Comparison of Marker- and Markerless-Derived Lower Body Three-Dimensional Gait Kinematics in Typically Developing Children
Abstract
1. Introduction
2. Materials and Methods
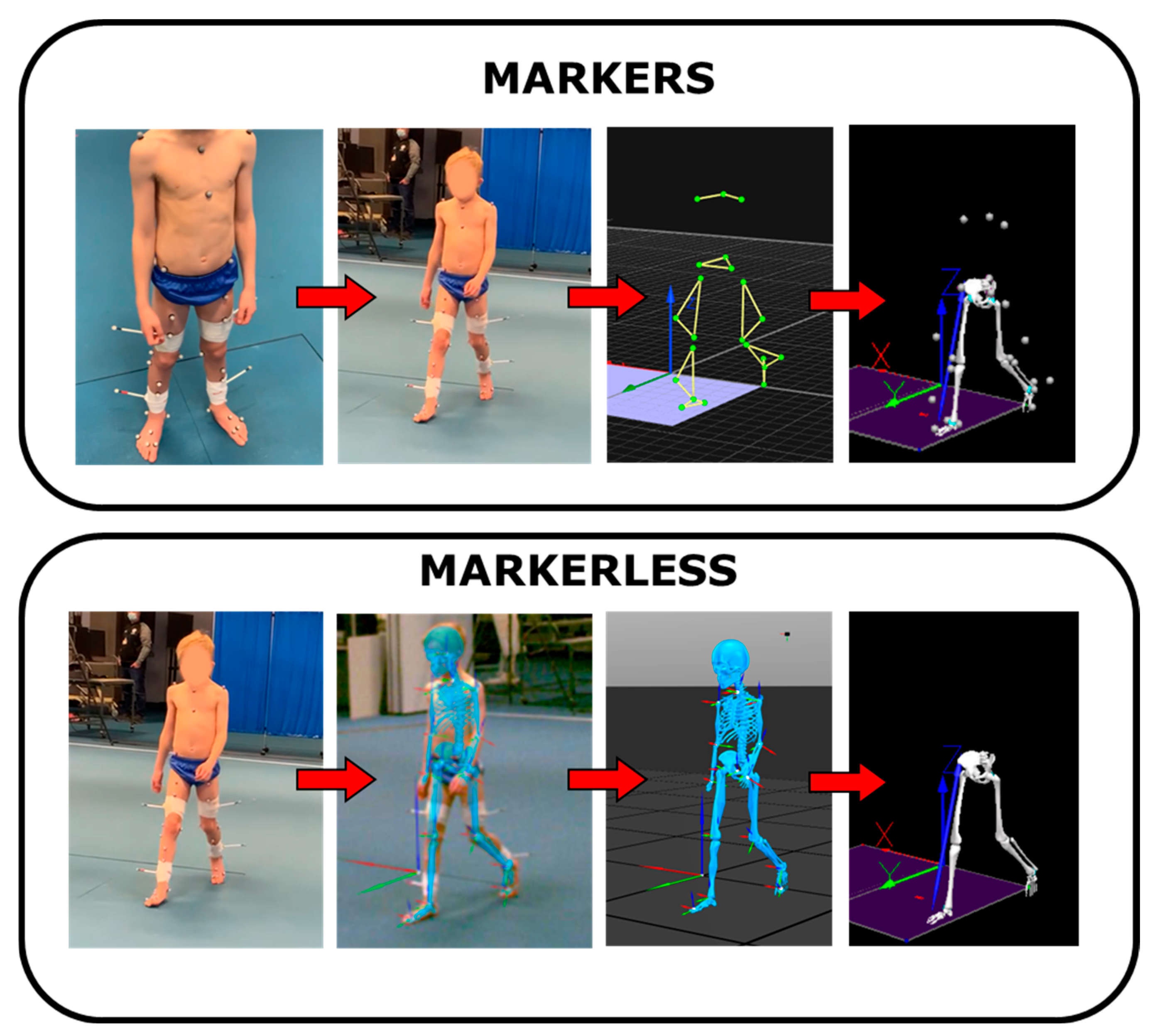
2.1. Participants and Data Collection
2.2. Data Analysis
2.3. Statistical Analysis
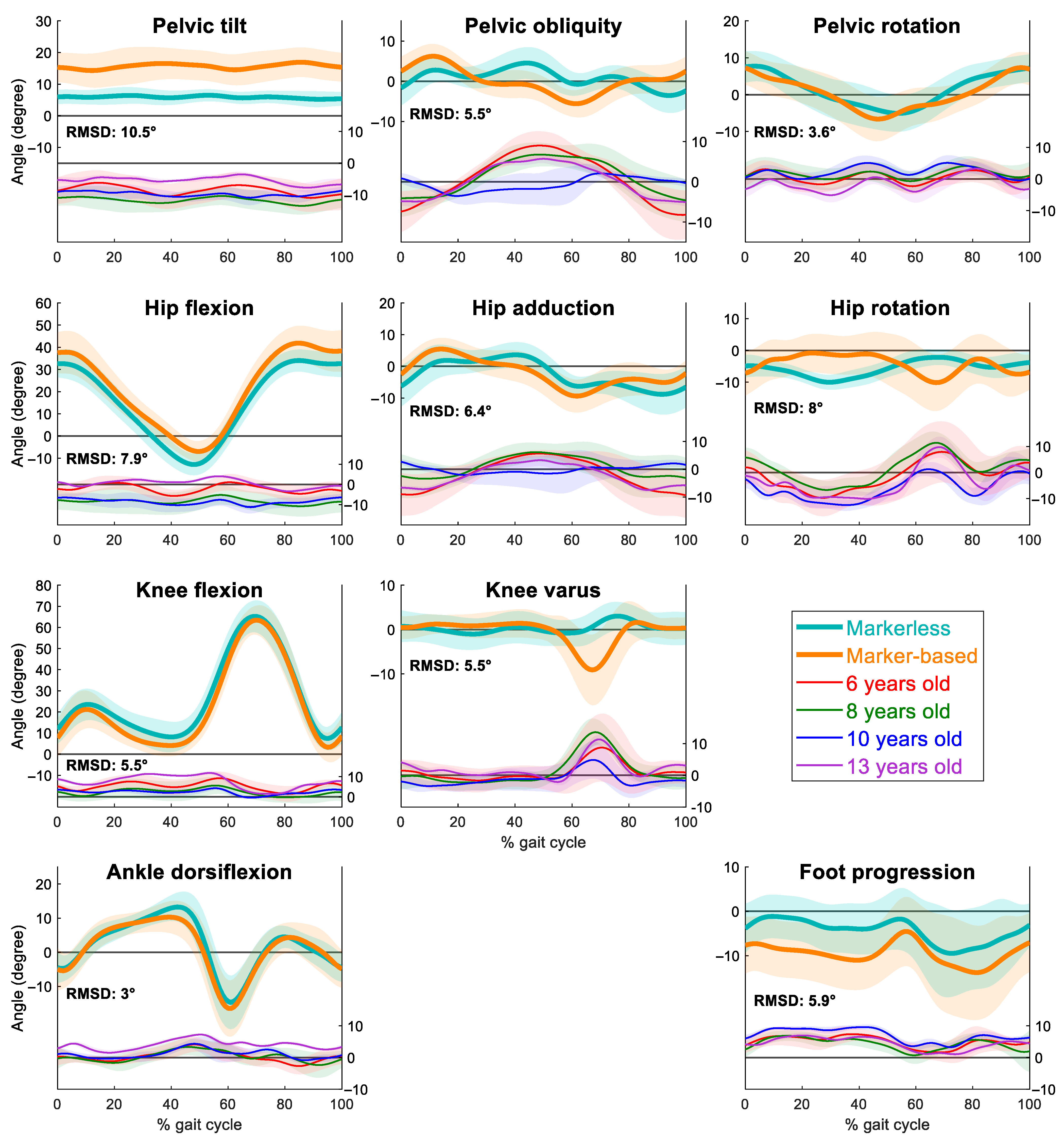
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, J.; Burnfield, J. Gait Analysis: Normal and Pathological Function, 2nd ed.; Slack Incorporated: New Jersey, NJ, USA, 2010. [Google Scholar]
- Armand, S.; Sawacha, Z.; Goudriaan, M.; Horsak, B.; van der Krogt, M.; Huenaerts, C.; Daly, C.; Kranzl, A.; Boehm, H.; Petrarca, M.; et al. Current practices in clinical gait analysis in Europe: A comprehensive survey-based study from the European society for movement analysis in adults and children (ESMAC) standard initiative. Gait Posture 2024, 111, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hallemans, A.; Van de Walle, P.; Wyers, L.; Verheyen, K.; Schoonjans, A.-S.; Desloovere, K.; Ceulemans, B. Clinical usefulness and challenges of instrumented motion analysis in patients with intellectual disabilities. Gait Posture 2019, 71, 105–115. [Google Scholar] [CrossRef]
- Hulleck, A.A.; Mohan, D.M.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, F.; Barre, A.; Aminian, K.; Sangeux, M. On the accuracy of the Conventional gait Model: Distinction between marker misplacement and soft tissue artefact errors. J. Biomech. 2023, 159, 111774. [Google Scholar] [CrossRef]
- Fonseca, M.; Gasparutto, X.; Leboeuf, F.; Dumas, R.; Armand, S. Impact of knee marker misplacement on gait kinematics of children with cerebral palsy using the Conventional Gait Model-A sensitivity study. PLoS ONE 2020, 15, e0232064. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Passmore, E.; Graham, H.K.; Sangeux, M. Defining the medial-lateral axis of the femur: Medical imaging, conventional and functional calibration methods lead to differences in hip rotation kinematics for children with torsional deformities. J. Biomech. 2018, 69, 156–163. [Google Scholar] [CrossRef]
- Colyer, S.L.; Evans, M.; Cosker, D.P.; Salo, A.I.T. A review of the evolution of vision-based motion analysis and the integration of advanced computer vision methods towards developing a markerless system. Sports Med. Open 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.; Needham, L.; McGuigan, P.; Bilzon, J. Applications and limitations of current markerless motion capture methods for clinical gait biomechanics. PeerJ 2022, 10, e12995. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Pua, Y.-H.; McGaw, R.; Heywood, S.; Clark, R.A. Gait assessment using the Microsoft Xbox One Kinect: Concurrent validity and inter-day reliability of spatiotemporal and kinematic variables. J. Biomech. 2015, 48, 2166–2170. [Google Scholar] [CrossRef]
- Cao, Z.; Simon, T.; Wei, S.E.; Sheikh, Y. Realtime multi-person 2d pose estimation using part affinity fields. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition 2017, Honolulu, HI, USA, 21–26 July 2017; pp. 7291–7299. [Google Scholar]
- Uhlrich, S.D.; Falisse, A.; Kidziński, Ł.; Muccini, J.; Ko, M.; Chaudhari, A.S.; Hicks, J.L.; Delp, S.L. OpenCap: Human movement dynamics from smartphone videos. PLOS Comput. Biol. 2023, 19, e1011462. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.K.; Davis, E.M.; Selbie, W.S.; Deluzio, K.J. Concurrent assessment of gait kinematics using marker-based and markerless motion capture. J. Biomech. 2021, 127, 110665. [Google Scholar] [CrossRef] [PubMed]
- Wren, T.A.L.; Isakov, P.; Rethlefsen, S.A. Comparison of kinematics between Theia markerless and conventional marker-based gait analysis in clinical patients. Gait Posture 2023, 104, 9–14. [Google Scholar] [CrossRef]
- McGuirk, T.E.; Perry, E.S.; Sihanath, W.B.; Riazati, S.; Patten, C. Feasibility of markerless motion capture for three-dimensional gait assessment in community settings. Front. Hum. Neurosci. 2022, 16, 867485. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, K.; Schallig, W.; van Dorst, M.H.; Buizer, A.I.; van der Krogt, M.M. The applicability of markerless motion capture for clinical gait analysis in children with cerebral palsy. Sci. Rep. 2024, 14, 11910. [Google Scholar] [CrossRef]
- D'Souza, S.; Siebert, T.; Fohanno, V. A comparison of lower body gait kinematics and kinetics between Theia3D markerless and marker-based models in healthy subjects and clinical patients. Sci. Rep. 2024, 14, 29154. [Google Scholar] [CrossRef]
- Flux, E.; van der Krogt, M.M.; Cappa, P.; Petrarca, M.; Desloovere, K.; Harlaar, J. The Human Body Model versus conventional gait models for kinematic gait analysis in children with cerebral palsy. Hum. Mov. Sci. 2020, 70, 102585. [Google Scholar] [CrossRef] [PubMed]
- Kainz, H.; Graham, D.; Edwards, J.; Walsh, H.P.J.; Maine, S.; Boyd, R.N.; Lloyd, D.G.; Modenese, L.; Carty, C.P. Reliability of four models for clinical gait analysis. Gait Posture 2017, 54, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D. The development of mature gait. Gait Posture 1997, 6, 163–170. [Google Scholar] [CrossRef]
- Oudenhoven, L.M.; Booth, A.T.C.; Buizer, A.I.; Harlaar, J.; van der Krogt, M.M. How normal is normal: Consequences of stride to stride variability, treadmill walking and age when using normative paediatric gait data. Gait Posture 2019, 70, 289–297. [Google Scholar] [CrossRef]
- Davis, R.B., III; Ounpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Steinwender, G.; Saraph, V.; Scheiber, S.; Zwick, E.B.; Uitz, C.; Hackl, K. Intrasubject repeatability of gait analysis data in normal and spastic children. Clin. Biomech. 2000, 15, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.A., Jr.; Richards, J.G.; Higginson, J.S. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC/Taylor & Francis: New York, NY, USA, 2017. [Google Scholar]
- Stewart, C.; Eve, L.; Durham, S.; Holmes, G.; Stebbins, J.; Harrington, M.; Corbett, M.; Kiernan, D.; Kidgell, V.; Jarvis, S.; et al. Clinical movement analysis society–UK and Ireland: Clinical movement analysis standards. Gait Posture 2023, 106, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kanko, R.M.; Outerleys, J.B.; Laende, E.K.; Selbie, W.S.; Deluzio, K.J. Comparison of Concurrent and Asynchronous Running Kinematics and Kinetics from Marker-Based and Markerless Motion Capture Under Varying Clothing Conditions. J. Appl. Biomech. 2024, 40, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, F.; Reay, J.; Jones, R.; Sangeux, M. The effect on conventional gait model kinematics and kinetics of hip joint centre equations in adult healthy gait. J. Biomech. 2019, 87, 167–171. [Google Scholar] [CrossRef]
- Mantovani, G.; Ng, K.C.G.; Lamontagne, M. Regression models to predict hip joint centers in pathological hip population. Gait Posture 2016, 44, 48–54. [Google Scholar] [CrossRef]
- Augustine, S.; Foster, R.; Barton, G.; Lake, M.J.; Sharir, R.; Robinson, M.A. The inter-trial and inter-session reliability of Theia3D-derived markerless gait analysis in tight versus loose clothing. PeerJ 2025, 13, e18613. [Google Scholar] [CrossRef]
| Signal | Nonlinear Trend over Time | Nonlinear Trend over Age | Interaction Between Age and Time | R2 | |
|---|---|---|---|---|---|
| Pelvis | Tilt | F(8.45, 9954.67) = 61.02 | F(2.99, 9954.67) = 899.35 | F(8.35, 9954.67) = 12.35 | 0.91 |
| p < 0.001 | p < 0.001 | p < 0.001 | |||
| Obliquity | F(8.61, 9949.58) = 964.12 | F(2.99, 9949.58) = 119.92 | F(8.95, 9949.58) = 340.67 | 0.78 | |
| p < 0.001 | p < 0.001 | p < 0.001 | |||
| Rotation | F(8.85, 9954.26) = 68.07 | F(2.97, 9954.26) = 129.16 | F(8.07, 9954.26) = 13.04 | 0.82 | |
| p < 0.001 | p < 0.001 | p < 0.001 | |||
| Hip | Flex/Ext | F(8.60, 9949.56) = 41.07 | F(2.99, 9949.56) = 939.66 | F(8.35, 9949.56) = 11.58 | 0.97 |
| p < 0.001 | p < 0.001 | p < 0.001 | |||
| Abd/ | F(8.34, 9952.34) = 340.36 | F(2.98, 9952.34) = 147.27 | F(8.88, 9952.34) = 153.28 | 0.78 | |
| Adduction | p < 0.001 | p < 0.001 | p < 0.001 | ||
| Int/ Ext | F(8.93, 9952.22) = 716.78 | F(3.00, 9952.22) = 700.88 | F(8.74, 9952.22) = 17.41 | 0.74 | |
| Rotation | p < 0.001 | p < 0.001 | p < 0.001 | ||
| Knee | Flex/Ext | F(8.60, 9942.91) = 47.45 | F(2.967, 9942.91) = 176.63 | F(8.27, 9942.91) = 8.07 | 0.96 |
| p < 0.001 | p < 0.001 | p < 0.001 | |||
| Varus/Valgus | F(8.96, 9949.27) = 579.42 | F(2.99, 9949.27) = 184.13 | F(8.91, 9949.27) = 77.50 | 0.73 | |
| p < 0.001 | p < 0.001 | p < 0.001 | |||
| Ankle | Plantar/ | F(8.42, 9951.03) = 66.90 | F(2.82, 9951.03) = 109.02 | F(4.26, 9951.03) = 3.12 | 0.89 |
| Dorsiflexion | p < 0.001 | p < 0.001 | p = 0.006 | ||
| Foot | F(8.59, 9947.86) = 89.44 | F(2.99, 9947.86) = 96.36 | F(7.22, 9947.86) = 3.01 | 0.87 | |
| Progression | p < 0.001 | p < 0.001 | p = 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greaves, H.; Eleuteri, A.; Barton, G.J.; Robinson, M.A.; Gibbon, K.C.; Foster, R.J. Comparison of Marker- and Markerless-Derived Lower Body Three-Dimensional Gait Kinematics in Typically Developing Children. Sensors 2025, 25, 4249. https://doi.org/10.3390/s25144249
Greaves H, Eleuteri A, Barton GJ, Robinson MA, Gibbon KC, Foster RJ. Comparison of Marker- and Markerless-Derived Lower Body Three-Dimensional Gait Kinematics in Typically Developing Children. Sensors. 2025; 25(14):4249. https://doi.org/10.3390/s25144249
Chicago/Turabian StyleGreaves, Henrike, Antonio Eleuteri, Gabor J. Barton, Mark A. Robinson, Karl C. Gibbon, and Richard J. Foster. 2025. "Comparison of Marker- and Markerless-Derived Lower Body Three-Dimensional Gait Kinematics in Typically Developing Children" Sensors 25, no. 14: 4249. https://doi.org/10.3390/s25144249
APA StyleGreaves, H., Eleuteri, A., Barton, G. J., Robinson, M. A., Gibbon, K. C., & Foster, R. J. (2025). Comparison of Marker- and Markerless-Derived Lower Body Three-Dimensional Gait Kinematics in Typically Developing Children. Sensors, 25(14), 4249. https://doi.org/10.3390/s25144249









