Electromyographic Diaphragm and Electrocardiographic Signal Analysis for Weaning Outcome Classification in Mechanically Ventilated Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Protocol
- Inclusion criteria:
- ‑
- Resolution of the underlying cause of respiratory failure, without the need for vasopressors or sedatives (suspended 24 h before the study)
- ‑
- Adequate oxygenation, PaO2 > 60 mmHg with an inspired oxygen fraction (FiO2) < 0.4 and a positive pressure at the end of the expiration (PEEP) < 8 cmH2O and PaO2/FiO2 > 150
- ‑
- Cardiovascular stability: heart rate < 130 beats per minute and average blood pressure > 60 mm Hg
- ‑
- Afebrile and hemodynamically stable
- ‑
- Adequate haemoglobin level > 8 g/dL
- ‑
- Adequate respiration muscle function
- ‑
- Normal basic acid and electrolyte measurements
- Exclusion criteria:
- ‑
- Age < 18 years, known pregnancy, protected adult
- ‑
- Brain damage defined by a Glasgow Coma Scale < 9
- ‑
- Severe obesity
- ‑
- Presence of a neuromuscular disease
- ‑
- Suspected or confirmed phrenic nerve lesion
- ‑
- Ongoing extracorporeal membrane oxygenation
- ‑
- Contraindication for surface electrode placement
2.2. Data Acquisition and Patients
2.3. Signal Analysis
2.4. Linear Analysis Methods
2.4.1. Frequency Domain Methods Characterisation
2.4.2. Coherence
2.5. Nonlinear Analysis Methods
2.5.1. Approximate Entropy
2.5.2. Sample Entropy
2.6. Statistical Analysis
2.7. Classification Methods
2.7.1. SVM Classifier
2.7.2. KNN Classifier
2.7.3. Naive Bayes Classifier
3. Results
3.1. Signal Analysis Results
3.2. Statistical and Correlation Analysis of Parameters Extracted from Linear and Nonlinear Methods
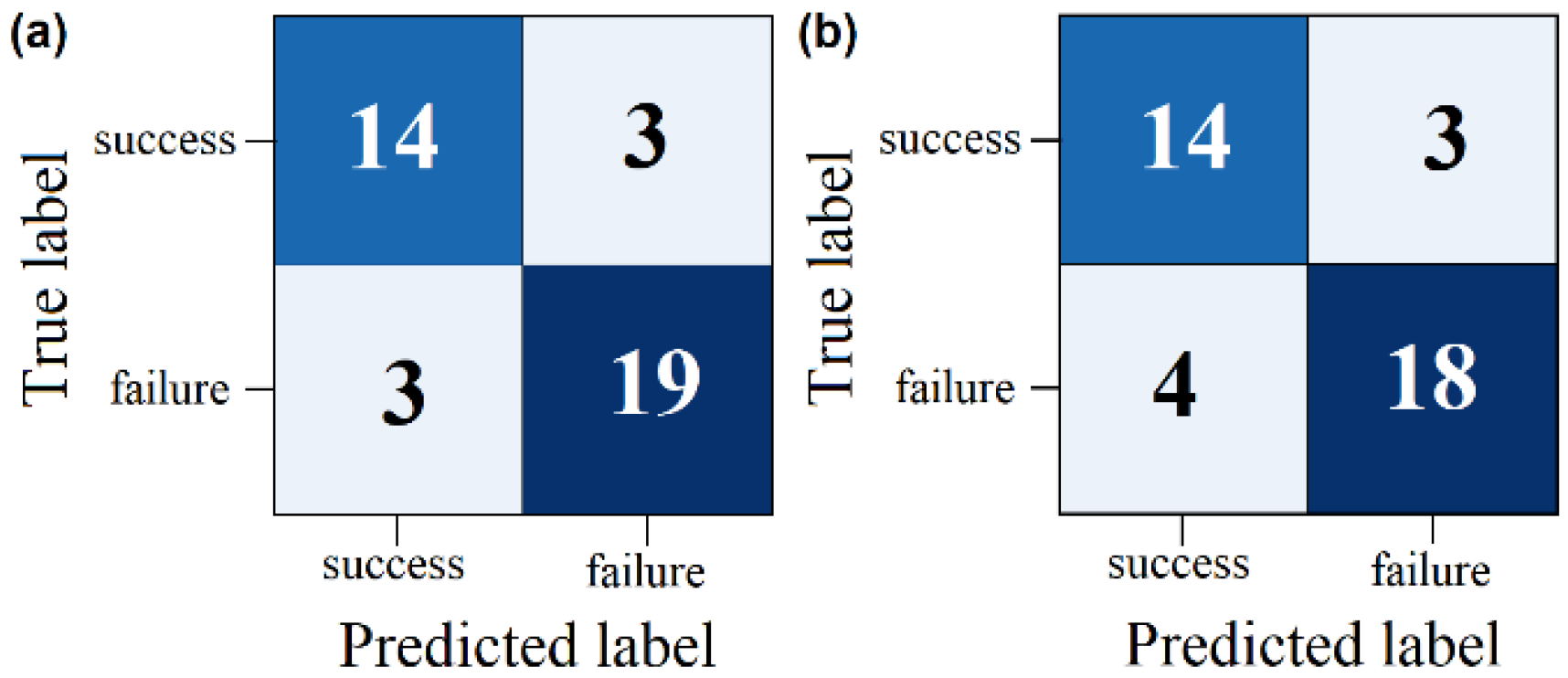
3.3. Classifier Performance Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piriyapatsom, A.; Trisukhonth, A.; Chintabanyat, O.; Chaiwat, O.; Kongsayreepong, S.; Thanakiattiwibun, C. Adherence to lung protective mechanical ventilation in patients admitted to a surgical intensive care unit and the associated increased mortality. Heliyon 2024, 10, e26220. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chasteen, B. Rapid review of ventilator-induced diaphragm dysfunction. Respir. Med. 2024, 223, 107541. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, H. Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology. Am. J. Respir. Crit. Care Med. 2020, 202, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.-M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Williams, E.E.; Thodika, F.M.S.A.; Chappelow, I.; Chapman-Hatchett, N.; Dassios, T.; Greenough, A. Diaphragmatic electromyography during a spontaneous breathing trial to predict extubation failure in preterm infants. Pediatr. Res. 2022, 92, 1064–1069. [Google Scholar] [CrossRef]
- Graßhoff, J.; Petersen, E.; Farquharson, F.; Kustermann, M.; Kabitz, H.-J.; Rostalski, P.; Walterspacher, S. Surface EMG-based quantification of inspiratory effort: A quantitative comparison with Pes. Crit. Care 2021, 25, 441. [Google Scholar] [CrossRef]
- de Vries, H.; Jonkman, A.; Shi, Z.-H.; Man, A.S.-D.; Heunks, L. Assessing breathing effort in mechanical ventilation: Physiology and clinical implications. Ann. Transl. Med. 2018, 6, 387. [Google Scholar] [CrossRef]
- Dres, M.; Schmidt, M.; Ferre, A.; Mayaux, J.; Similowski, T.; Demoule, A. Diaphragm electromyographic activity as a predictor of weaning failure. Intensive Care Med. 2012, 38, 2017–2025. [Google Scholar] [CrossRef]
- van Leuteren, R.W.; Hutten, G.J.; de Waal, C.G.; Dixon, P.; van Kaam, A.H.; de Jongh, F.H. Processing transcutaneous electromyography measurements of respiratory muscles, a review of analysis techniques. J. Electromyogr. Kinesiol. 2019, 48, 176–186. [Google Scholar] [CrossRef]
- AbuNurah, H.Y.; Russell, D.W.; Lowman, J.D. The validity of surface EMG of extra-diaphragmatic muscles in assessing respiratory responses during mechanical ventilation: A systematic review. Pulmonology 2020, 26, 378–385. [Google Scholar] [CrossRef]
- Karthick, P.A.; Ghosh, D.M.; Ramakrishnan, S. Surface electromyography based muscle fatigue detection using high-resolution time-frequency methods and machine learning algorithms. Comput. Methods Programs Biomed. 2018, 154, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Bronco, A.; Marocco, S.A.; Pozzi, M.; Sala, V.; Eronia, N.; Villa, G.; Foti, G.; Tagliabue, G.; Eger, M.; et al. Measurement of Diaphragmatic Electrical Activity by Surface Electromyography in Intubated Subjects and Its Relationship with Inspiratory Effort. Respir. Care 2018, 63, 1341–1349. [Google Scholar] [CrossRef]
- Latremouille, S.; Bhuller, M.; Shalish, W.; Sant’Anna, G. Cardiorespiratory measures shortly after extubation and extubation outcomes in extremely preterm infants. Pediatr. Res. 2023, 93, 1687–1693. [Google Scholar] [CrossRef]
- Arboleda, A.; Franco, M.; Amado, L.; Naranjo, F.; Giraldo, B.F. Coherence Analysis between the Surface Diaphragm EMG Envelope Signal and the Respiratory Signal derived from the ECG in Patients assisted by Mechanical Ventilation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 1923–1926. [Google Scholar] [CrossRef]
- Arboleda, A.; Amado, L.; Rodriguez, J.; Naranjo, F.; Giraldo, B.F. A new protocol to compare successful versus failed patients using the electromyographic diaphragm signal in extubation process. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 5646–5649. [Google Scholar] [CrossRef]
- Pinto, J.; González, H.; Arizmendi, C.; González, H.; Muñoz, Y.; Giraldo, B.F. Analysis of the Cardiorespiratory Pattern of Patients Undergoing Weaning Using Artificial Intelligence. Int. J. Environ. Res. Public Health 2023, 20, 4430. [Google Scholar] [CrossRef]
- Arcentales, A.; Caminal, P.; Diaz, I.; Benito, S.; Giraldo, B.F. Classification of patients undergoing weaning from mechanical ventilation using the coherence between heart rate variability and respiratory flow signal. Physiol. Meas. 2015, 36, 1439–1452. [Google Scholar] [CrossRef]
- Giraldo, B.; Arizmendi, C.; Romero, E.; Alquezar, R.; Caminal, P.; Benito, S.; Ballesteros, D. Patients on weaning trials from mechanical ventilation classified with neural networks and feature selection. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology—Proceedings, New York, NY, USA, 30 August–3 September 2006; pp. 2195–2198. [Google Scholar] [CrossRef]
- Chaparro, J.A.; Giraldo, B.F. Power index of the inspiratory flow signal as a predictor of weaning in intensive care units. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 78–81. [Google Scholar] [CrossRef]
- Sarlabous, L.; Aquino-Esperanza, J.; Magrans, R.; de Haro, C.; López-Aguilar, J.; Subirà, C.; Batlle, M.; Rué, M.; Gomà, G.; Ochagavia, A.; et al. Development and validation of a sample entropy-based method to identify complex patient-ventilator interactions during mechanical ventilation. Sci. Rep. 2020, 10, 13911. [Google Scholar] [CrossRef]
- Giordano, G.; Alessandri, F.; Tosi, A.; Zullino, V.; Califano, L.; Petramala, L.; Galardo, G.; Pugliese, F. Heart Rate Variability During Weaning from Invasive Mechanical Ventilation: A Systematic Review. J. Clin. Med. 2024, 13, 7634. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, J.; Deng, Y.; Tu, L.; Ding, Y.; Zhang, Y.; Yuan, X.; Xu, K.; Guo, L.; Gao, N. Advances in Machine Learning for Mechanically Ventilated Patients. Int. J. Gen. Med. 2025, 18, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Hao, A.T.-H.; Liu, S.-C.; Chang, Y.-C.; Tsai, Y.-T.; Weng, S.-J.; Chan, M.-C.; Wang, C.-Y.; Xu, Y.-Y. Prediction of Spontaneous Breathing Trial Outcome in Critically Ill-Ventilated Patients Using Deep Learning: Development and Verification Study. JMIR Med. Inform. 2025, 13, e64592. [Google Scholar] [CrossRef] [PubMed]
- Sidek, K.A.; Khalil, I. Enhancement of low sampling frequency recordings for ECG biometric matching using interpolation. Comput. Methods Programs Biomed. 2013, 109, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Garde, A.; Giraldo, B.F.; Jané, R.; Latshang, T.D.; Turk, A.J.; Hess, T.; Bosch, M.M.; Barthelmes, D.; Merz, T.M.; Hefti, J.P.; et al. Time-varying signal analysis to detect high-altitude periodic breathing in climbers ascending to extreme altitude. Med. Biol. Eng. Comput. 2015, 53, 699–712. [Google Scholar] [CrossRef]
- Giraldo, B.F.; Tellez, J.P.; Herrera, S.; Benito, S. Study of the oscillatory breathing pattern in elderly patients. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 5228–5231. [Google Scholar] [CrossRef]
- Czanner, G.; Sarma, S.V.; Ba, D.; Eden, U.T.; Wu, W.; Eskandar, E.; Lim, H.H.; Temereanca, S.; Suzuki, W.A.; Brown, E.N. Measuring the signal-to-noise ratio of a neuron. Proc. Natl. Acad. Sci. USA 2015, 112, 7141–7146. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Mercer, V.S.; Stergiou, N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: A methodological report. J. Neuroeng. Rehabil. 2007, 4, 42. [Google Scholar] [CrossRef]
- Chon, K.; Scully, C.; Lu, S. Approximate entropy for all signals. IEEE Eng. Med. Biol. Mag. 2009, 28, 18–23. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar] [CrossRef]
- Lahmiri, S. Integrating convolutional neural networks, kNN, and Bayesian optimization for efficient diagnosis of Alzheimer’s disease in magnetic resonance images. Biomed. Signal Process Control 2023, 80, 104375. [Google Scholar] [CrossRef]
- Elsayad, A.M.; Nassef, A.M.; Al-Dhaifallah, M. Bayesian optimization of multiclass SVM for efficient diagnosis of erythemato-squamous diseases. Biomed. Signal Process. Control 2022, 71, 103223. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V.; Saitta, L. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Patgiri, C.; Ganguly, A. Adaptive thresholding technique based classification of red blood cell and sickle cell using Naïve Bayes Classifier and K-nearest neighbor classifier. Biomed. Signal Process. Control 2021, 68, 102745. [Google Scholar] [CrossRef]
- Setsirichok, D.; Piroonratana, T.; Wongseree, W.; Usavanarong, T.; Paulkhaolarn, N.; Kanjanakorn, C.; Sirikong, M.; Limwongse, C.; Chaiyaratana, N. Classification of complete blood count and haemoglobin typing data by a C4.5 decision tree, a naïve Bayes classifier and a multilayer perceptron for thalassaemia screening. Biomed. Signal Process. Control 2012, 7, 202–212. [Google Scholar] [CrossRef]
- Chang, K.M.; Liu, P.T.; Wei, T.S. Electromyography Parameter Variations with Electrocardiography Noise. Sensors 2022, 22, 5948. [Google Scholar] [CrossRef] [PubMed]
- Farago, E.; Chan, A.D.C. Detection and Reconstruction of Poor-Quality Channels in High-Density EMG Array Measurements. Sensors 2023, 23, 4759. [Google Scholar] [CrossRef]
- Smital, L.; Haider, C.R.; Vitek, M.; Leinveber, P.; Jurak, P.; Nemcova, A.; Smisek, R.; Marsanova, L.; Provaznik, I.; Felton, C.L.; et al. Real-Time Quality Assessment of Long-Term ECG Signals Recorded by Wearables in Free-Living Conditions. IEEE Trans. Biomed. Eng. 2020, 67, 2721–2734. [Google Scholar] [CrossRef]
- Rahman, S.; Karmakar, C.; Natgunanathan, I.; Yearwood, J.; Palaniswami, M. Robustness of electrocardiogram signal quality índices. J. R. Soc. Interface 2022, 19, 20220012. [Google Scholar] [CrossRef]
- Pozzi, M.; Rezoagli, E.; Bronco, A.; Rabboni, F.; Grasselli, G.; Foti, G.; Bellani, G. Accessory and Expiratory Muscles Activation During Spontaneous Breathing Trial: A Physiological Study by Surface Electromyography. Front. Med. 2022, 9, 814219. [Google Scholar] [CrossRef]
- Barwing, J.; Pedroni, C.; Olgemöller, U.; Quintel, M.; Moerer, O. Electrical activity of the diaphragm (EAdi) as a monitoring parameter in difficult weaning from respirator: A pilot study. Crit. Care 2013, 17, R182. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Yang, Y.; Huang, Y.; Liu, S.; Beck, J.; Slutsky, A.S.; Sinderby, C.; Qiu, H. Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit. Care 2012, 16, R143. [Google Scholar] [CrossRef]
- Huang, C.T.; Tsai, Y.J.; Lin, J.W.; Ruan, S.Y.; Wu, H.D.; Yu, C.J. Application of heart-rate variability in patients undergoing weaning from mechanical ventilation. Crit. Care 2014, 18, R21. [Google Scholar] [CrossRef]
- da Silva, R.B.; Neves, V.R.; Montarroyos, U.R.; Silveira, M.S.; Filho, D.C.S. Heart rate variability as a predictor of mechanical ventilation weaning outcomes. Heart Lung 2023, 59, 33–36. [Google Scholar] [CrossRef]
- Salah, H.M.; Goldberg, L.R.; Molinger, J.; Felker, G.M.; Applefeld, W.; Rassaf, T.; Tedford, R.J.; Mirro, M.; Cleland, J.G.; Fudim, M. Diaphragmatic Function in Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 80, 1647–1659. [Google Scholar] [CrossRef]
- Pincus, S.M.; Goldberger, A.L. Physiological time-series analysis: What does regularity quantify? Am. J. Physiol. 1994, 266 Pt 2, H1643–H1656. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Yu, H.Y.; Jeon, H.J. Entropy analysis of heart rate variability and its application to recognize major depressive disorder: A pilot study. Technol. Health Care 2019, 27 (Suppl. S1), 407. [Google Scholar] [CrossRef] [PubMed]
- Leistedt, S.J.J.; Linkowski, P.; Lanquart, J.-P.; E Mietus, J.; Davis, R.B.; Goldberger, A.L.; Costa, M.D. Decreased neuroautonomic complexity in men during an acute major depressive episode: Analysis of heart rate dynamics. Transl. Psychiatry 2011, 1, e27. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Kliem, A.; Yeragani, V.; Bär, K.J. Cardio-respiratory coupling in untreated patients with major depression. J. Affect. Disord. 2012, 139, 166–171. [Google Scholar] [CrossRef]
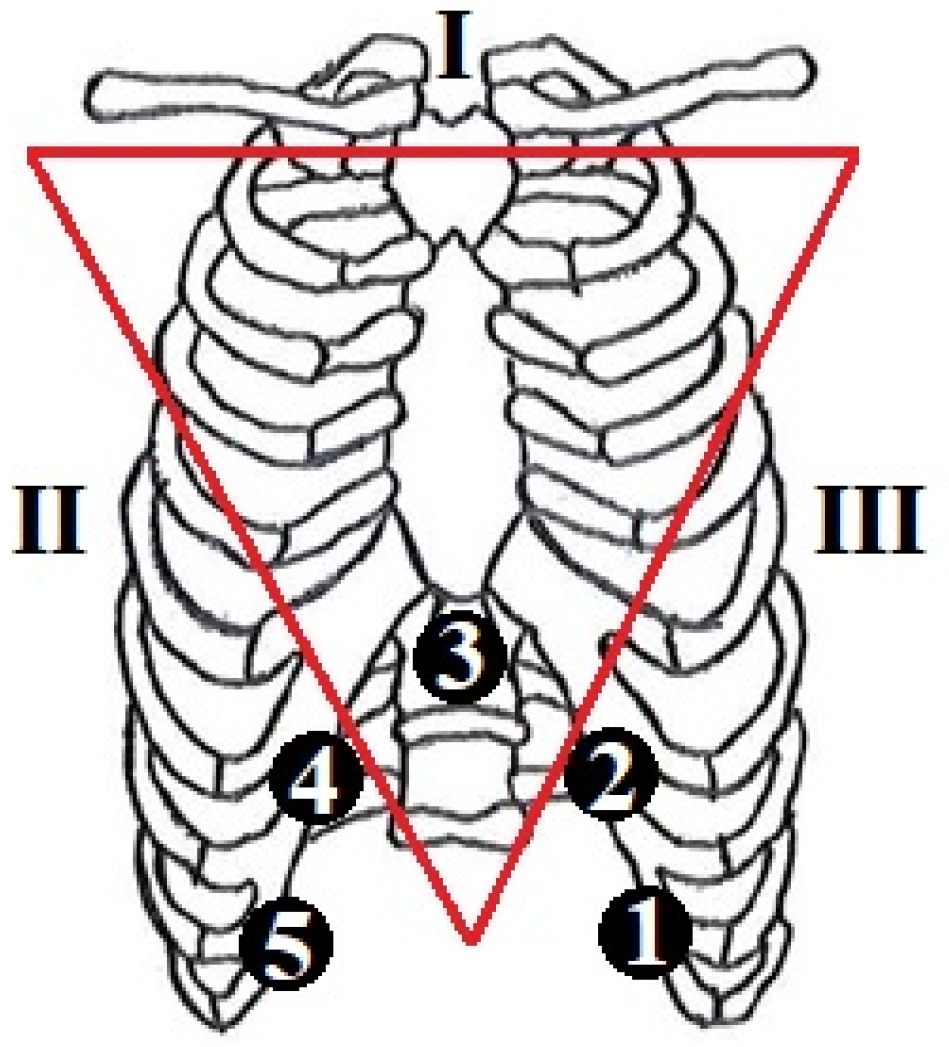
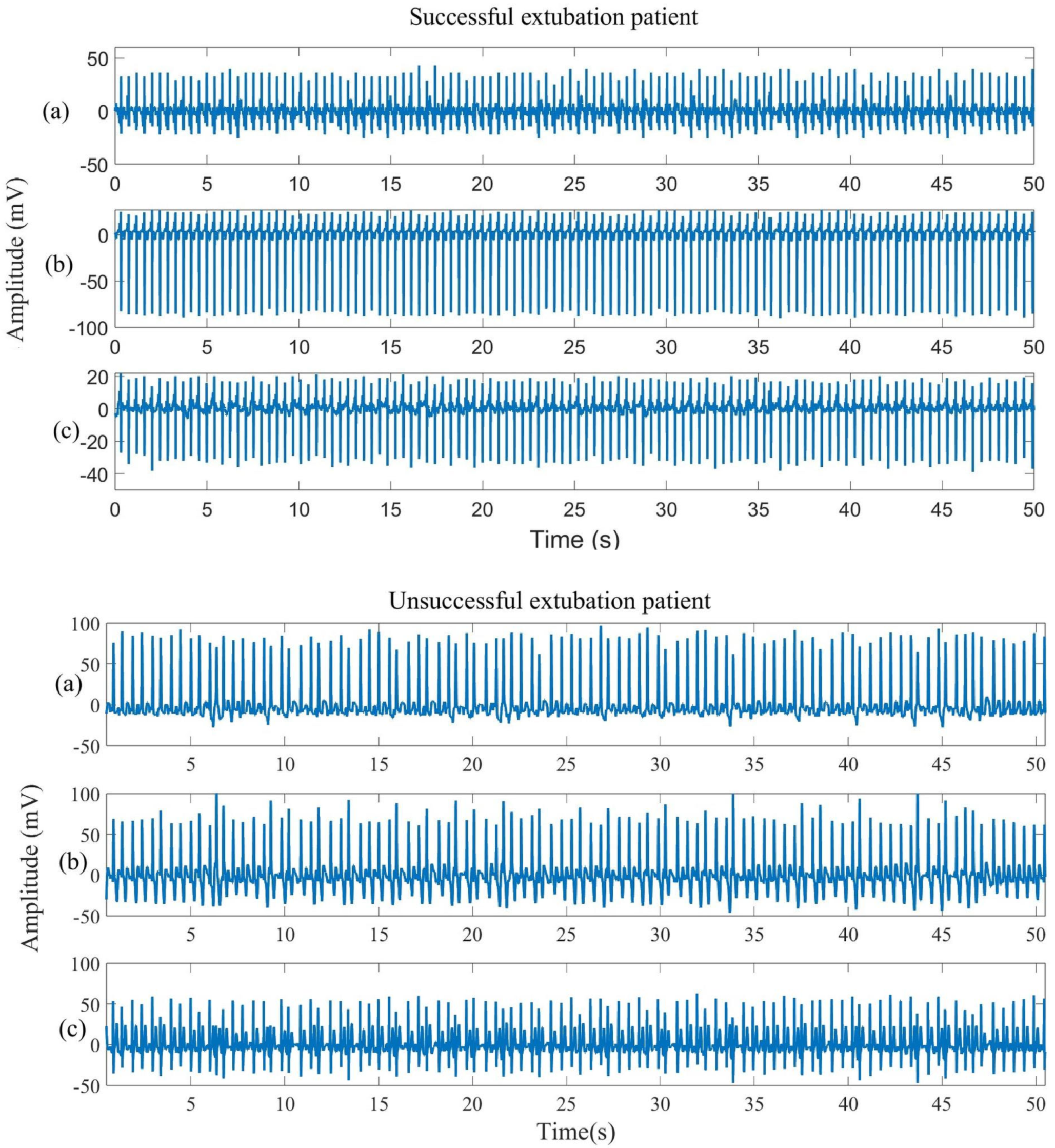

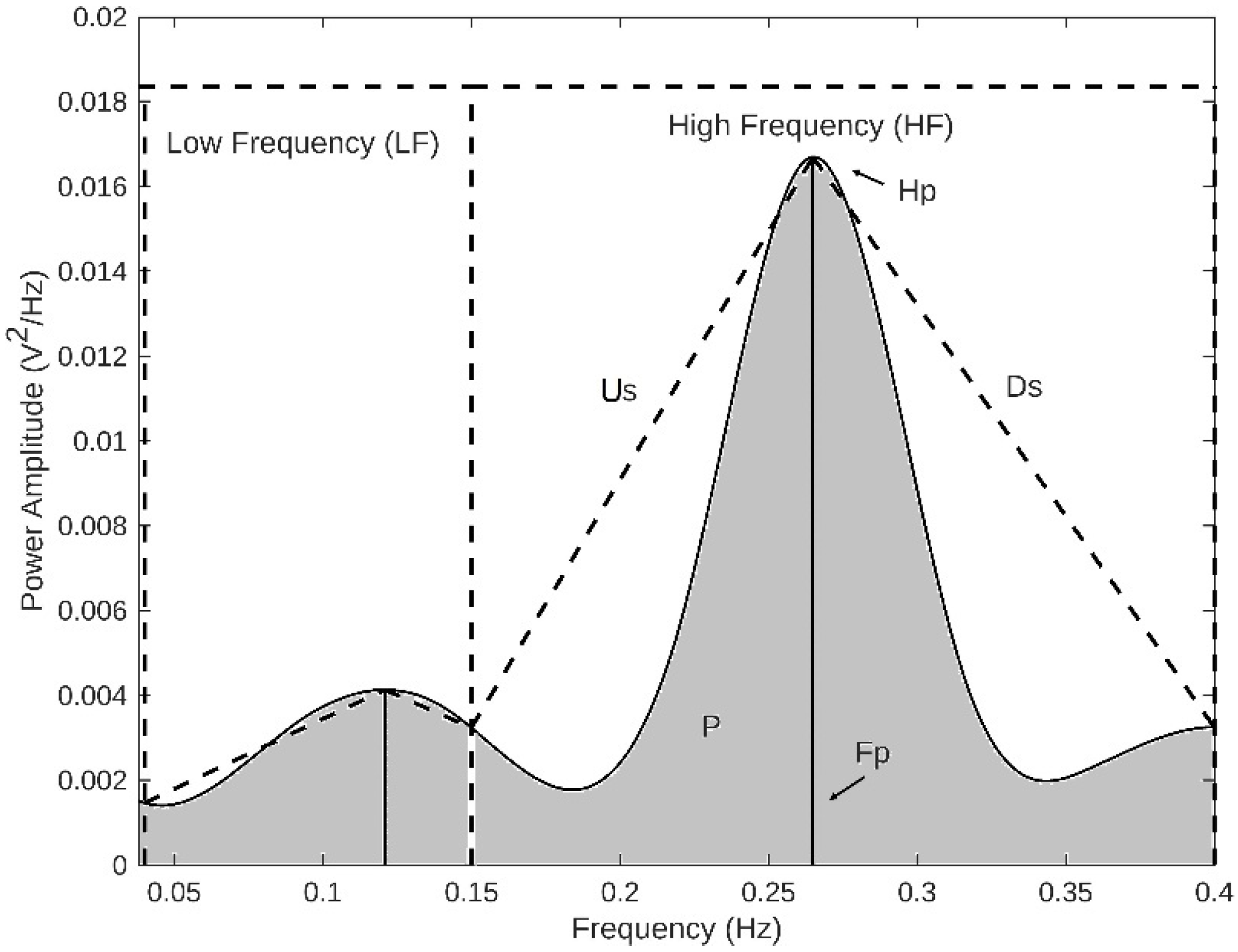


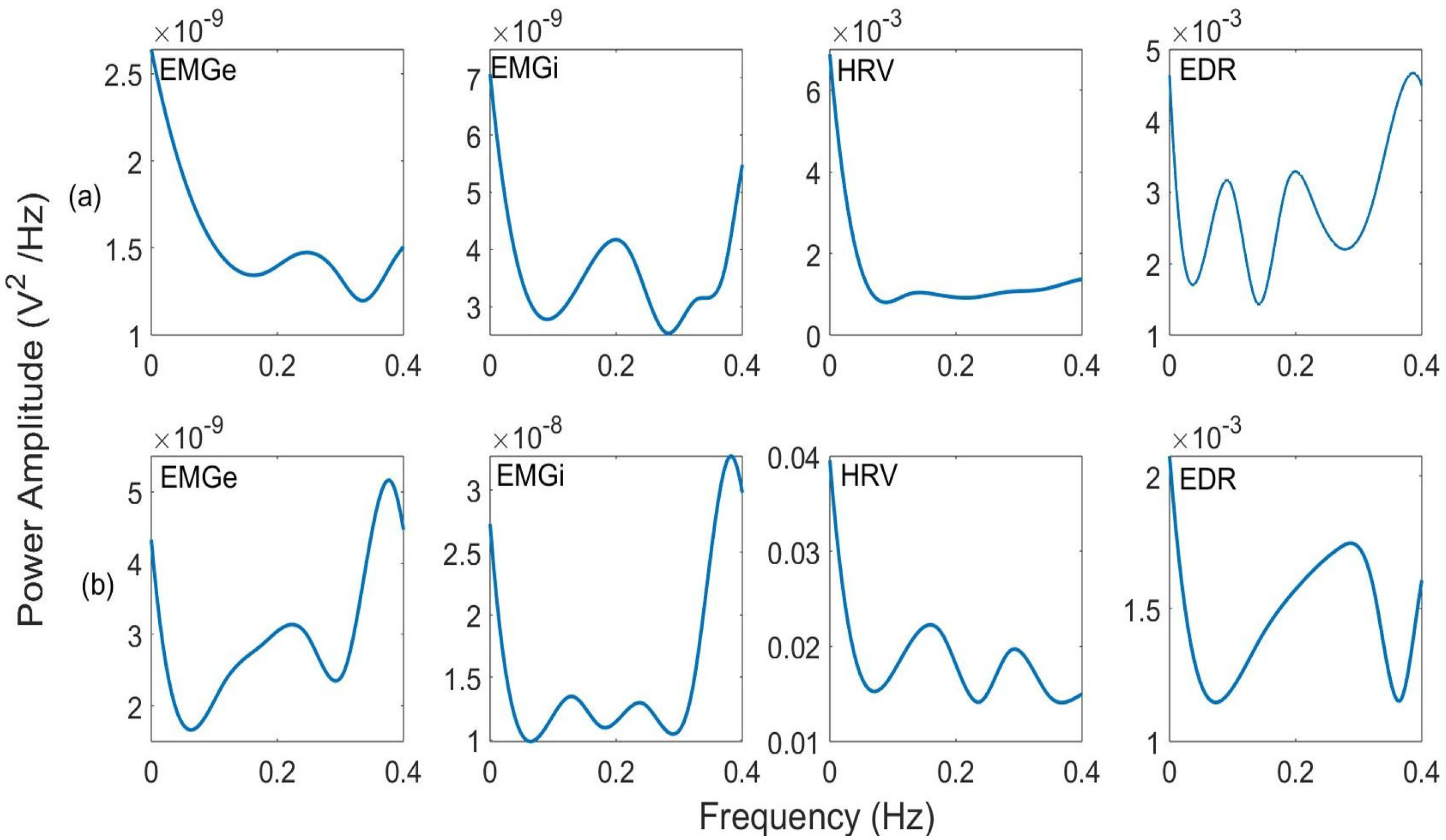
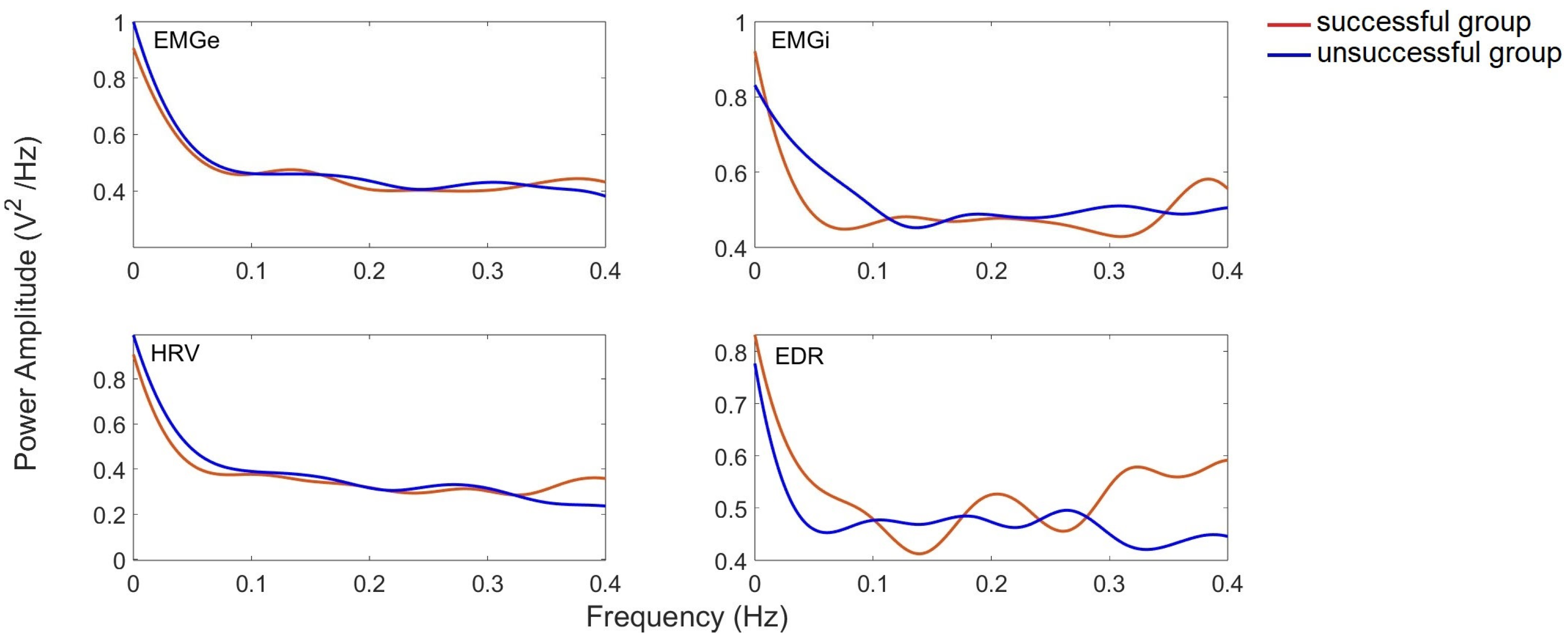
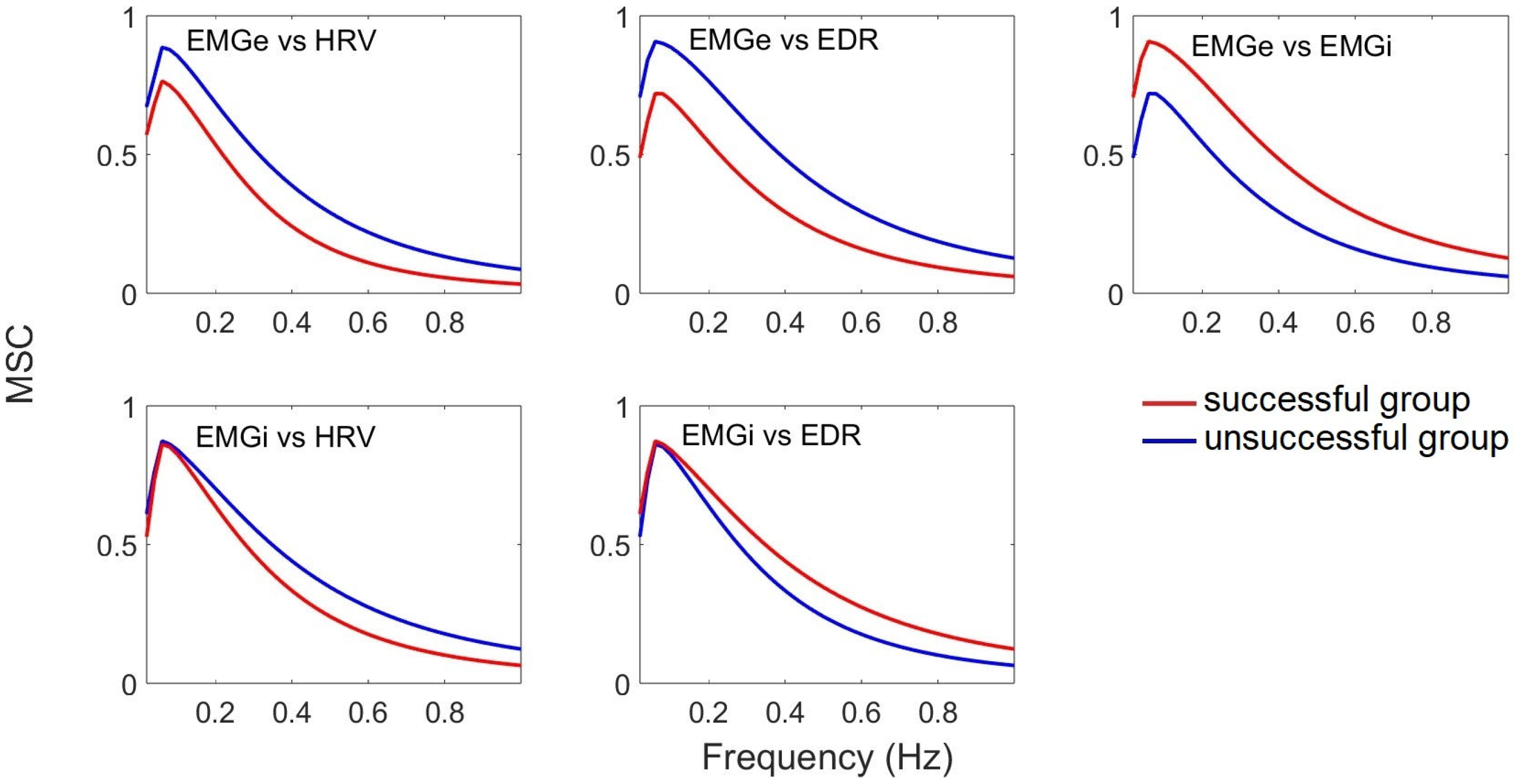


| Class | Sex (M: Male; F: Female) | Age (Years) (Mean ± SD) | VT (mL) | RR (rpm) |
|---|---|---|---|---|
| Successful group | 12 M, 7 F | 53.7 ± 23.3 | 539 ± 151.0 | 18.7 ± 3.2 |
| Failure Group | 13 M, 8 F | 68.6 ± 15.7 | 498.3 ± 100.5 | 21.2 ± 3.6 |
| Signal Analysis | Parameter | Definition |
|---|---|---|
| ECG-derived respiratory (EDR) | Highest peak modulation band at LF | |
| Power of modulation band at LF | ||
| Modulation frequency peak at LF | ||
| Slope between modulation frequency peak and start of the modulation frequency band at LF | ||
| Slope between modulation frequency peak and end of the modulation frequency band at LF | ||
| Highest peak modulation band at HF | ||
| Power of modulation band at HF | ||
| Modulation frequency peak at HF | ||
| Slope between modulation frequency peak and start of the modulation frequency band at HF | ||
| Slope between modulation frequency peak and end of the modulation frequency band at HF | ||
| Heart rate variability (HRV) | Highest peak modulation band at LF | |
| Cardiac power at LF | ||
| Modulation frequency peak at LF | ||
| Slope between modulation frequency peak and start of the modulation frequency band at LF | ||
| Slope between modulation frequency peak and end of the modulation frequency band at LF | ||
| Highest peak modulation band at HF | ||
| Cardiac power in HF | ||
| Modulation frequency peak at HF | ||
| Slope between modulation frequency peak and start of the modulation frequency band at HF | ||
| Slope between modulation frequency peak and end of the modulation frequency band at HF | ||
| EMG enveloped (EMGe) | Highest peak modulation band at LF | |
| Power of modulation band at LF | ||
| Modulation frequency peak at LF | ||
| Slope between modulation frequency peak and start of the modulation frequency band at LF | ||
| Slope between modulation frequency peak and end of the modulation frequency band at LF | ||
| Highest peak modulation band at HF | ||
| Power of modulation band at HF | ||
| Modulation frequency peak at HF | ||
| Slope between modulation frequency peak and start of the modulation frequency band at HF | ||
| Slope between modulation frequency peak and end of the modulation frequency band at HF | ||
| EMG Interpolated (EMGi) | The highest peak modulation band at LF | |
| Power of the modulation band at LF | ||
| Modulation frequency peak at LF | ||
| Slope between the modulation frequency peak and the start of the modulation frequency band at LF | ||
| Slope between the modulation frequency peak and the end of the modulation frequency band at LF | ||
| Highest peak modulation band at HF | ||
| Power of the modulation band at HF | ||
| Modulation frequency peak at HF | ||
| Slope between the modulation frequency peak and the start of the modulation frequency band at HF | ||
| Slope between the modulation frequency peak and the end of the modulation frequency band at HF |
| Analysis | Parameters | Definition |
|---|---|---|
| Cardiac and diaphragmatic interaction | Power of coherence between EMGe and EDR | |
| Power of coherence between EMGe and HRV | ||
| Power of coherence between EMGi and EDR | ||
| Power of coherence between EMGi and HRV | ||
| Root mean square of the coherence between EMGe and EDR | ||
| Root mean square of the coherence between EMGe and HRV | ||
| Root mean square of the coherence between EMGi and EDR | ||
| Root mean square of the coherence between EMGi and HRV | ||
| Modulation frequency peak of the coherence between EMGe and EDR | ||
| Modulation frequency peak of the coherence between EMGe and HVR | ||
| Modulation frequency peak of the coherence between EMGi and EDR | ||
| Modulation frequency peak of the coherence between EMGi and HVR | ||
| Highest peak modulation band of the coherence between EMGe and EDR | ||
| Highest peak modulation band of the coherence between EMGe and HRV | ||
| Highest peak modulation band of the coherence between EMGi and EDR | ||
| Highest peak modulation band of the coherence between EMGi and HRV | ||
| Slope between modulation frequency peak and start of the modulation frequency band of the coherence between EMGe and EDR | ||
| Slope between the modulation frequency peak and the start of the modulation frequency band of the coherence between EMGe and HRV | ||
| Slope between modulation frequency peak and start of the modulation frequency band of the coherence between EMGi and EDR | ||
| Slope between the modulation frequency peak and the start of the modulation frequency band of the coherence between EMGi and HRV | ||
| Slope between modulation frequency peak and end of the modulation frequency band of coherence between EMGe and EDR | ||
| Slope between the modulation frequency peak and the end of the modulation frequency band of the coherence between EMGe and HRV | ||
| Slope between modulation frequency peak and end of the modulation frequency band of coherence between EMGi and EDR | ||
| Slope between the modulation frequency peak and the end of the modulation frequency band of the coherence between EMGi and HRV | ||
| (j) | Highest peak modulation band of coherence between EMGe and EDR. | |
| (j) | Highest peak modulation band of coherence between EMGe and HRV. | |
| (j) | Highest peak modulation band of coherence between EMGi and EDR. | |
| (j) | Highest peak modulation band of coherence between EMGi and HRV. |
| Analysis | Index | Definition |
|---|---|---|
| Cardiac and diaphragmatic complexity | Sample entropy applied to ECG-derived respiration | |
| Approximate entropy applied to ECG-derived respiration | ||
| Sample entropy applied to heart rate | ||
| Approximate entropy applied to heart rate | ||
| Sample entropy applied to EMG enveloped | ||
| Approximate entropy applied to EMG enveloped | ||
| Sample entropy applied to EMG Interpolated | ||
| Approximate entropy applied to EMG Interpolated |
| Feature | Detail | Formula | Equation No. |
|---|---|---|---|
| Mean | On average, the signal, it just adds all the samples in the signal and divides by the total number of samples n. In the discrete set of samples, the central values are the key values. | (4) | |
| Coefficient of variation | Normalised measures of distribution of data and defined as the ratio of standard deviation to the mean. | (5) | |
| Kurtosis | It defines the peaks of the data distribution in our data. If the value of K is higher means, the peak is very sharp. We get the smooth curve of the data point if the value of K is less. | (6) | |
| Interquartile range | Measure of dispersion based on the lower and upper quartile. Distance between the 75th and 25th percentile in the sample | (7) |
| No. | Parameter | Successful Group (Mean [IQR]) | Failure Group (Mean [IQR]) | p-Value |
|---|---|---|---|---|
| 1 | 50.61 [29.07–72.16] | 50.36 [24.47–76.24] | 0.0474 | |
| 2 | 2.19 × 10−1 [1.62–2.77] × 10−1 | 2.77 × 10−1 [2.08–3.48] × 10−1 | 0.0078 | |
| 3 | 9.78 × 10−9 [−0.12–2.08] × 10−8 | 3.91 × 10−9 [0.28–7.54] × 10−9 | 0.0487 | |
| 4 | 8.90 × 10−7 [−0.07–1.86] × 10−8 | 3.68 × 10−7 [1.32–6.05] × 10−7 | 0.0020 | |
| 5 | −3.00 [−4.00–−2.00] | −2.99 [−4.82–−1.16] | 0.0288 | |
| 6 | 1.48 × 10−6 [0.09–2.86] × 10−6 | 7.12 × 10−7 [0.24–1.18] × 10−6 | 0.0069 | |
| 7 | 1.89 × 10−8 [−0.01–3.81] × 10−8 | 9.17 × 10−9 [0.38–1.44] × 10−8 | 0.0069 | |
| 8 | 1.71 × 10−6 [0.15–3.27] × 10−6 | 9.24 × 10−7 [0.46–1.39] × 10−6 | 0.0106 | |
| 9 | 1.38 × 10−8 [0.30–2.47] × 10−8 | 9.54 × 10−9 [0.41–1.49] × 10−8 | 0.0356 | |
| 10 | 3.01 × 10−6 [0.81–5.21] × 10−6 | 2.08 × 10−6 [1.09–3.07] × 10−6 | 0.0094 | |
| 11 | 0.86 [8.04–9.30] × 10−1 | 0.73 [5.57–9.20] × 10−1 | 0.0003 | |
| 12 | 0.88 [8.26–9.42] × 10−1 | 0.72 [5.26–9.16] × 10−1 | 0.0034 | |
| 13 | 0.81 [7.56–8.82] × 10−1 | 0.17 [−0.07–0.35] × 10−2 | 0.0373 | |
| 14 | 0.84 [7.32–9.53] × 10−1 | 0.87 [8.16–9.36] × 10−1 | 0.0470 |
| No. | Feature | Parameter | Successful Group (Mean ± SD) | Failure Group (Mean ± SD) | p-Value |
|---|---|---|---|---|---|
| 15 | CV | (0.4 Hz) | 1.54 ± 0.0400 | 1.29 ± 0.0300 | 0.0021 |
| 16 | 1.90 ± 0.0200 | 1.39 ± 0.0200 | <0.0001 | ||
| 17 | K | 2.03 ± 0.0100 | 2.15 ± 0.0200 | 0.0003 | |
| 18 | 4.93 ± 0.0910 | 4.75 ± 0.1020 | 0.0199 | ||
| 19 | (0.4 Hz) | 3.01 ± 0.0830 | 3.85 ± 0.0980 | <0.0001 | |
| 20 | (0.4 Hz) | 1.87 ± 0.0030 | 2.49 ± 0.0050 | <0.0001 | |
| 21 | 1.98 ± 0.0090 | 3.12 ± 0.0150 | <0.0001 | ||
| 22 | (0.02 Hz) | 2.21 ± 0.0300 | 2.50 ± 0.0400 | 0.0015 | |
| 23 | (0.02 Hz) | 2.43 ± 0.0300 | 2.61 ± 0.0200 | 0.0088 | |
| 24 | (0.4 Hz) | 2.74 ± 0.0400 | 3.75 ± 0.0300 | <0.0001 | |
| 25 | 4.93 ± 0.0500 | 4.40 ± 0.0600 | <0.0001 | ||
| 26 | (0.02 Hz) | 1.98 ± 0.0080 | 2.52 ± 0.0090 | <0.0001 | |
| 27 | (0.4 Hz) | 1.56 ± 0.0090 | 2.54 ± 0.0180 | <0.0001 | |
| 28 | 1.62 ± 0.0080 | 2.89 ± 0.0060 | <0.0001 | ||
| 29 | IQR | 0.03 ± 0.0016 | 0.10 ± 0.0024 | <0.0001 | |
| 30 | 0.12 ± 0.0023 | 0.20 ± 0.0034 | 0.0204 | ||
| 31 | 0.13 ± 0.0029 | 0.24 ± 0.0035 | 0.0040 | ||
| 32 | 0.09 ± 0.0006 | 0.26 ± 0.0008 | 0.0405 | ||
| 33 | 0.00 ± 0.0003 | 0.02 ± 0.0003 | 0.0109 | ||
| 34 | (0.02 Hz) | 0.27 ± 0.0050 | 0.47 ± 0.0070 | 0.0170 | |
| 35 | 14.90 ± 0.1870 | 7.26 ± 0.2430 | 0.0361 | ||
| 36 | 0.38 ± 0.0100 | 0.19 ± 0.0600 | 0.0153 | ||
| 37 | 0.20 ± 0.0041 | 0.11 ± 0.0024 | 0.0182 | ||
| 38 | 21.7 ± 2.1500 | 10.8 ± 2.2800 | 0.0073 | ||
| 39 | (0.4 Hz) | 0.48 ± 0.0100 | 0.25 ± 0.0300 | 0.0044 | |
| 40 | 0.93 ± 0.0390 | 0.47 ± 0.0350 | 0.0020 |
| No. | Parameter | Successful Group (Mean ± SD) | Failure Group (Mean ± SD) | p-Value |
|---|---|---|---|---|
| 41 | 1.8049 ± 0.3369 | 1.5611 ± 0.3493 | 0.0366 | |
| 42 | 1.9057 ± 0.2892 | 1.6335 ± 0.3408 | 0.0335 | |
| 43 | 1.5415 ± 0.3088 | 1.5197 ± 0.3808 | 0.0406 |
| No. | 1 | 4 | 5 | 10 | 41 | 42 | 43 | 17 | 29 | 30 | 31 | 32 | 33 | 39 | 40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −0.1 | −0.3 | 0.0 | −0.1 | 0.1 | 0.0 | −0.3 | −0.1 | −0.1 | −0.2 | −0.5 | −0.5 | −0.2 | 0.1 | |
| 4 | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | −0.2 | −0.1 | 0.1 | −0.2 | 0.0 | ||
| 5 | 0.0 | 0.1 | −0.1 | 0.0 | 0.2 | 0.1 | −0.1 | 0.0 | 0.3 | 0.1 | −0.1 | −0.1 | |||
| 10 | −0.1 | −0.2 | −0.1 | 0.0 | −0.1 | 0.1 | −0.1 | 0.1 | −0.1 | 0.1 | 0.2 | ||||
| 41 | 0.5 | 0.0 | −0.1 | 0.1 | 0.2 | −0.2 | 0.0 | −0.2 | −0.2 | −0.1 | |||||
| 42 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | −0.3 | 0.0 | 0.0 | 0.0 | ||||||
| 43 | 0.0 | 0.3 | 0.1 | 0.0 | −0.2 | 0.1 | −0.4 | −0.1 | |||||||
| 17 | −0.1 | 0.0 | 0.2 | 0.0 | 0.3 | −0.1 | 0.0 | ||||||||
| 29 | 0.0 | 0.0 | −0.1 | 0.0 | −0.2 | 0.0 | |||||||||
| 30 | 0.2 | −0.3 | 0.0 | −0.3 | 0.2 | ||||||||||
| 31 | 0.0 | 0.4 | −0.1 | 0.2 | |||||||||||
| 32 | −0.1 | 0.4 | 0.0 | ||||||||||||
| 33 | 0.1 | 0.1 | |||||||||||||
| 39 | −0.1 | ||||||||||||||
| 40 |
| Naive Bayes Classifier (Mean ± SD) | ||||
|---|---|---|---|---|
| Attribute Set * | Accuracy | Specificity | Sensibility | F Score |
| Complete | 0.481 ± 0.16 | 0.738 ± 0.35 | 0.395 ± 0.22 | 0.514 ± 0.27 |
| Without No. 1 | 0.678 ± 0.16 | 0.566 ± 0.28 | 0.672 ± 0.26 | 0.615 ± 0.27 |
| Without No. 1-4 | 0.691 ± 0.16 | 0.590 ± 0.27 | 0.718 ± 0.24 | 0.648 ± 0.25 |
| Without No. 1-4-5 | 0.722 ± 0.16 | 0.602 ± 0.26 | 0.731 ± 0.24 | 0.660 ± 0.25 |
| Without No. 1-4-5-42 | 0.773 ± 0.16 | 0.610 ± 0.27 | 0.737 ± 0.24 | 0.668 ± 0.25 |
| Without No. 1-4-5-42-43 | 0.784 ± 0.16 | 0.628 ± 0.26 | 0.728 ± 0.25 | 0.674 ± 0.26 |
| Without No. 1-4-5-42-43-31 | 0.798 ± 0.16 | 0.637 ± 0.26 | 0.757 ± 0.25 | 0.692 ± 0.25 |
| Without No. 1-4-5-42-43-30-31 | 0.830 ± 0.17 | 0.643 ± 0.25 | 0.800 ± 0.24 | 0.713 ± 0.24 |
| Without No. 1-4-5-42-43-30-31-40 | 0.855 ± 0.12 | 0.847 ± 0.21 | 0.834 ± 0.21 | 0.840 ± 0.21 |
| Without No. 1-4-5-42-43-17-30-31-40 | 0.835 ± 0.16 | 0.788 ± 0.26 | 0.614 ± 0.23 | 0.841 ± 0.24 |
| KNN Classifier (Mean ± SD) | ||||
|---|---|---|---|---|
| Attribute Set * | Accuracy | Specificity | Sensibility | F Score |
| Complete | 0.485 ± 0.15 | 0.157 ± 0.20 | 0.731 ± 0.22 | 0.258 ± 0.21 |
| Without No. 40 | 0.560 ± 0.14 | 0.335 ± 0.27 | 0.676 ± 0.21 | 0.448 ± 0.24 |
| Without No. 43-40 | 0.641 ± 0.16 | 0.508 ± 0.27 | 0.741 ± 0.21 | 0.603 ± 0.24 |
| Without No. 43-30-40 | 0.685 ± 0.16 | 0.520 ± 0.28 | 0.774 ± 0.19 | 0.622 ± 0.23 |
| Without No. 43-29-30-40 | 0.694 ± 0.15 | 0.465 ± 0.27 | 0.831 ± 0.17 | 0.596 ± 0.22 |
| Without No. 5-43-29-30-40 | 0.675 ± 0.14 | 0.458 ± 0.27 | 0.831 ± 0.17 | 0.591 ± 0.21 |
| Without No. 5-10-43-29-30-40 | 0.679 ± 0.16 | 0.487 ± 0.29 | 0.824 ± 0.17 | 0.612 ± 0.21 |
| Without No. 5-10-43-17-29-30-40 | 0.674 ± 0.16 | 0.495 ± 0.27 | 0.808 ± 0.21 | 0.614 ± 0.23 |
| SVM Classifier (Mean ± SD) | ||||
|---|---|---|---|---|
| Attribute Set * | Accuracy | Specificity | Sensibility | F Score |
| Complete | 0.478 ± 0.14 | 0.615 ± 0.39 | 0.375 ± 0.32 | 0.466 ± 0.35 |
| Without No. 5 | 0.578 ± 0.16 | 0.412 ± 0.28 | 0.703 ± 0.28 | 0.519 ± 0.28 |
| Without No. 5-39 | 0.609 ± 0.16 | 0.397 ± 0.30 | 0.716 ± 0.27 | 0.511 ± 0.29 |
| Without No. 5-33-39 | 0.649 ± 0.16 | 0.487 ± 0.28 | 0.626 ± 0.28 | 0.548 ± 0.28 |
| Without No. 5-31-33-39 | 0.657 ± 0.17 | 0.412 ± 0.31 | 0.736 ± 0.28 | 0.528 ± 0.29 |
| Without No. 5-42-31-33-39 | 0.670 ± 0.17 | 0.458 ± 0.26 | 0.753 ± 0.26 | 0.570 ± 0.26 |
| Without No. 5-42-17-31-33-39 | 0.648 ± 0.16 | 0.495 ± 0.27 | 0.723 ± 0.27 | 0.588 ± 0.27 |
| Classifier | Accuracy | Specificity | Sensibility | F Score |
|---|---|---|---|---|
| (Mean ± SD) | ||||
| KNN | 0.855 ± 0.12 | 0.847 ± 0.21 | 0.834 ± 0.21 | 0.840 ± 0.21 |
| SVM | 0.831 ± 0.15 | 0.848 ± 0.22 | 0.818 ± 0.19 | 0.833 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arboleda, A.; Franco, M.; Naranjo, F.; Giraldo, B.F. Electromyographic Diaphragm and Electrocardiographic Signal Analysis for Weaning Outcome Classification in Mechanically Ventilated Patients. Sensors 2025, 25, 6000. https://doi.org/10.3390/s25196000
Arboleda A, Franco M, Naranjo F, Giraldo BF. Electromyographic Diaphragm and Electrocardiographic Signal Analysis for Weaning Outcome Classification in Mechanically Ventilated Patients. Sensors. 2025; 25(19):6000. https://doi.org/10.3390/s25196000
Chicago/Turabian StyleArboleda, Alejandro, Manuel Franco, Francisco Naranjo, and Beatriz Fabiola Giraldo. 2025. "Electromyographic Diaphragm and Electrocardiographic Signal Analysis for Weaning Outcome Classification in Mechanically Ventilated Patients" Sensors 25, no. 19: 6000. https://doi.org/10.3390/s25196000
APA StyleArboleda, A., Franco, M., Naranjo, F., & Giraldo, B. F. (2025). Electromyographic Diaphragm and Electrocardiographic Signal Analysis for Weaning Outcome Classification in Mechanically Ventilated Patients. Sensors, 25(19), 6000. https://doi.org/10.3390/s25196000






