Experimental Study on the Effects of Cockpit Noise on Physiological Indicators of Pilots
Abstract
1. Introduction
2. Materials and Methods
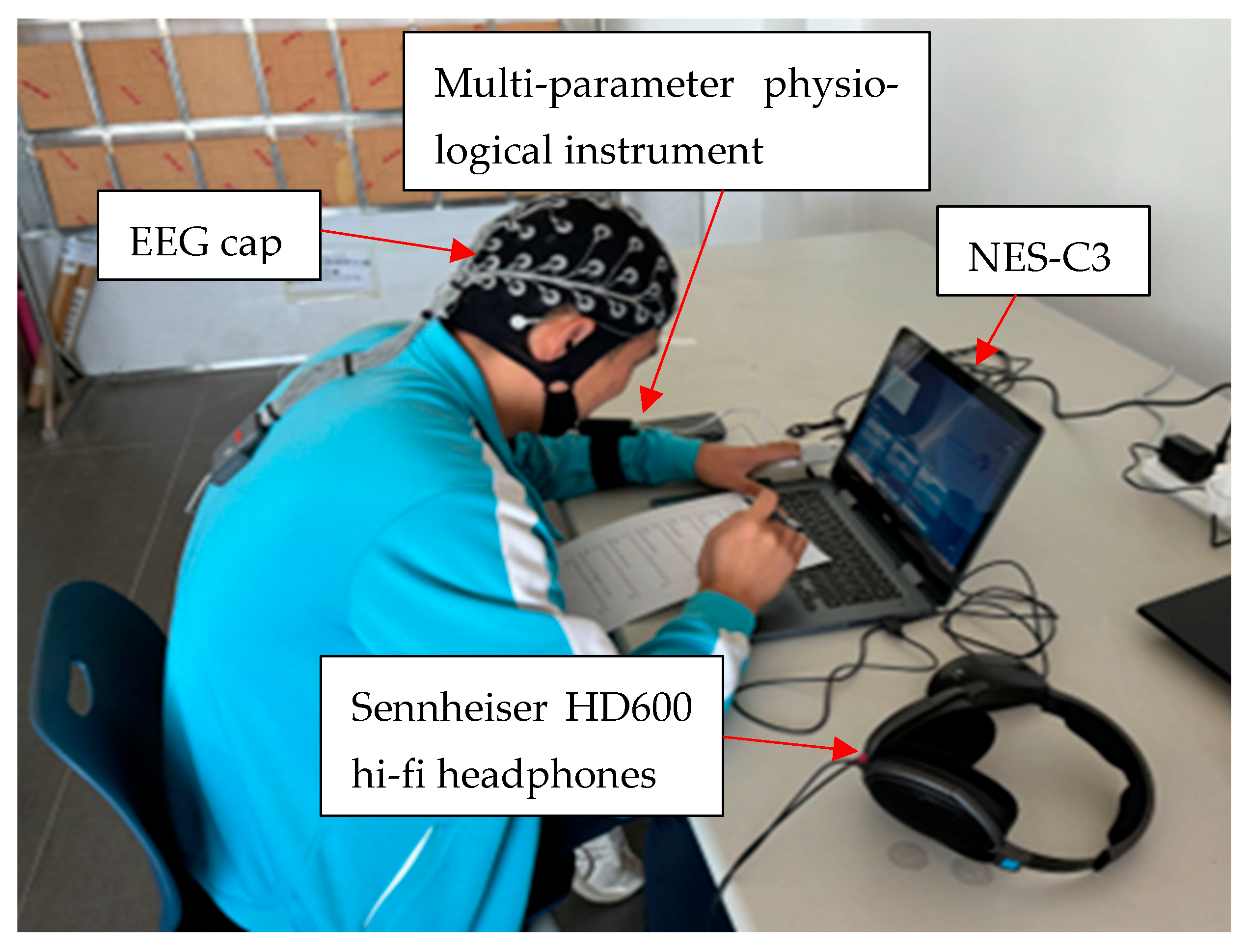
2.1. Experimental Condition
2.2. Physiological Data Acquisition
- (1)
- Attentional breadth
- (2)
- Visual retention
- (3)
- Visual complex reaction time
- (4)
- Space perception
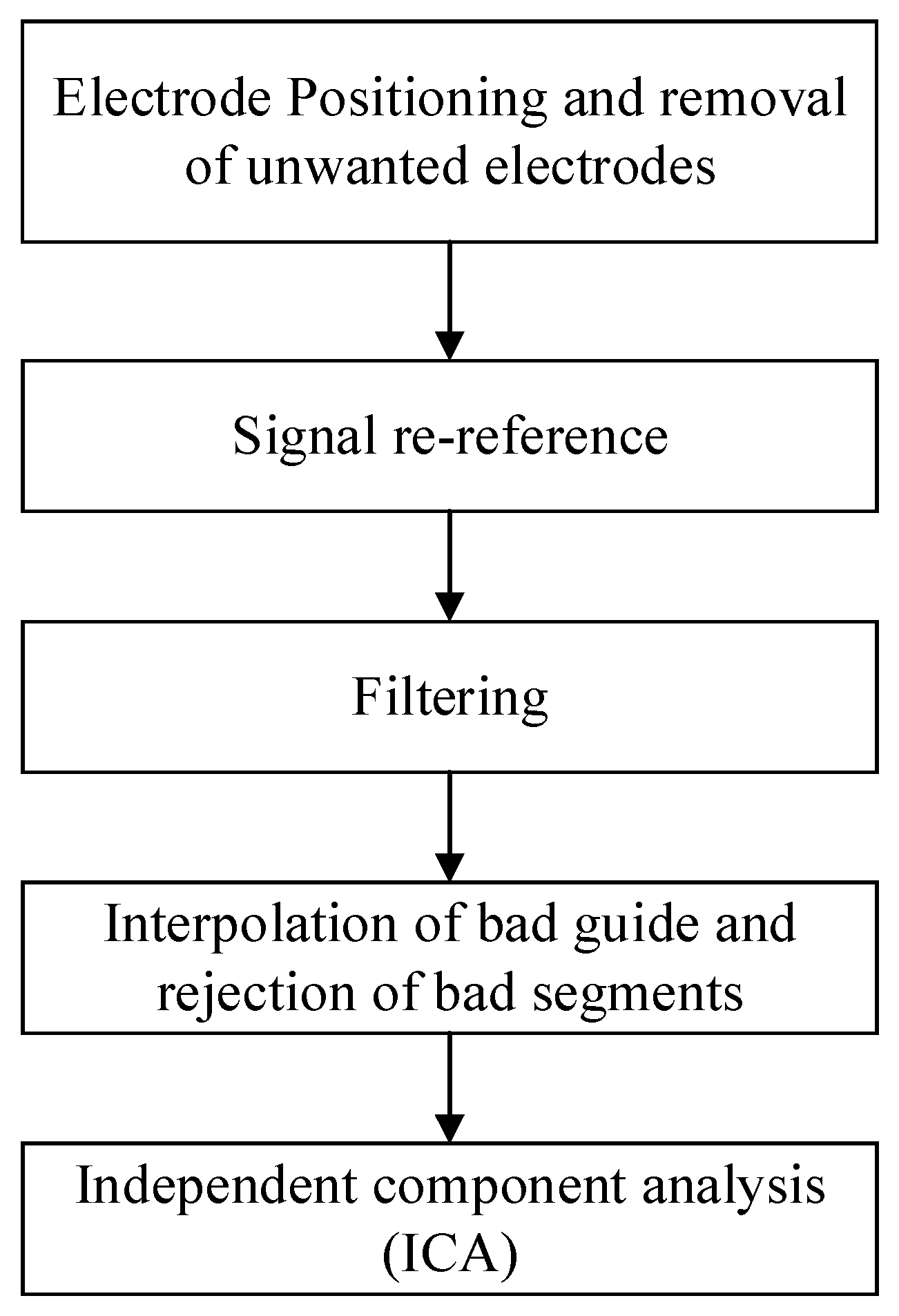
2.3. Physiological Signal Processing
- (1)
- Electrode positioning and removal of unwanted electrodes
- (2)
- Signal re-reference
- (3)
- Filtering
- (4)
- Interpolation of bad guide and rejection of bad segments
- (5)
- Independent component analysis (ICA)
3. Results
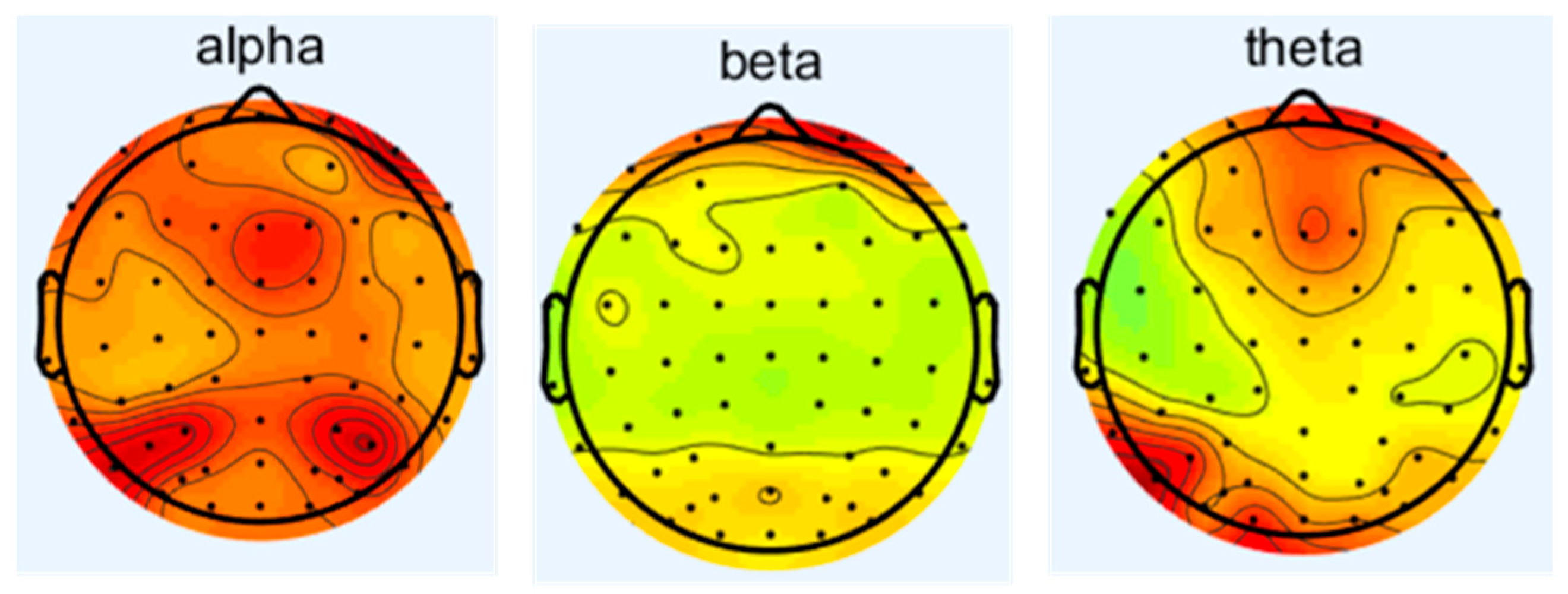
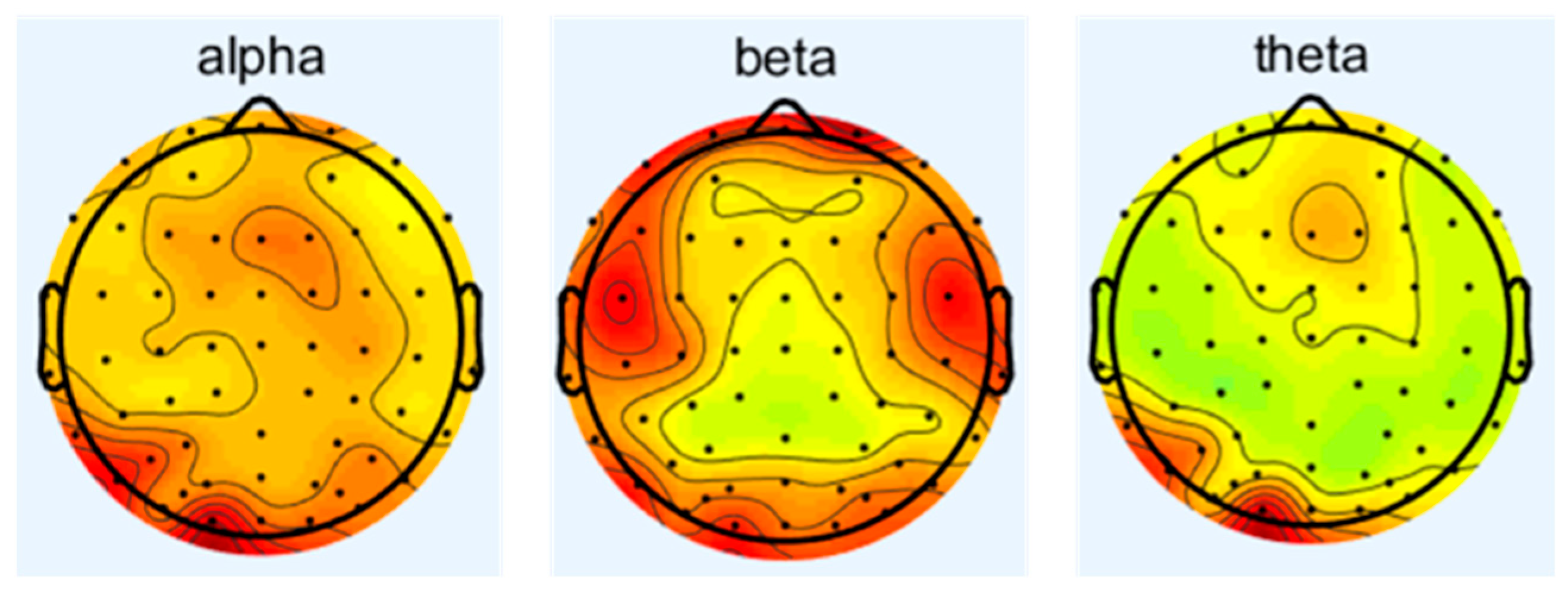
3.1. The Effect of Noise on Electroencephalogram (EEG)
3.2. The Effect of Noise on Electrocardiogram (ECG)
3.3. Effect of Noise on Electrodermal Activity (EDA)
3.4. Correlation Analysis Between Physiological Indicators
4. Discussion
5. Conclusions
- (1)
- The increase in the cockpit noise level will activate the sympathetic nervous system to induce a stress response, promote the secretion of related stress hormones, directly lead to abnormal cardiovascular indicators such as an accelerated heart rate, and increase sweat gland secretion, thus indirectly affecting the skin conductance level.
- (2)
- Cockpit noise acts on the central nervous system through the auditory pathway, interferes with the balance between the excitation and inhibition of the cerebral cortex, and leads to changes in the power spectral density of the main frequency of EEG (such as α wave, β wave), which is manifested as the inhibition of the α wave (8–13 Hz) and enhancement of the β wave (14–30 Hz).
- (3)
- Based on the Pearson correlation analysis, several physiological indicators of pilot trainees in the noise environment exhibited significant correlations. While strong correlations were observed within certain individual indicators, the correlation coefficients (|r|) between different types of indicators generally ranged from 0 to 0.5, indicating low-to-moderate levels of association.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goujard, B.; Sakout, A.; Valeau, V. Acoustic comfort on board ships: An evaluation based on a questionnaire. Appl. Acoust. 2005, 66, 1063–1073. [Google Scholar] [CrossRef]
- Lee, H.P.; Kumar, S.; Garg, S.; Lim, K.M. Assessment of in-cabin noise of wide-body aircrafts. Appl. Acoust. 2022, 194, 108809. [Google Scholar] [CrossRef] [PubMed]
- Orikpete, O.F.; Dennis, N.M.; Kikanme, K.N.; Ewim, D.R.E. Advancing noise management in aviation: Strategic approaches for preventing noise-induced hearing loss. J. Environ. Manag. 2024, 363, 121413. [Google Scholar] [CrossRef] [PubMed]
- Shuicheng, T.I.; Hongyan, L.I.; Yanbin, S.H.; Mengfei, D.U.; Wei, L.I.; Qingyan, W.A. Research on the Impact of Noise Level on Human Unsafe Behavior. Chin. J. Saf. Sci. 2017, 27, 19–24. [Google Scholar]
- Borelli, D.; Gaggero, T.; Rizzuto, E.; Schenone, C. Onboard ship noise: Acoustic comfort in cabins. Appl. Acoust. 2021, 177, 107912. [Google Scholar] [CrossRef]
- Masri, J.; Amer, M.; Salman, S.; Ismail, M.; Elsisi, M. A survey of modern vehicle noise, vibration, and harshness: A state-of-the-art. Ain Shams Eng. J. 2024, 15, 102957. [Google Scholar] [CrossRef]
- Bo, Y.; Xiao, T.; Shiliang, S. Simulation experiment study on the impact of noise on human safety behavior. Min. Eng. Res. 2017, 32, 14–19. [Google Scholar]
- GBZ 2.2-2007; Limits of Occupational Exposure to Hazardous Factors in the Workplace Part 2: Physical Factors. People’s Republic of China Ministry of Health: Beijing, China, 2007.
- GJB 1357-1992; Noise Levels in Aircraft. Commission of Science, Technology and Industry for National Defense: Beijing, China, 1992.
- Genyin, C.; Shaojie, C.; Zhiyong, W.; Fei, H. Analysis of the Psychological and Physiological Effects of Underground Noise on Human Health. J. Xi’an Univ. Sci. Technol. 2011, 31, 850–853. [Google Scholar]
- Zhu, S. The effect of white noise on sleep quality and physiological parameters of premature infants in neonatal intensive care unit. Chin. J. Matern. Child Health 2025, 40, 444–447. [Google Scholar] [CrossRef]
- Cheng, X. Study on the Effects of Wind Power Characteristic Noise on the Behavioral Physiology of Sea Cucumber. Ph.D. Thesis, Qingdao University of Science and Technology, Qingdao, China, 2024. [Google Scholar] [CrossRef]
- Guo, J. Research on the Effects of Vibration Noise on the Behavior and Physiology of Large Yellow Croaker. Ph.D. Thesis, Dalian Ocean University, Dalian, China, 2024. [Google Scholar] [CrossRef]
- Guoxun, J.; Min, W. Research on the Impact of Noise on Cognitive Performance of Workers Based on Physiological Indicators. Coal Mine Saf. 2021, 52, 243–247. [Google Scholar]
- Fu, C. Research on the Impact of Indoor Environmental Noise in Street facing Buildings on Human Physiological Parameters. Ph.D. Thesis, Chongqing University, Chongqing, China, 2005. [Google Scholar]
- Xu, G.; Wang, Y.; Liu, W. A comparative study of attention allocation between flight students and flight instructors during flight training. J. Civ. Aviat. Flight Sch. China 2024, 35, 5–8+14. [Google Scholar]
- MIL-STD-1474E; Noise Limits. U.S. Department of Defense: Washington, DC, USA, 2015.
- Ikkai, A.; Dandekar, S.; Curtis, C.E. Lateralization in Alpha-Band Oscillations Predicts the Locus and Spatial Distribution of Attention. PLoS ONE 2016, 11, e0154796. [Google Scholar] [CrossRef]
- Rodriguez-Larios, J.; Haegens, S. Genuine beta bursts in human working memory: Controlling for the influence of lower-frequency rhythms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gehmacher, Q.; Reisinger, P.; Hartmann, T.; Keintzel, T.; Rösch, S.; Schwarz, K.; Weisz, N. Direct cochlear recordings in humans show a theta rhythmic modulation of auditory nerve activity by selective attention. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Morillas-Romero, A.; Tortella-Feliu, M.; Bornas, X.; Putman, P. Spontaneous EEG theta/beta ratio and delta-beta coupling in relation to attentional network functioning and self-reported attentional control. Cogn. Affect. Behav. Neurosci. 2015, 15, 598–606. [Google Scholar] [CrossRef]
- Liu, N.-H.; Chiang, C.-Y.; Chu, H.-C. Recognizing the Degree of Human Attention Using EEG Signals from Mobile Sensors. Sensors 2013, 13, 10273–10286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X. Estimating mental fatigue based on electroencephalogram and heart rate variability. Pol. J. Med. Phys. Eng. 2010, 16, 67–84. [Google Scholar] [CrossRef]
- Love, J.; Sung, W.; Francis, A.L. Psychophysiological responses to potentially annoying heating, ventilation, and air conditioning noise during mentally demanding work. J. Acoust. Soc. Am. 2021, 150, 3149. [Google Scholar] [CrossRef]
- Shen, H.; Hao, M.; Ren, J.; An, Z.; Long, Z. Experimental study on human HRV under different ventilation conditions in aircraft cockpit. IET Conf. Proc. 2024, 2024, 489–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Zhu, L.; Zhang, J.; Wei, L.; Wang, M.; Wang, Q.; Yu, J. Study on the correlation between nighttime blood pressure drop rate and HRV and DC in female patients with primary hypertension. Sensors 2024, 53, 44–49. [Google Scholar]
- You, B.; Shi, S.; Liu, H. Simulation experiment on physiological effects of confined space noise on human body. Min. Eng. Res. 2018, 33, 56–60. [Google Scholar]
- Zhao, H. Experimental Study on Unsafe Behavior of Miners Based on Physiological Psychological Measurements. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2012. [Google Scholar]




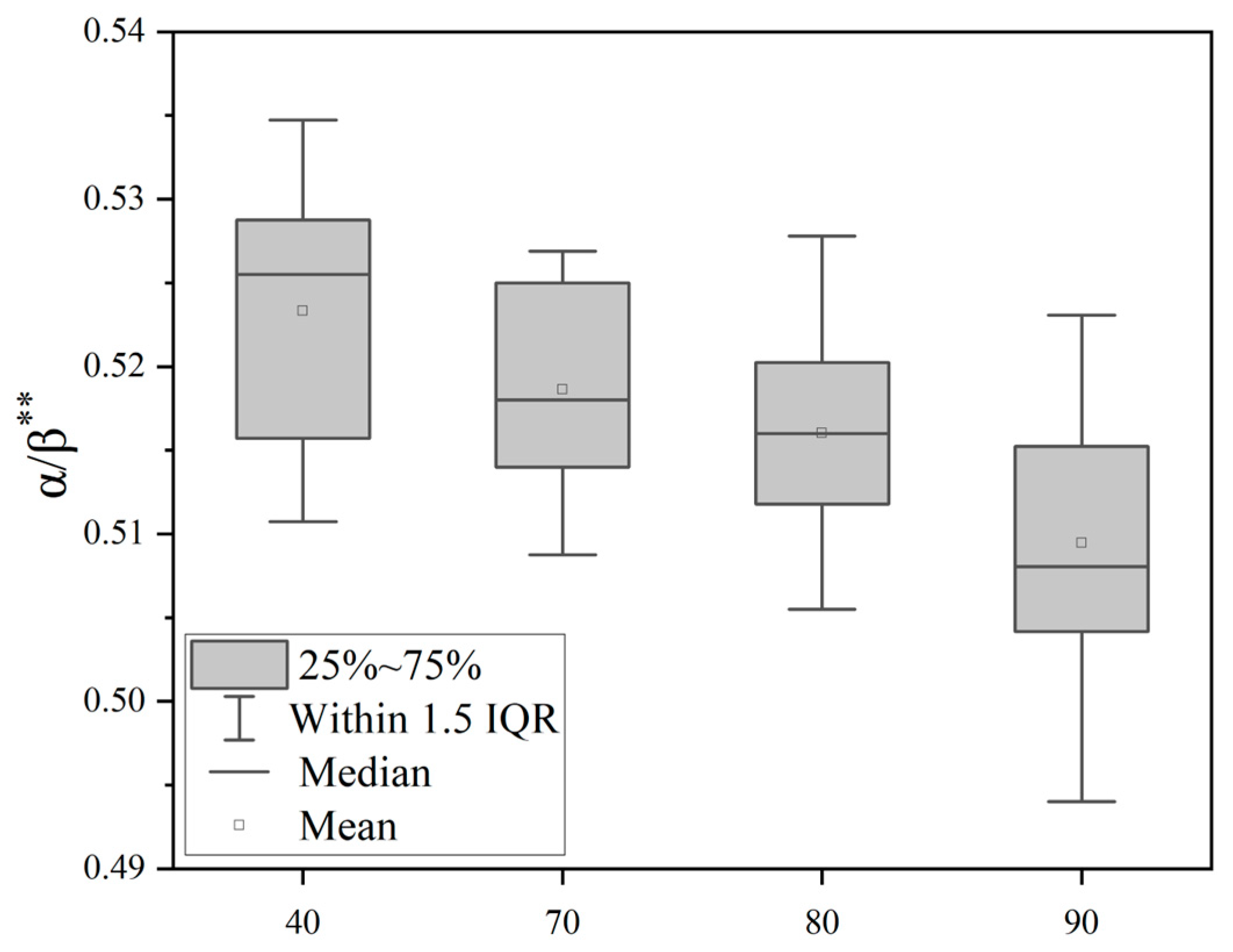

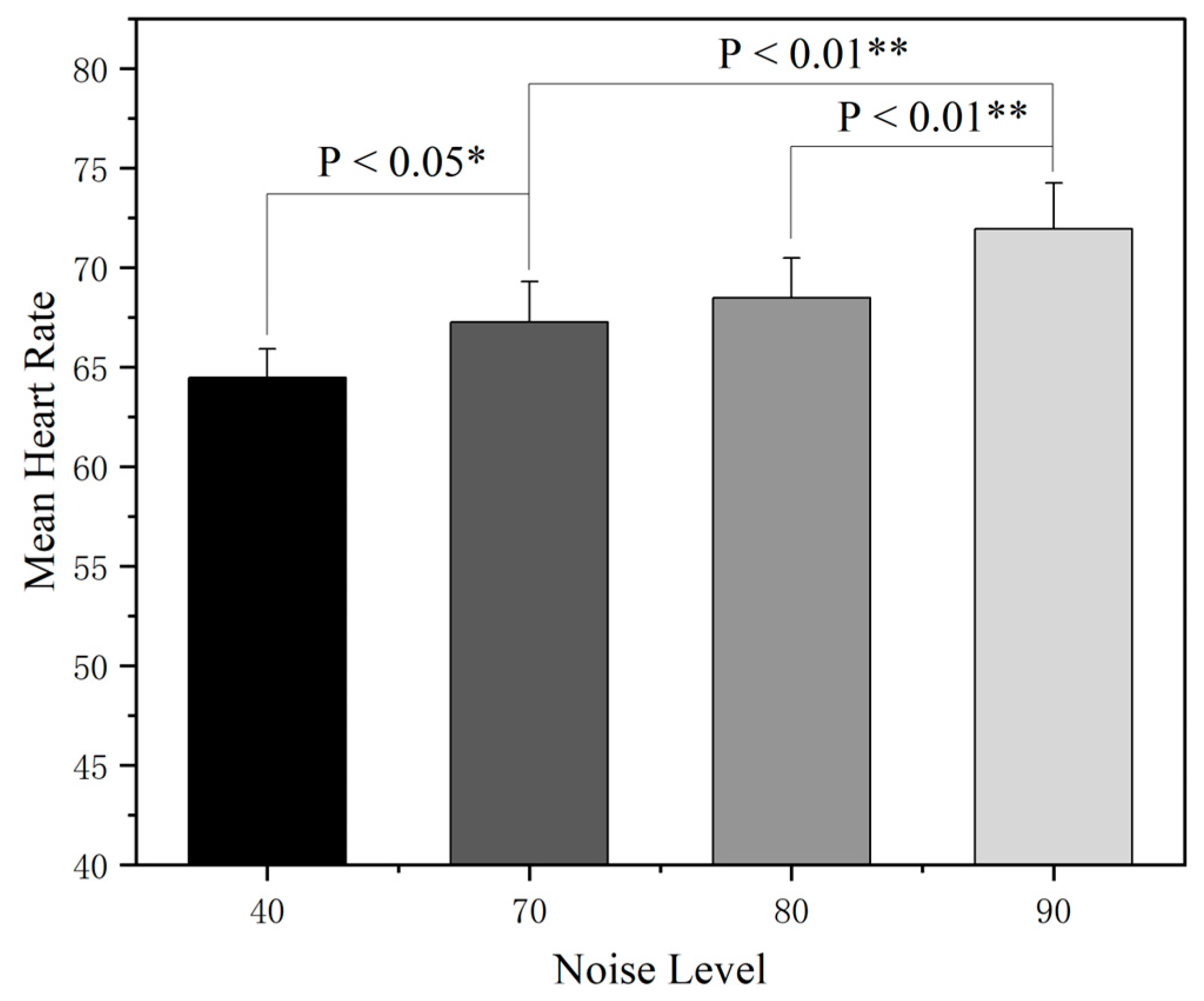
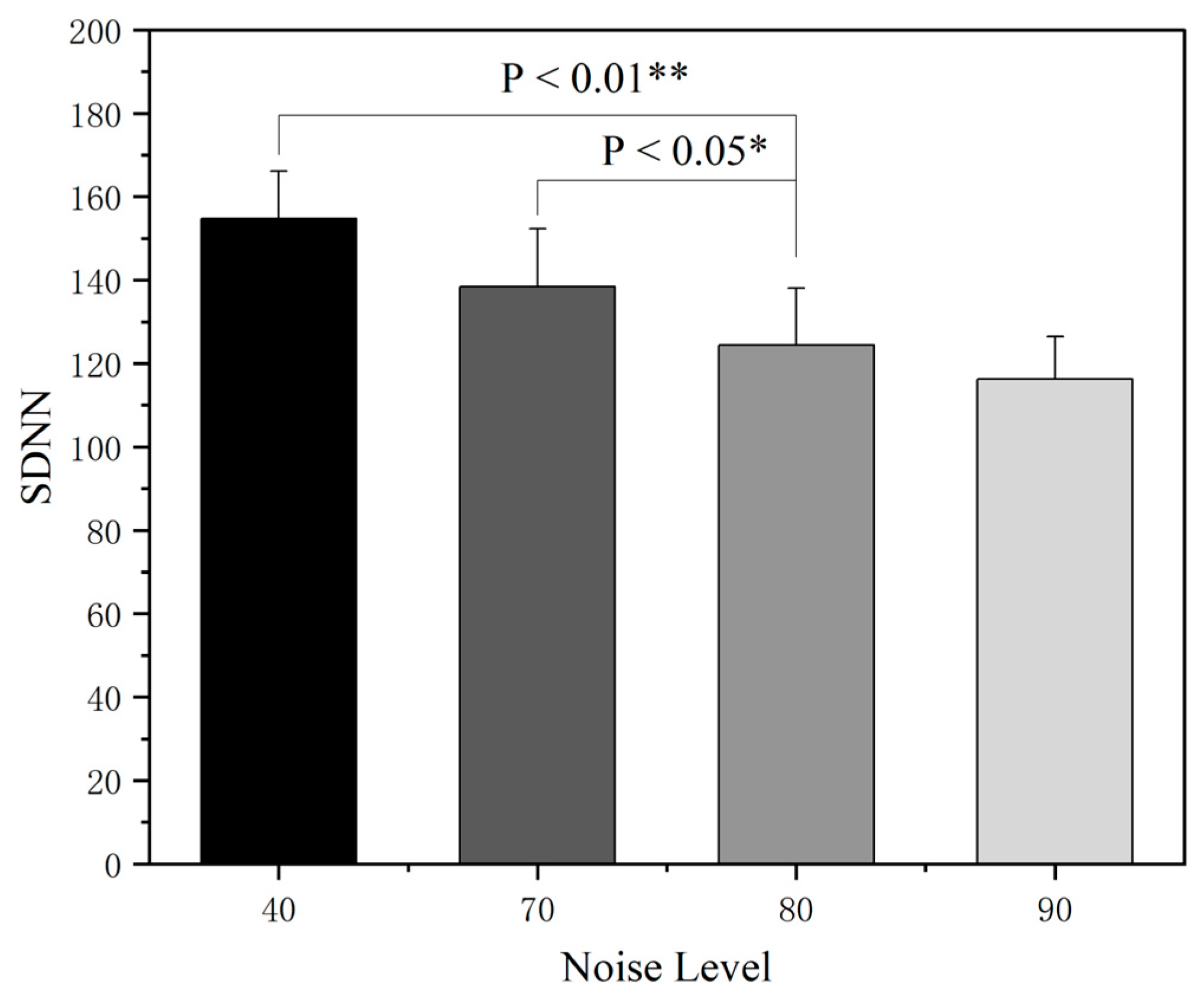
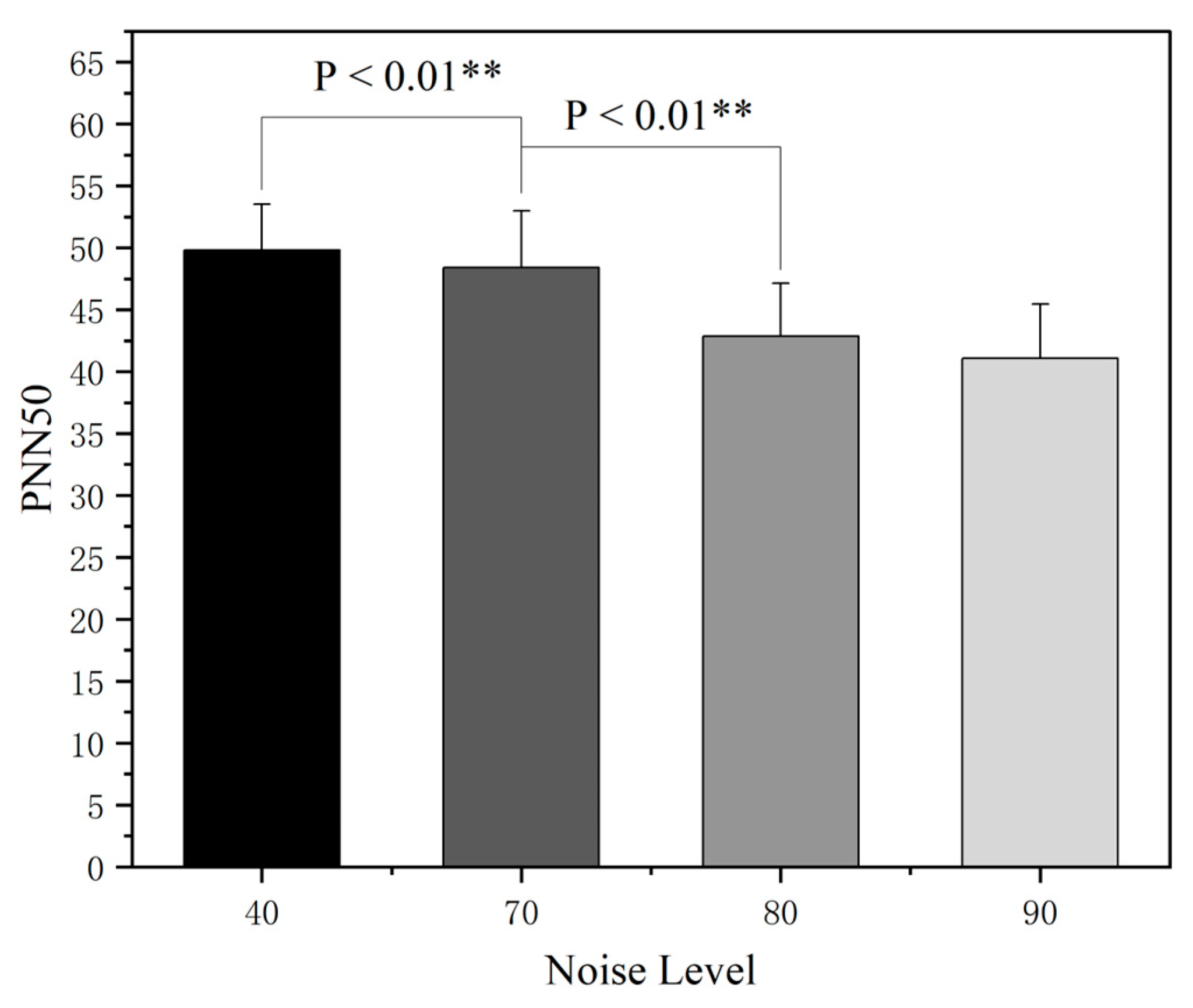


| EEG | N | F | P |
|---|---|---|---|
| θ/β | 16 | 10.624 | 0.000 ** |
| α/β | 16 | 10.704 | 0.000 ** |
| β/(θ + α) | 16 | 10.684 | 0.000 ** |
| Null Hypothesis | Test | Significance | Decision |
|---|---|---|---|
| The distributions of heart rate under 40 dB(A), 70 dB(A), 80 dB(A), and 90 dB(A) are identical. | Related-sample Friedman’s two-way analysis of variance by ranks | p = 0.000 ** | Reject the null hypothesis. |
| Noise Level | N | ± s | F | p |
|---|---|---|---|---|
| 40 | 16 | 0.94 ± 1.46 | 3.245 | 0.029 * |
| 70 | 16 | 1.25 ± 1.48 | ||
| 80 | 16 | 2.09 ± 1.78 | ||
| 90 | 16 | 2.63 ± 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Hao, M.; Ren, J.; Chen, K.; Gao, Y. Experimental Study on the Effects of Cockpit Noise on Physiological Indicators of Pilots. Sensors 2025, 25, 4175. https://doi.org/10.3390/s25134175
Shen H, Hao M, Ren J, Chen K, Gao Y. Experimental Study on the Effects of Cockpit Noise on Physiological Indicators of Pilots. Sensors. 2025; 25(13):4175. https://doi.org/10.3390/s25134175
Chicago/Turabian StyleShen, Haiming, Meiqing Hao, Jiawei Ren, Kun Chen, and Yang Gao. 2025. "Experimental Study on the Effects of Cockpit Noise on Physiological Indicators of Pilots" Sensors 25, no. 13: 4175. https://doi.org/10.3390/s25134175
APA StyleShen, H., Hao, M., Ren, J., Chen, K., & Gao, Y. (2025). Experimental Study on the Effects of Cockpit Noise on Physiological Indicators of Pilots. Sensors, 25(13), 4175. https://doi.org/10.3390/s25134175





