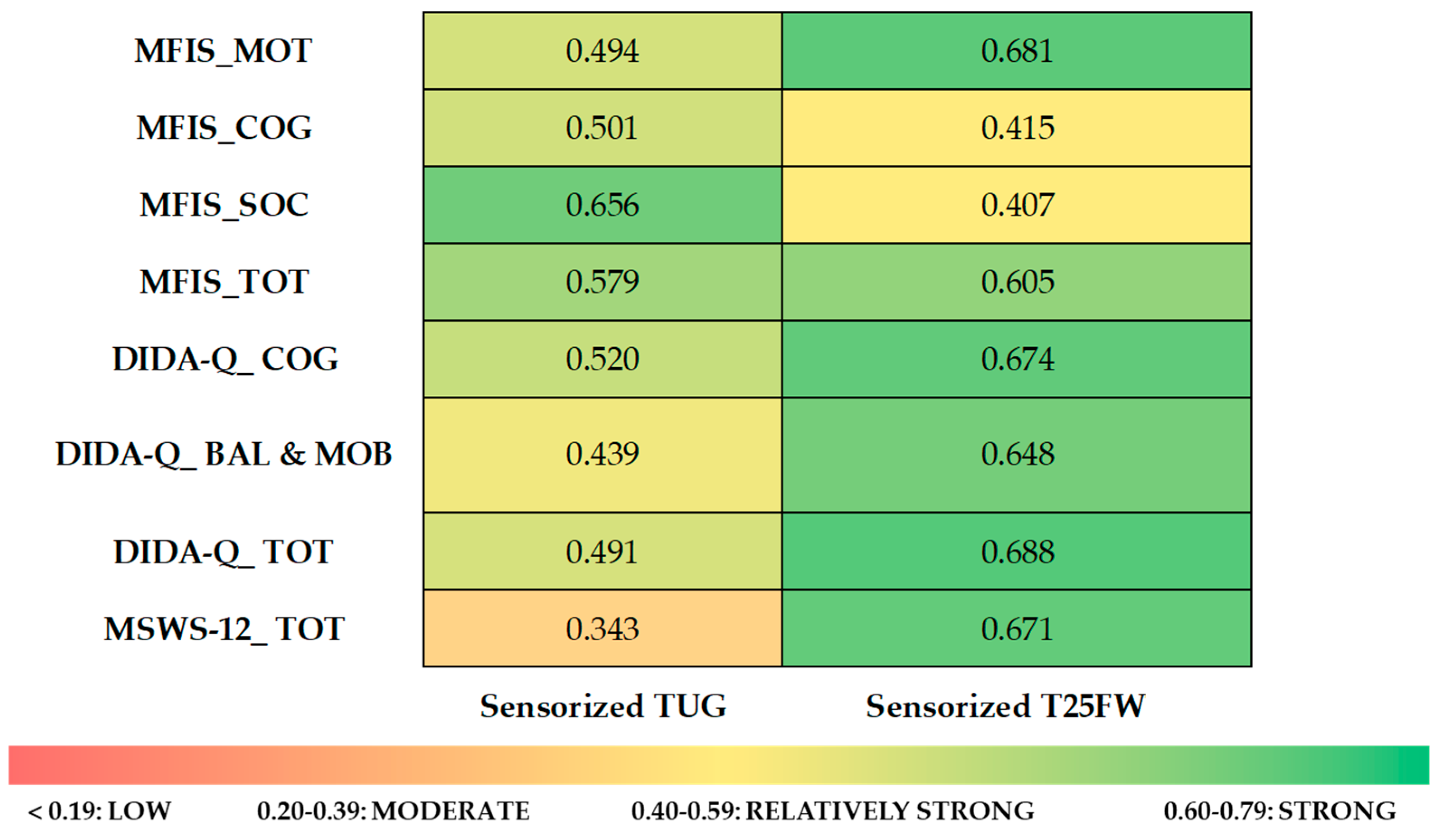
| MFIS_MOT | MFIS_COG | MFIS_SOC | MFIS_TOT | DIDA-Q_COG | DIDA-Q_BAL & MOB | DIDA-Q_TOT | MSWS-12_TOT | ||
|---|---|---|---|---|---|---|---|---|---|
| Sensorized T25FW | r | 0.681 | 0.415 | 0.407 | 0.605 | 0.674 | 0.648 | 0.688 | 0.671 |
| p | 0.004 | 0.110 | 0.118 | 0.013 | 0.004 | 0.007 | 0.003 | 0.004 | |
| Sensorized TUG | r | 0.494 | 0.501 | 0.656 | 0.579 | 0.520 | 0.439 | 0.491 | 0.343 |
| p | 0.052 | 0.048 | 0.006 | 0.019 | 0.039 | 0.089 | 0.053 | 0.194 | |
| KPI | Subgroup | M | SE | 95% CI | p | Partial η2 | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| cadence (steps/min) | MSWS < 25 | 99.733 | 4.073 | 90.859 | 108.607 | 0.99 | 0.210 |
| MSWS ≥ 25 | 89.433 | 4.073 | 80.559 | 98.307 | |||
| cycle_duration_left (s) | MSWS < 25 | 1.126 | 0.045 | 1.029 | 1.223 | 0.01 | 0.441 |
| MSWS ≥ 25 | 1.320 | 0.045 | 1.223 | 1.417 | |||
| cycle_duration_right (s) | MSWS < 25 | 1.130 | 0.037 | 1.049 | 1.211 | 0.002 | 0.570 |
| MSWS ≥ 25 | 1.339 | 0.037 | 1.258 | 1.419 | |||
| stride_length_left (m) | MSWS < 25 | 0.730 | 0.052 | 0.616 | 0.843 | 0.223 | 0.121 |
| MSWS ≥ 25 | 0.635 | 0.052 | 0.522 | 0.749 | |||
| stride_length_right (m) | MSWS < 25 | 0.754 | 0.066 | 0.610 | 0.897 | 0.635 | 0.019 |
| MSWS ≥ 25 | 0.708 | 0.066 | 0.565 | 0.852 | |||
| max_angular_velocity_left (deg/s) | MSWS < 25 | 2.064 | 0.193 | 1.643 | 2.485 | 0.648 | 0.018 |
| MSWS ≥ 25 | 1.936 | 0.193 | 1.516 | 2.357 | |||
| max_angular_velocity_right (deg/s) | MSWS < 25 | 2.569 | 0.163 | 2.214 | 2.923 | 0.034 | 0.321 |
| MSWS ≥ 25 | 2.020 | 0.163 | 1.666 | 2.375 | |||
| swing_width_left (% of stride length) | MSWS < 25 | 32.473 | 8.991 | 12.883 | 52.063 | 0.404 | 0.059 |
| MSWS ≥ 25 | 43.477 | 8.991 | 23.887 | 63.067 | |||
| swing_width_right (% of stride length) | MSWS < 25 | 44.793 | 6.754 | 30.077 | 59.510 | 0.542 | 0.032 |
| MSWS ≥ 25 | 38.798 | 6.754 | 24.081 | 53.515 | |||
| path_length_left (% of stride length) | MSWS < 25 | 111.450 | 11.931 | 85.454 | 137.446 | 0.632 | 0.020 |
| MSWS ≥ 25 | 119.737 | 11.931 | 93.741 | 145.733 | |||
| path_length_right (% of stride length) | MSWS < 25 | 126.712 | 11.134 | 102.452 | 150.971 | 0.405 | 0.058 |
| MSWS ≥ 25 | 113.119 | 11.134 | 88.859 | 137.378 | |||
| stance_phase_left (% of cycle) | MSWS < 25 | 62.007 | 0.659 | 60.571 | 63.443 | 0.020 | 0.377 |
| MSWS ≥ 25 | 64.517 | 0.659 | 63.082 | 65.953 | |||
| stance_phase_right (% of cycle) | MSWS < 25 | 64.123 | 1.211 | 61.484 | 66.763 | 0.281 | 0.096 |
| MSWS ≥ 25 | 66.058 | 1.211 | 63.418 | 68.697 | |||
| swing_phase_left (% of cycle) | MSWS < 25 | 37.993 | 0.659 | 36.557 | 39.429 | 0.020 | 0.377 |
| MSWS ≥ 25 | 35.483 | 0.659 | 34.047 | 36.918 | |||
| swing_phase_right (% of cycle) | MSWS < 25 | 35.877 | 1.211 | 33.237 | 38.516 | 0.281 | 0.096 |
| MSWS ≥ 25 | 33.942 | 1.211 | 31.303 | 36.582 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podda, J.; Grange, E.; Latella, C.; Tacchino, A.; Valli, E.; Danovaro, L.; Milani, G.; Forleo, M.; Tatarelli, A.; Gorbani, D.; et al. A Novel Whole-Body Wearable Technology for Motor Assessment in Multiple Sclerosis: Feasibility and Usability Pilot Study. Sensors 2025, 25, 6214. https://doi.org/10.3390/s25196214
Podda J, Grange E, Latella C, Tacchino A, Valli E, Danovaro L, Milani G, Forleo M, Tatarelli A, Gorbani D, et al. A Novel Whole-Body Wearable Technology for Motor Assessment in Multiple Sclerosis: Feasibility and Usability Pilot Study. Sensors. 2025; 25(19):6214. https://doi.org/10.3390/s25196214
Chicago/Turabian StylePodda, Jessica, Erica Grange, Claudia Latella, Andrea Tacchino, Enrico Valli, Ludovica Danovaro, Gianluca Milani, Marco Forleo, Antonella Tatarelli, Davide Gorbani, and et al. 2025. "A Novel Whole-Body Wearable Technology for Motor Assessment in Multiple Sclerosis: Feasibility and Usability Pilot Study" Sensors 25, no. 19: 6214. https://doi.org/10.3390/s25196214
APA StylePodda, J., Grange, E., Latella, C., Tacchino, A., Valli, E., Danovaro, L., Milani, G., Forleo, M., Tatarelli, A., Gorbani, D., Coppola, A., Pedullà, L., Brichetto, G., & Pucci, D. (2025). A Novel Whole-Body Wearable Technology for Motor Assessment in Multiple Sclerosis: Feasibility and Usability Pilot Study. Sensors, 25(19), 6214. https://doi.org/10.3390/s25196214








