Mobile App–Induced Mental Fatigue Affects Strength Asymmetry and Neuromuscular Performance Across Upper and Lower Limbs
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Research Tools
2.2.1. Soma NPT Application, Stroop Task, and Psychomotor Vigilance Task (PVT)
2.2.2. Mental Fatigue Visual Analog Scale (M-VAS)
2.2.3. NASA Task Load Index (NASA-TLX)
2.2.4. Rating of Perceived Exertion (RPE)
2.2.5. My Jump Lab Application (My Jump 3)
2.2.6. Takei 5401 Hand Grip Digital Dynamometer
2.2.7. Takei 5402 Back Muscle Dynamometer
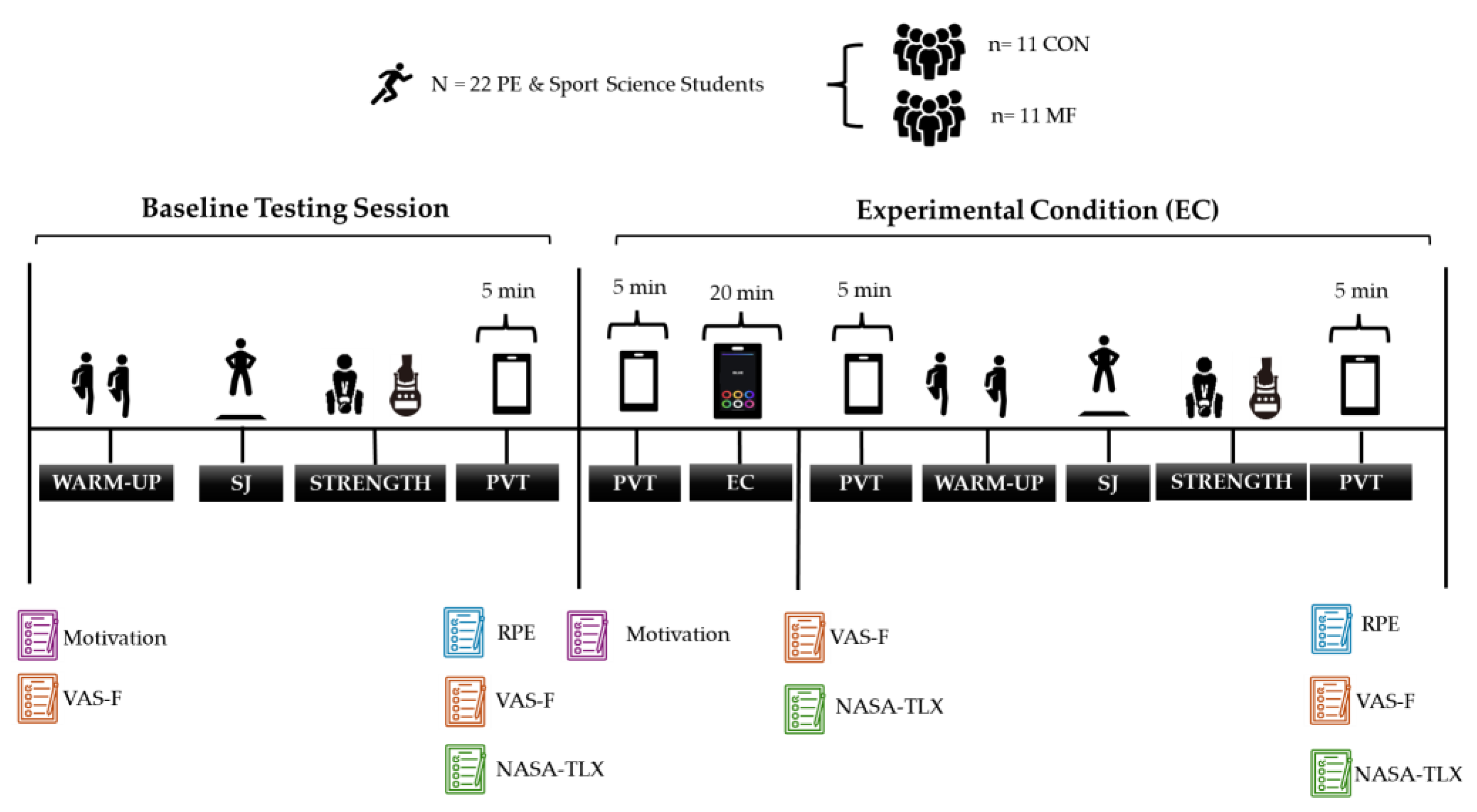
2.3. Research Procedure
2.4. Statistical Analysis
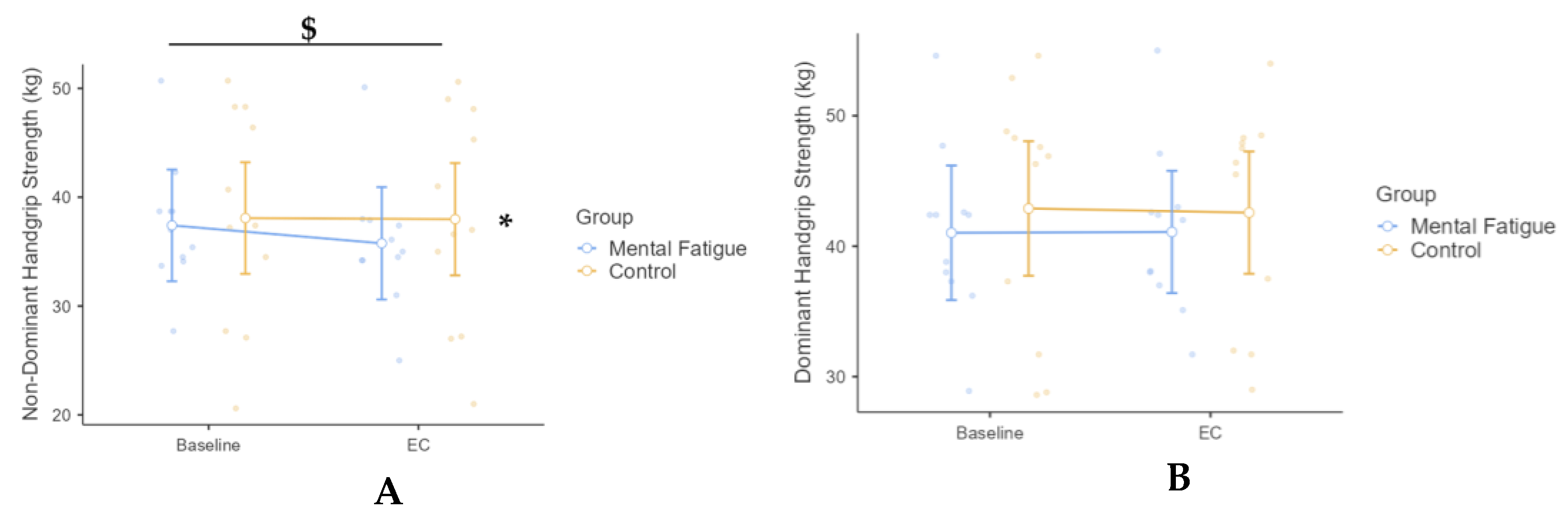
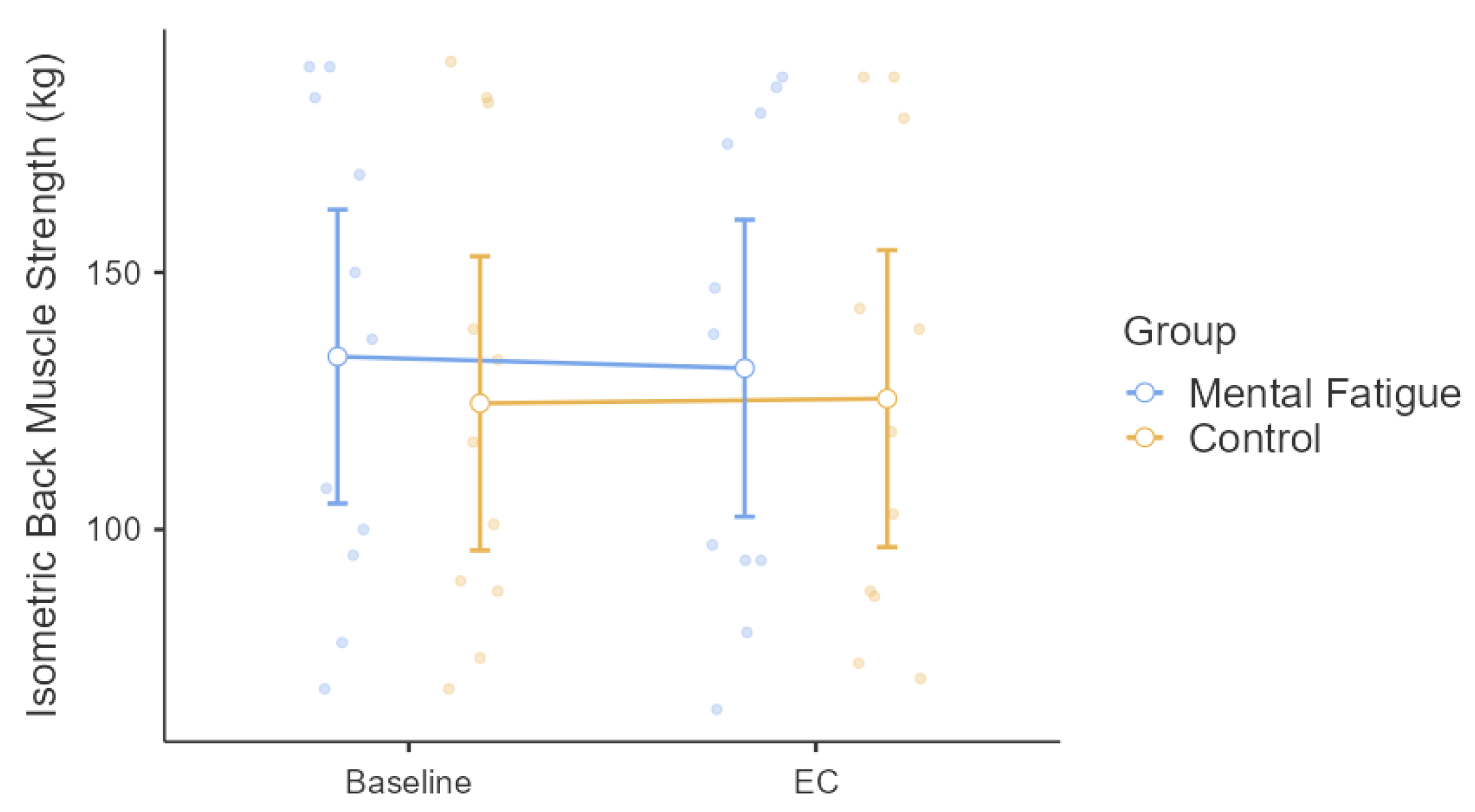
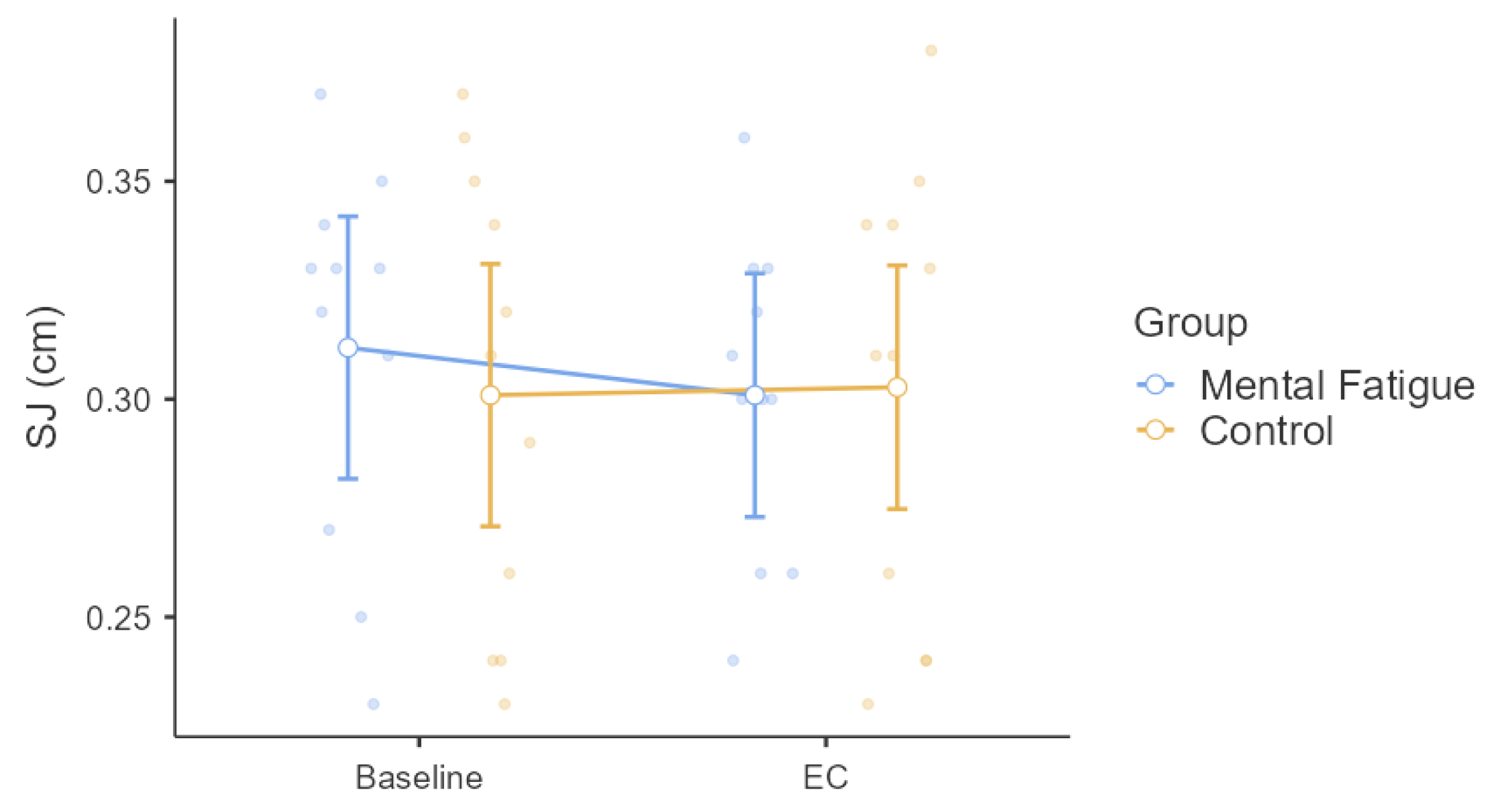
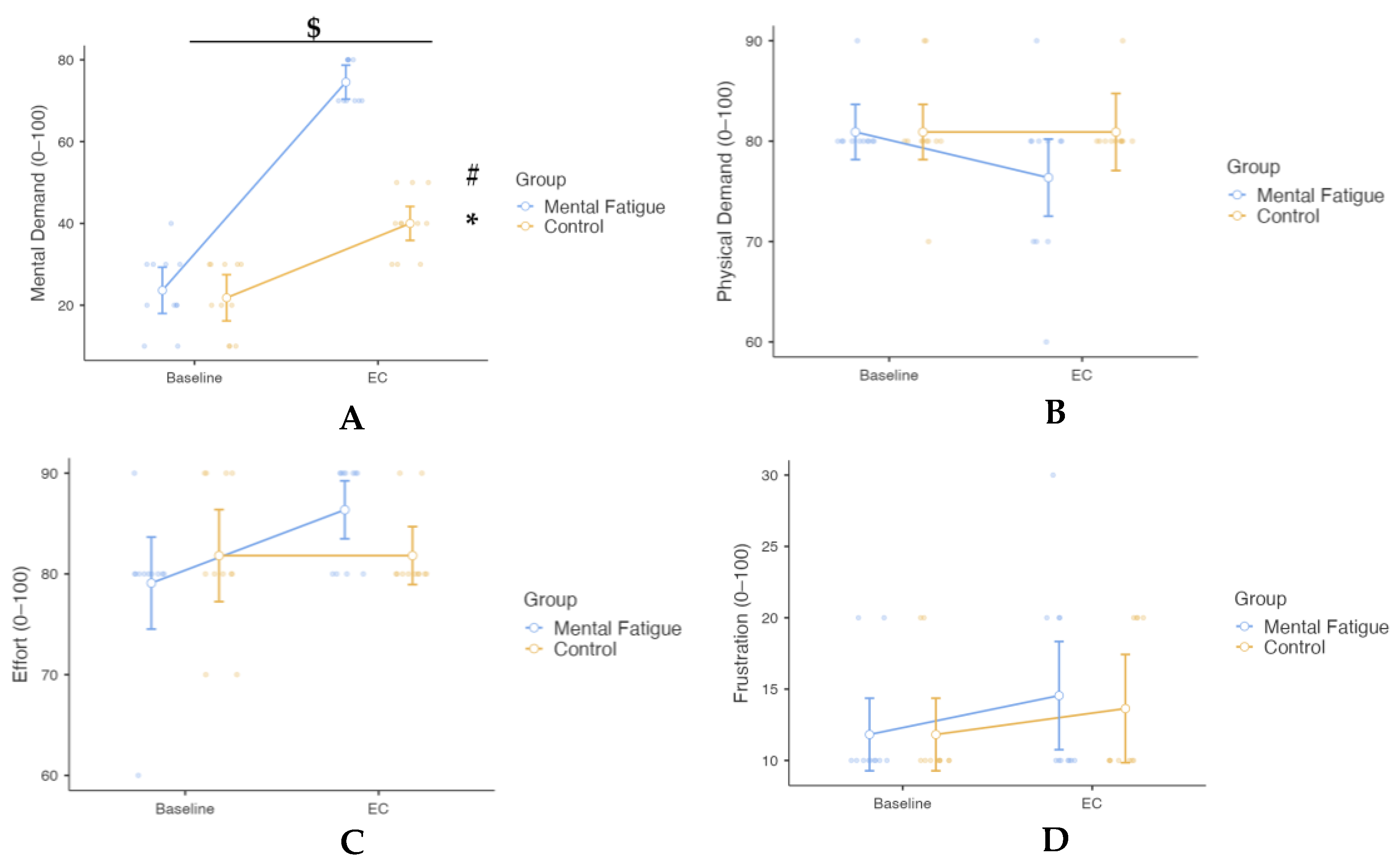
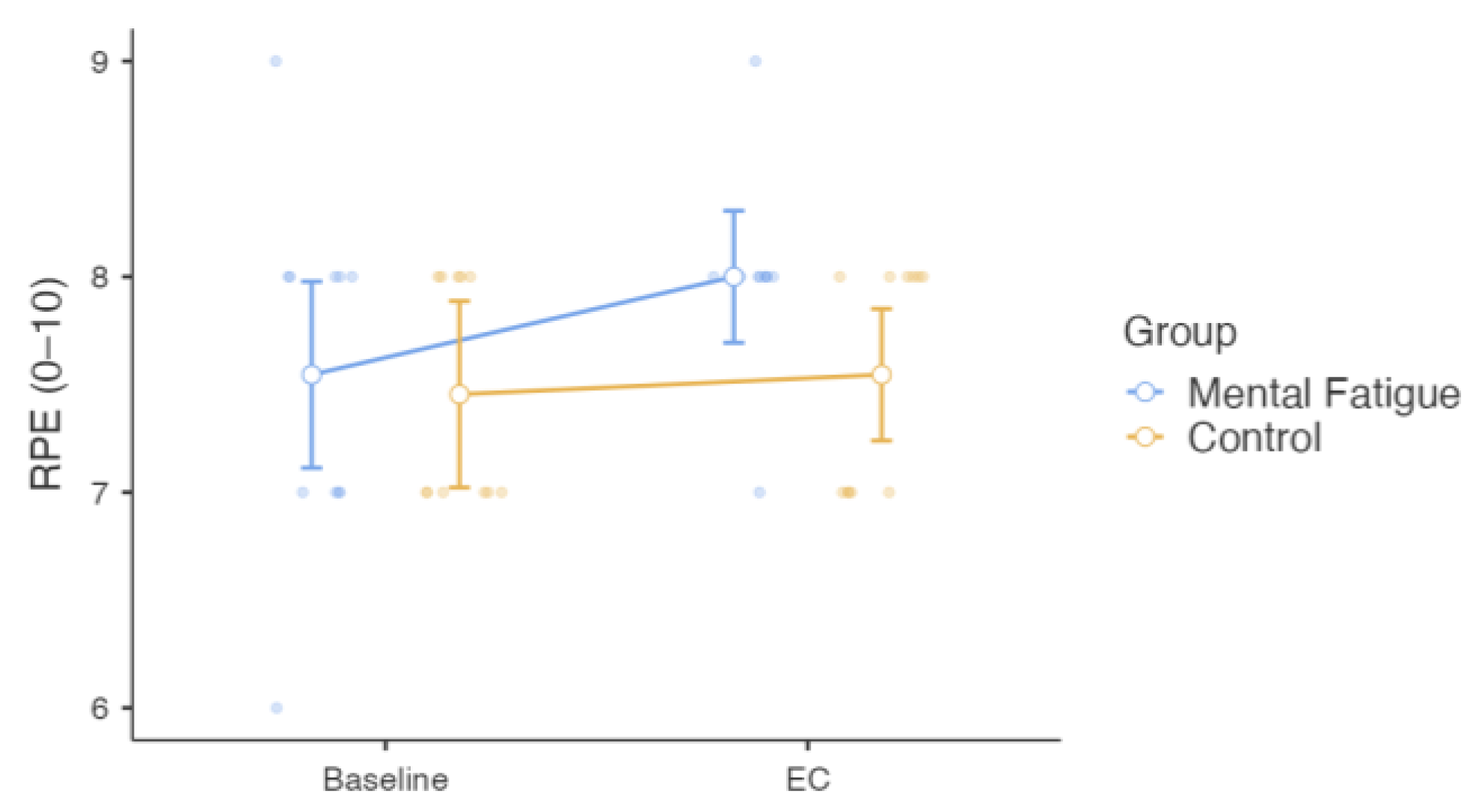
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boksem, M.A.; Tops, M. Mental fatigue: Costs and benefits. Brain Res. Rev. 2008, 59, 125–139. [Google Scholar] [CrossRef]
- Boksem, M.A.; Meijman, T.F.; Lorist, M.M. Mental fatigue, motivation and action monitoring. Biol. Psychol. 2006, 72, 123–132. [Google Scholar] [CrossRef]
- Pageaux, B.; Lepers, R. The effects of mental fatigue on sport-related performance. Prog. Brain Res. 2018, 240, 291–315. [Google Scholar]
- Russell, S.; Jenkins, D.; Smith, M.; Halson, S.; Kelly, V. The application of mental fatigue research to elite team sport performance: New perspectives. J. Sci. Med. Sport 2019, 22, 723–728. [Google Scholar] [CrossRef]
- Russell, S.; Jenkins, D.; Rynne, S.; Halson, S.L.; Kelly, V. What is mental fatigue in elite sport? Perceptions from athletes and staff. Eur. J. Sport Sci. 2019, 19, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Sun, H.; Soh, K.G.; Mohammadi, A.; Toumi, Z.; Zhang, Z. The effects of mental fatigue on sport-specific motor performance among team sport athletes: A systematic scoping review. Front. Psychol. 2023, 14, 1143618. [Google Scholar] [CrossRef] [PubMed]
- Habay, J.; Van Cutsem, J.; Verschueren, J.; De Bock, S.; Proost, M.; De Wachter, J.; Roelands, B. Mental fatigue and sport-specific psychomotor performance: A systematic review. Sports Med. 2021, 51, 1527–1548. [Google Scholar] [CrossRef]
- Smith, M.R.; Thompson, C.; Marcora, S.M.; Skorski, S.; Meyer, T.; Coutts, A.J. Mental fatigue and soccer: Current knowledge and future directions. Sports Med. 2018, 48, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Marcora, S.M.; Coutts, A.J. Mental fatigue impairs intermittent running performance. Med. Sci. Sports Exerc. 2015, 47, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Staiano, W.; Bonet, L.R.S.; Romagnoli, M.; Ring, C. Mental fatigue: The cost of cognitive loading on weight lifting, resistance training, and cycling performance. Int. J. Sports Physiol. Perform. 2023, 18, 465–473. [Google Scholar] [CrossRef]
- Li, T.; Zhang, D.; Wang, Y.; Cheng, S.; Wang, J.; Zhang, Y.; Chen, X. Research on mental fatigue during long-term motor imagery: A pilot study. Sci. Rep. 2024, 14, 18454. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, T.; Poulin-Charronnat, B.; Bard, P.; Lepers, R. Effect of mental fatigue on hand force production capacities. PLoS ONE 2024, 19, e0298958. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Baz-Valle, E.; González-Cano, H.; Jiménez-Martínez, P.; Balsalobre-Fernández, C. Mental fatigue from smartphone use or Stroop task does not affect bench press force–velocity profile, one-repetition maximum, or vertical jump performance. Mot. Control 2023, 27, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Holgado, D.; Jolidon, L.; Borragán, G.; Sanabria, D.; Place, N. Individualized mental fatigue does not impact neuromuscular function and exercise performance. Med. Sci. Sports Exerc. 2023, 55, 1823. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Alizadeh, S.; Summers, D.; Hodder, A.; Behm, D.G. The effect of a mental task versus unilateral physical fatigue on non-local muscle fatigue in recreationally active young adults. J. Sports Sci. Med. 2023, 22, 549. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Grgic, J.; Jiménez-Martínez, P.; Baz-Valle, E.; Balsalobre-Fernández, C. Effects of mental fatigue on strength endurance: A systematic review and meta-analysis. Mot. Control 2022, 27, 442–461. [Google Scholar] [CrossRef]
- Fortes, L.S.; Gantois, P.; de Lima-Júnior, D.; Barbosa, B.T.; Ferreira, M.E.C.; Nakamura, F.Y.; Fonseca, F.S. Playing videogames or using social media applications on smartphones causes mental fatigue and impairs decision-making performance in amateur boxers. Appl. Neuropsychol. Adult 2023, 30, 227–238. [Google Scholar] [CrossRef]
- Türkmen, O.B.; Akçay, B.; Demir, C.; Kurtoğlu, A.; Alotaibi, M.H.; Elkholi, S.M. Does the effect of mental fatigue created by motor imagery on upper extremity functions change with diaphragmatic breathing exercises? A randomized, controlled, single-blinded trial. Medicina 2024, 60, 1069. [Google Scholar] [CrossRef]
- Severijns, D.; Lamers, I.; Kerkhofs, L.; Feys, P. Hand grip fatigability in persons with multiple sclerosis according to hand dominance and disease progression. J. Rehabil. Med. 2015, 47, 154–160. [Google Scholar] [CrossRef]
- Jacquet, T.; Lepers, R.; Poulin-Charronnat, B.; Bard, P.; Pfister, P.; Pageaux, B. Mental fatigue induced by prolonged motor imagery increases perception of effort and the activity of motor areas. Neuropsychologia 2021, 150, 107701. [Google Scholar] [CrossRef]
- Staiano, W.; Bonet, L.R.S.; Romagnoli, M.; Ring, C. Mental fatigue impairs repeated sprint and jump performance in team sport athletes. J. Sci. Med. Sport 2024, 27, 105–112. [Google Scholar] [CrossRef]
- Díaz-García, J.; Clemente-Suárez, V.J.; Fuentes-García, J.P.; Villafaina, S. Combining HIIT plus cognitive task increased mental fatigue but not physical workload in tennis players. Appl. Sci. 2023, 13, 7046. [Google Scholar] [CrossRef]
- Angius, L.; Merlini, M.; Hopker, J.; Bianchi, M.; Fois, F.; Piras, F.; Cugia, P.; Russell, J.; Marcora, S.M. Physical and mental fatigue reduce psychomotor vigilance in professional football players. Int. J. Sports Physiol. Perform. 2022, 17, 1391–1398. [Google Scholar] [CrossRef]
- Dallaway, N.; Mortimer, H.; Gore, A.; Ring, C. Brain endurance training improves dynamic calisthenic exercise and benefits novel exercise and cognitive performance: Evidence of performance enhancement and near transfer of training. J. Strength Cond. Res. 2024, 38, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Daub, B.D.; McLean, B.D.; Heishman, A.D.; Peak, K.M.; Coutts, A.J. The relationship between mental fatigue and shooting performance over the course of a National Collegiate Athletic Association Division I basketball season. J. Strength Cond. Res. 2022, 38, 334–341. [Google Scholar] [CrossRef]
- Daub, B.D.; McLean, B.D.; Heishman, A.D.; Peak, K.M.; Coutts, A.J. Impacts of mental fatigue and sport-specific film sessions on basketball shooting tasks. Eur. J. Sport Sci. 2023, 23, 1500–1508. [Google Scholar] [CrossRef]
- López-Rodriguez, R.; Ring, C.; Díaz-García, J. The detrimental effects of mental fatigue on cognitive and physical performance in older adults are accentuated by age and attenuated by habitual physical activity. J. Aging Phys. Act. 2025, 1, 1–12. [Google Scholar] [CrossRef]
- Mortimer, H.; Dallaway, N.; Ring, C. Effects of isolated and combined mental and physical fatigue on motor skill and endurance exercise performance. Psychol. Sport Exerc. 2024, 75, 102720. [Google Scholar] [CrossRef]
- Staiano, W.; Díaz-García, J.; García-Calvo, T.; Ring, C. Brain endurance training improves soccer-specific technical skills and cognitive performance in fatigued professional soccer players. J. Sci. Med. Sport 2025, 28, 69–76. [Google Scholar] [CrossRef]
- Staiano, W.; Marcora, S.; Romagnoli, M.; Kirk, U.; Ring, C. Brain Endurance Training Improves Endurance and Cognitive tPerformance in Road Cyclists. J. Sci. Med. Sport 2023, 26, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Staiano, W.; Merlini, M.; Romagnoli, M.; Kirk, U.; Ring, C.; Marcora, S. Brain endurance training improves physical, cognitive, and multitasking performance in professional football players. Int. J. Sports Physiol. Perform. 2022, 17, 1732–1740. [Google Scholar] [CrossRef]
- Staiano, W.; Romagnoli, M.; Salazar Bonet, L.R.; Ferri-Caruana, A. Adaptive cognitive tasks for mental fatigue: An innovative paradigm for cognitive loading in human performance. J. Sci. Med. Sport 2024, 27, 883–889. [Google Scholar] [CrossRef]
- Waldman, H.S.; O’Neal, E.K.; Barker, G.A.; Witt, C.R.; Lara, D.A.; Huber, A.K.; Egan, B. No benefit of ingesting a low-dose ketone monoester supplement on markers of cognitive performance in females. J. Cogn. Enhanc. 2023, 7, 193–202. [Google Scholar] [CrossRef]
- Waldman, H.S.; O’Neal, E.K.; Barker, G.A.; Witt, C.R.; Lara, D.A.; Huber, A.K.; Egan, B. A ketone monoester with carbohydrate improves cognitive measures postexercise, but not performance in trained females. Med. Sci. Sports Exerc. 2024, 56, 725–736. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Migliaccio, G.M.; Di Filippo, G.; Russo, L.; Orgiana, T.; Ardigò, L.P.; Casal, M.Z.; Padulo, J. Effects of mental fatigue on reaction time in sportsmen. Int. J. Environ. Res. Public Health 2022, 19, 14360. [Google Scholar] [CrossRef]
- Penna, E.M.; Filho, E.; Wanner, S.P.; Campos, B.T.; Quinan, G.R.; Mendes, T.T.; Smith, M.R.; Prado, L.S. Mental fatigue impairs physical performance in young swimmers. Pediatr. Exerc. Sci. 2018, 30, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Rauch, W.A.; Schmitt, K. Fatigue of cognitive control in the Stroop-task. In Proceedings of the 31st Annual Conference of the Cognitive Science Society, Amsterdam, The Netherlands, 29 July–1 August 2009; pp. 750–755. [Google Scholar]
- Scarpina, F.; Tagini, S. The Stroop color and word test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Rubio-Morales, A.; Díaz-García, J.; Barbosa, C.; Habay, J.; López-Gajardo, M.Á.; García-Calvo, T. Do cognitive, physical, and combined tasks induce similar levels of mental fatigue? Testing the effects of different moderating variables. Mot. Control 2022, 26, 630–648. [Google Scholar] [CrossRef]
- Proost, M.; Habay, J.; De Wachter, J.; De Pauw, K.; Rattray, B.; Meeusen, R.; Van Cutsem, J. How to tackle mental fatigue: A systematic review of potential countermeasures and their underlying mechanisms. Sports Med. 2022, 52, 2129–2158. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. In Advances in Psychology; North-Holland: Amsterdam, The Netherlands, 1988; Volume 52, pp. 139–183. [Google Scholar]
- Grier, R.A. How high is high? A meta-analysis of NASA-TLX global workload scores. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting; Sage: Los Angeles, CA, USA, 2015; Volume 59, pp. 1727–1731. [Google Scholar]
- Díaz-García, J.; González-Ponce, I.; Ponce-Bordón, J.C.; López-Gajardo, M.Á.; Ramírez-Bravo, I.; Rubio-Morales, A.; García-Calvo, T. Mental load and fatigue assessment instruments: A systematic review. Int. J. Environ. Res. Public Health 2021, 19, 419. [Google Scholar] [CrossRef]
- Borg, G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre-Fernández, C.; Varela-Olalla, D. The validity and reliability of the My Jump Lab App for the measurement of vertical jump performance using artificial intelligence. Sensors 2024, 24, 7897. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Glaister, M.; Lockey, R.A. The validity and reliability of an iPhone app for measuring vertical jump performance. J. Sports Sci. 2015, 33, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Bogataj, Š.; Pajek, M.; Andrašić, S.; Trajković, N. Concurrent validity and reliability of My Jump 2 app for measuring vertical jump height in recreationally active adults. Appl. Sci. 2020, 10, 3805. [Google Scholar] [CrossRef]
- Puljić, D.; Karavas, C.; Mandroukas, A.; Stafylidis, A. Validity of the Enode Sensor and My Jump 3 App for assessing countermovement jump performance. Appl. Sci. 2024, 14, 11989. [Google Scholar] [CrossRef]
- Ding, L.; Lyu, M.; Chen, Z.; Wu, J.; Wang, Y.; Bishop, C.; Li, Y. Associations between inter-limb asymmetry in lower limb strength and jump performance in 14–15-year-old basketball players. Symmetry 2024, 16, 1421. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bohannon, R.W.; Li, X.; Yen, S.C.; Sindhu, B.; Kapellusch, J. Summary of grip strength measurements obtained in the 2011–2012 and 2013–2014 National Health and Nutrition Examination Surveys. J. Hand Ther. 2019, 32, 489–496. [Google Scholar] [CrossRef]
- Balogun, J.A.; Onigbinde, A.T. Intratester reliability and validity of the Takei Kiki Kogo hand grip dynamometer. J. Phys. Ther. Sci. 1991, 3, 55–60. [Google Scholar]
- Watanabe, T.; Owashi, K.; Kanauchi, Y.; Mura, N.; Takahara, M.; Ogino, T. The short-term reliability of grip strength measurement and the effects of posture and grip span. J. Hand Surg. 2005, 30, 603–609. [Google Scholar] [CrossRef]
- Coldwells, A.; Atkinson, G.; Reilly, T. Sources of variation in back and leg dynamometry. Ergonomics 1994, 37, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, N.; Hunwicks, R.; Jones, B.; Till, K.; Highton, J.; Twist, C. Criterion and construct validity of an isometric midthigh-pull dynamometer for assessing whole-body strength in professional rugby league players. Int. J. Sports Physiol. Perform. 2018, 13, 235–239. [Google Scholar] [CrossRef]
- Imagama, S.; Matsuyama, Y.; Hasegawa, Y.; Sakai, Y.; Ito, Z.; Ishiguro, N.; Hamajima, N. Back muscle strength and spinal mobility are predictors of quality of life in middle-aged and elderly males. Eur. Spine J. 2011, 20, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Kroll, P.G.; Machado, L.; Happy, C.; Leong, S.; Chen, B. The relationship between five measures of trunk strength. J. Back Musculoskelet. Rehabil. 2000, 14, 89–97. [Google Scholar] [CrossRef]
- Díaz-García, J.; García-Calvo, T.; López-Gajardo, M.A.; Rubio-Morales, A.; Parraca, J.A. Physical fatigue exacerbates the negative effects of mental fatigue on soccer performance in practitioners. Eur. J. Hum. Mov. 2023, 50, 62–69. [Google Scholar] [CrossRef]
- Queirós, V.S.D.; Dantas, M.; Fortes, L.D.S.; Silva, L.F.D.; Silva, G.M.D.; Dantas, P.M.S.; Cabral, B.G.D.A.T. Mental fatigue reduces training volume in resistance exercise: A cross-over and randomized study. Percept. Mot. Ski. 2021, 128, 409–423. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Lakens, D. Improving Your Statistical Inferences. 2016. Available online: https://lakens.github.io/statistical_inferences/ (accessed on 1 June 2025).
- Wuensch, K.L. SPSS Programs (Syntax); Department of Psychology, East Carolina University: Greenville, NC, USA, 2017. [Google Scholar]
- Steiger, J.H. Beyond the F Test: Effect Size Confidence Intervals and Tests of Close Fit in the Analysis of Variance and Contrast Analysis. Psychol. Methods 2004, 9, 164–182. [Google Scholar] [CrossRef]
- Smithson, M. Applications in ANOVA and Regression. In Confidence Intervals; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2003; pp. 42–66. [Google Scholar]
- Sainburg, R. Evidence for a Dynamic-Dominance Hypothesis of Handedness. Exp. Brain Res. 2002, 142, 241–258. [Google Scholar] [CrossRef]
- Gao, Z.; Fekete, G.; Baker, J.S.; Liang, M.; Xuan, R.; Gu, Y. Effects of Running Fatigue on Lower Extremity Symmetry among Amateur Runners: From a Biomechanical Perspective. Front. Physiol. 2022, 13, 899818. [Google Scholar] [CrossRef] [PubMed]
- Heil, J.; Loffing, F.; Büsch, D. The Influence of Exercise-Induced Fatigue on Inter-Limb Asymmetries: A Systematic Review. Sports Med. Open 2020, 6, 39. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and Human Performance: An Updated Framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009, 106, 857–864. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafylidis, A.; Staiano, W.; Mandroukas, A.; Michailidis, Y.; Bonet, L.R.S.; Romagnoli, M.; Metaxas, T.I. Mobile App–Induced Mental Fatigue Affects Strength Asymmetry and Neuromuscular Performance Across Upper and Lower Limbs. Sensors 2025, 25, 4758. https://doi.org/10.3390/s25154758
Stafylidis A, Staiano W, Mandroukas A, Michailidis Y, Bonet LRS, Romagnoli M, Metaxas TI. Mobile App–Induced Mental Fatigue Affects Strength Asymmetry and Neuromuscular Performance Across Upper and Lower Limbs. Sensors. 2025; 25(15):4758. https://doi.org/10.3390/s25154758
Chicago/Turabian StyleStafylidis, Andreas, Walter Staiano, Athanasios Mandroukas, Yiannis Michailidis, Lluis Raimon Salazar Bonet, Marco Romagnoli, and Thomas I. Metaxas. 2025. "Mobile App–Induced Mental Fatigue Affects Strength Asymmetry and Neuromuscular Performance Across Upper and Lower Limbs" Sensors 25, no. 15: 4758. https://doi.org/10.3390/s25154758
APA StyleStafylidis, A., Staiano, W., Mandroukas, A., Michailidis, Y., Bonet, L. R. S., Romagnoli, M., & Metaxas, T. I. (2025). Mobile App–Induced Mental Fatigue Affects Strength Asymmetry and Neuromuscular Performance Across Upper and Lower Limbs. Sensors, 25(15), 4758. https://doi.org/10.3390/s25154758










