The Impact of a Modality Switch During Isokinetic Leg Extensions on Performance Fatigability and Neuromuscular Patterns of Response
Abstract
Highlights
- Bilateral and unilateral muscle actions elicited similar neuromuscular activation strategies.
- Incorporating unilateral following bilateral muscle actions may potentiate greater metabolic stress.
- Practitioners should consider selecting exercises based on sport-specific needs.
- Following bilateral with unilateral muscle actions could be a method of eliciting greater muscular adaptations via metabolic stimulation of hypertrophic mechanisms.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Familiarization Visit
2.3. Test Visits
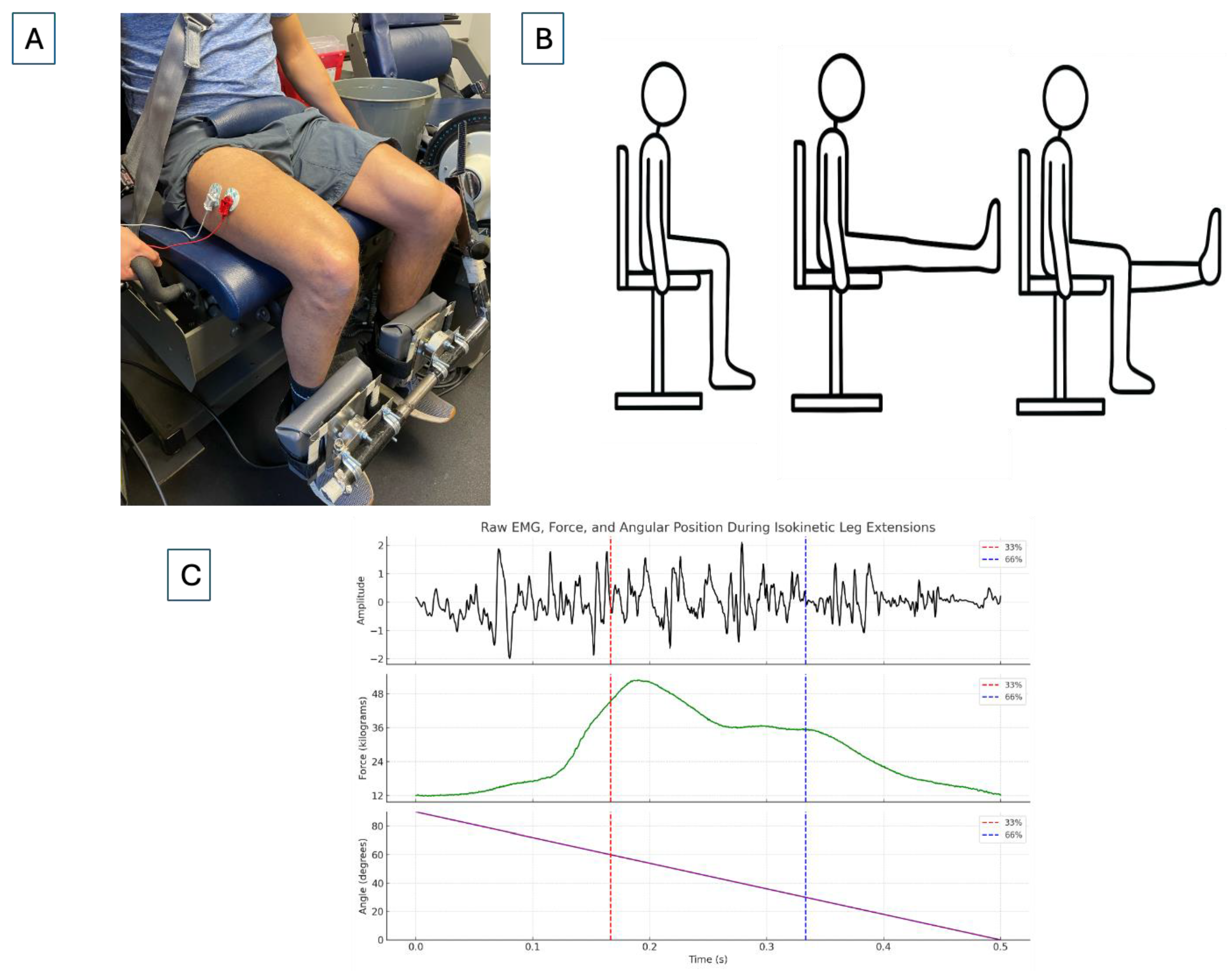
2.4. Electromyography and Force Acquisition
2.5. Statistical Analysis
3. Results
3.1. Pre- and Post-Fatigue Neuromuscular Assessments
3.2. Patterns of Response for Normalized Isokinetic Peak Force
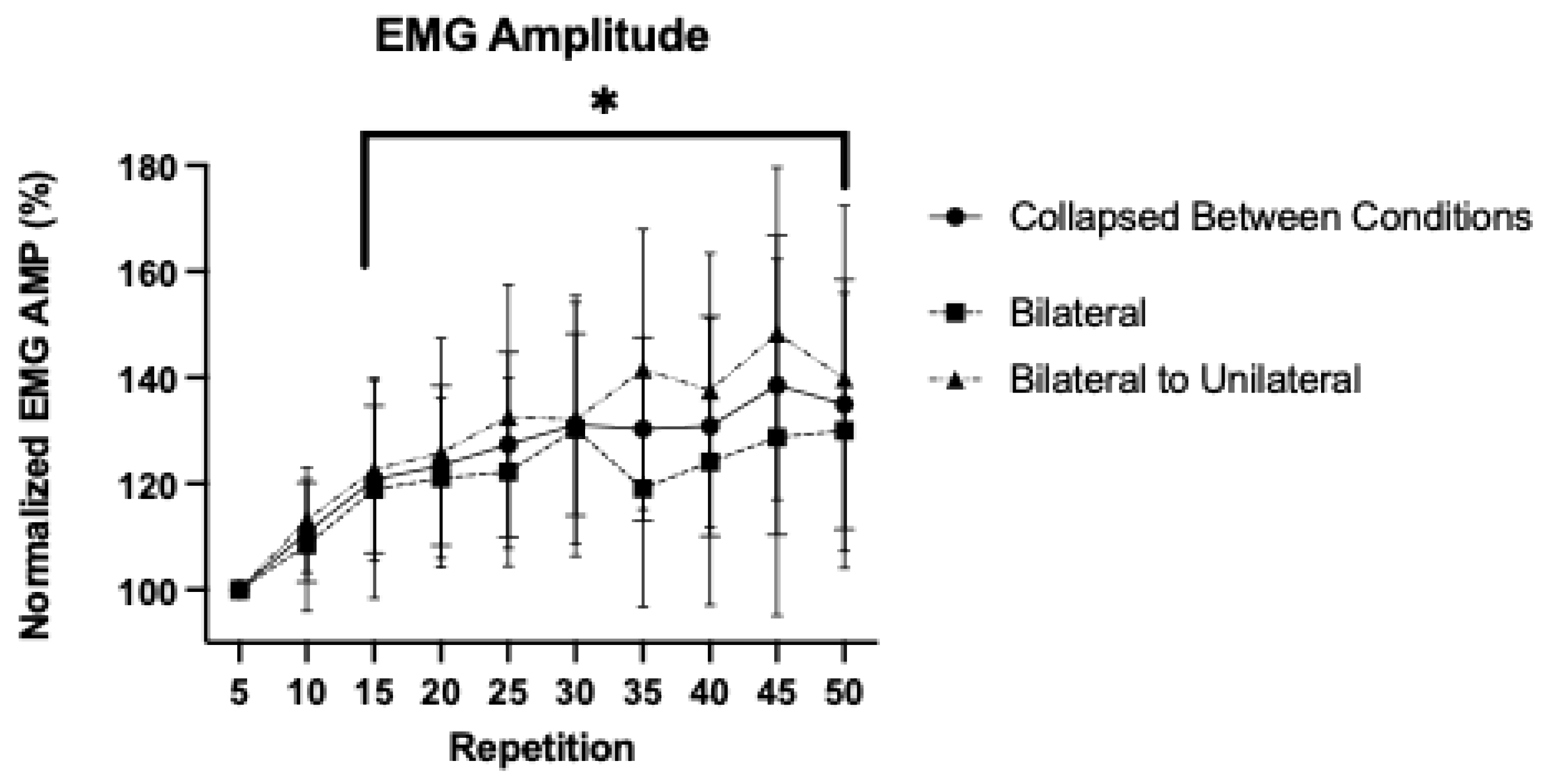
3.3. Patterns of Response for Normalized Electromyographic Amplitude
3.4. Patterns of Response for Normalized Electromyographic Mean Power Frequency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95% CI | 95% Confidence Interval |
| AMP | Amplitude |
| ANOVA | Analysis of Variance |
| ANCOVA | Analysis of Covariance |
| BL | Bilateral |
| EMG | Electromyography |
| IHI | Interhemispheric Inhibition |
| MPF | Mean Power Frequency |
| MVC | Maximum Voluntary Contraction |
| UL | Unilateral |
References
- Thomas, K.; Goodall, S.; Howatson, G. Performance Fatigability Is Not Regulated to A Peripheral Critical Threshold. Exerc. Sport Sci. Rev. 2018, 46, 240–246. [Google Scholar] [CrossRef]
- Anders, J.P.V.; Keller, J.L.; Neltner, T.J.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Task-specific performance fatigability and the bilateral deficit during isokinetic leg extensions. J. Musculoskelet. Neuronal Interact. 2021, 21, 4–12. [Google Scholar] [PubMed]
- Matkowski, B.; Place, N.; Martin, A.; Lepers, R. Neuromuscular fatigue differs following unilateral vs bilateral sustained submaximal contractions. Scand. J. Med. Sci. Sports 2011, 21, 268–276. [Google Scholar] [CrossRef]
- Rossman, M.J.; Garten, R.S.; Venturelli, M.; Amann, M.; Richardson, R.S. The role of active muscle mass in determining the magnitude of peripheral fatigue during dynamic exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 306, R934–R940. [Google Scholar] [CrossRef]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef]
- Oda, S. Motor control for bilateral muscular contractions in humans. Jpn. J. Physiol. 1997, 47, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Škarabot, J.; Cronin, N.; Strojnik, V.; Avela, J. Bilateral deficit in maximal force production. Eur. J. Appl. Physiol. 2016, 116, 2057–2084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, X.; Xu, K.; Xie, H.; Li, D.; Ding, S.; Sun, J. Effect of unilateral training and bilateral training on physical performance: A meta-analysis. Front. Physiol. 2023, 14, 1128250. [Google Scholar] [CrossRef]
- Liao, K.-F.; Nassis, G.P.; Bishop, C.; Yang, W.; Bian, C.; Li, Y.-M. Effects of unilateral vs. bilateral resistance training interventions on measures of strength, jump, linear and change of direction speed: A systematic review and meta-analysis. Biol. Sport 2022, 39, 485–497. [Google Scholar] [CrossRef]
- Botton, C.E.; Radaelli, R.; Wilhelm, E.N.; Rech, A.; Brown, L.E.; Pinto, R.S. Neuromuscular Adaptations to Unilateral vs. Bilateral Strength Training in Women. J. Strength Cond. Res. 2016, 30, 1924–1932. [Google Scholar] [CrossRef]
- Taniguchi, Y. Lateral specificity in resistance training: The effect of bilateral and unilateral training. Eur. J. Appl. Physiol. 1997, 75, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef]
- Basmajian, J.V.; De Luca, C.J. Muscles Alive: Their Functions Revealed by Electromyography, 5th ed.; Williams and Wilkins: Baltimore, MD, USA, 1985. [Google Scholar]
- Koral, J.; Oranchuk, D.J.; Wrightson, J.G.; Twomey, R.; Millet, G.Y. Mechanisms of neuromuscular fatigue and recovery in unilateral versus bilateral maximal voluntary contractions. J. Appl. Physiol. 2020, 128, 785–794. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Krantz, M.M.; Åström, M.; Drake, A.M. Strength and fatigue measurements of the hip flexor and hip extensor muscles: Test-retest reliability and limb dominance effect. Int. J. Sports Phys. Ther. 2020, 15, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.T.; Ponte, J.P.G. Divergent muscle fatigue during unilateral isometric contractions of dominant and non-dominant quadriceps. J. Sci. Med. Sport 2013, 16, 240–244. [Google Scholar] [CrossRef]
- Costa, D.C.; Valente-Dos-Santos, J.; Celis-Moreno, J.M.; Sousa-E-Silva, P.; Martinho, D.V.; Duarte, J.P.; Oliveira, T.; Tavares, O.M.; Castanheira, J.M.; Soles-Gonçalves, R.; et al. Learning effect on an isokinetic knee strength test protocol among male adolescent athletes. PLoS ONE 2023, 18, e0288382. [Google Scholar] [CrossRef]
- Lund, H.; Søndergaard, K.; Zachariassen, T.; Christensen, R.; Bülow, P.; Henriksen, M.; Bartels, E.M.; Danneskiold-Samsøe, B.; Bliddal, H. Learning effect of isokinetic measurements in healthy subjects, and reliability and comparability of Biodex and Lido dynamometers. Clin. Physiol. Funct. Imaging 2005, 25, 75–82. [Google Scholar] [CrossRef]
- Thorstensson, A.; Karlsson, J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiol. Scand. 1976, 98, 318–322. [Google Scholar] [CrossRef]
- Anders, J.P.V.; Keller, J.L.; Smith, C.M.; Hill, E.C.; Neltner, T.J.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Performance fatigability and neuromuscular responses for bilateral and unilateral leg extensions in men. J. Musculoskelet. Neuronal Interact. 2020, 20, 325–331. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E. Isokinetics in Human Performance; Human Kinetics: Champaign, IL, USA, 2000; ISBN 978-0-7360-0005-5. [Google Scholar]
- Amann, M.; Venturelli, M.; Ives, S.J.; McDaniel, J.; Layec, G.; Rossman, M.J.; Richardson, R.S. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J. Appl. Physiol. (1985) 2013, 115, 355–364. [Google Scholar] [CrossRef]
- Broxterman, R.M.; Hureau, T.J.; Layec, G.; Morgan, D.E.; Bledsoe, A.D.; Jessop, J.E.; Amann, M.; Richardson, R.S. Influence of group III/IV muscle afferents on small muscle mass exercise performance: A bioenergetics perspective. J. Physiol. 2018, 596, 2301–2314. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, K.T.; O’Connor, K.M.; Wier, A.P. Mechanomyographic and electromyographic responses to repeated concentric muscle actions of the quadriceps femoris. J. Electromyogr. Kinesiol. 2006, 16, 149–157. [Google Scholar] [CrossRef]
- Smith, C.M.; Housh, T.J.; HILL, E.C.; Schmidt, R.J.; Johnson, G.O. Time Course of Changes in Neuromuscular Responses at 30% versus 70% 1 Repetition Maximum during Dynamic Constant External Resistance Leg Extensions to Failure. Int. J. Exerc. Sci. 2017, 10, 365–378. [Google Scholar] [CrossRef]
- Van Dieën, J.H.; Ogita, F.; De Haan, A. Reduced neural drive in bilateral exertions: A performance-limiting factor? Med. Sci. Sports Exerc. 2003, 35, 111–118. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Kim, J. Electromyography-signal-based muscle fatigue assessment for knee rehabilitation monitoring systems. Biomed. Eng. Lett. 2018, 8, 345–353. [Google Scholar] [CrossRef]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation–contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef]
- Dimitrova, N.A.; Dimitrov, G.V. Interpretation of EMG changes with fatigue: Facts, pitfalls, and fallacies. J. Electromyogr. Kinesiol. 2003, 13, 13–36. [Google Scholar] [CrossRef]
- González-Izal, M.; Malanda, A.; Gorostiaga, E.; Izquierdo, M. Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 2012, 22, 501–512. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Mills, K.R. The relationship between mean power frequency of the EMG spectrum and muscle fibre conduction velocity. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 130–134. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 1984, 11, 251–279. [Google Scholar] [PubMed]
- Fortune, E.; Lowery, M.M. Effect of extracellular potassium accumulation on muscle fiber conduction velocity: A simulation study. Ann. Biomed. Eng. 2009, 37, 2105–2117. [Google Scholar] [CrossRef]
- Laurent, D.; Portero, P.; Goubel, F.; Rossi, A. Electromyogram spectrum changes during sustained contraction related to proton and diprotonated inorganic phosphate accumulation: A 31P nuclear magnetic resonance study on human calf muscles. Eur. J. Appl. Physiol. 1993, 66, 263–268. [Google Scholar] [CrossRef]
- Watanabe, K.; Sakai, T.; Kato, S.; Hashizume, N.; Horii, N.; Yoshikawa, M.; Hasegawa, N.; Iemitsu, K.; Tsuji, K.; Uchida, M.; et al. Conduction Velocity of Muscle Action Potential of Knee Extensor Muscle During Evoked and Voluntary Contractions After Exhaustive Leg Pedaling Exercise. Front. Physiol. 2020, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Casolo, A.; Maeo, S.; Balshaw, T.G.; Lanza, M.B.; Martin, N.R.W.; Nuccio, S.; Moro, T.; Paoli, A.; Felici, F.; Maffulli, N.; et al. Non-invasive estimation of muscle fibre size from high-density electromyography. J. Physiol. 2023, 601, 1831–1850. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.; Sjöström, M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J. Neurol. Sci. 1986, 72, 211–222. [Google Scholar] [CrossRef]
- Fink, J.; Kikuchi, N.; Nakazato, K. Effects of rest intervals and training loads on metabolic stress and muscle hypertrophy. Clin. Physiol. Funct. Imaging 2018, 38, 261–268. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.L.; Lehti, M.; Hulmi, J.J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. (1985) 2019, 126, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Felici, F.; Del Vecchio, A. Surface Electromyography: What Limits Its Use in Exercise and Sport Physiology? Front. Neurol. 2020, 11, 578504. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: 2004–2024. J. Appl. Physiol. 2025, 138, 121–135. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anders, J.P.V.; Neltner, T.J.; Smith, R.W.; Arnett, J.E.; Schmidt, R.J.; Housh, T.J. The Impact of a Modality Switch During Isokinetic Leg Extensions on Performance Fatigability and Neuromuscular Patterns of Response. Sensors 2025, 25, 4013. https://doi.org/10.3390/s25134013
Anders JPV, Neltner TJ, Smith RW, Arnett JE, Schmidt RJ, Housh TJ. The Impact of a Modality Switch During Isokinetic Leg Extensions on Performance Fatigability and Neuromuscular Patterns of Response. Sensors. 2025; 25(13):4013. https://doi.org/10.3390/s25134013
Chicago/Turabian StyleAnders, John Paul V., Tyler J. Neltner, Robert W. Smith, Jocelyn E. Arnett, Richard J. Schmidt, and Terry J. Housh. 2025. "The Impact of a Modality Switch During Isokinetic Leg Extensions on Performance Fatigability and Neuromuscular Patterns of Response" Sensors 25, no. 13: 4013. https://doi.org/10.3390/s25134013
APA StyleAnders, J. P. V., Neltner, T. J., Smith, R. W., Arnett, J. E., Schmidt, R. J., & Housh, T. J. (2025). The Impact of a Modality Switch During Isokinetic Leg Extensions on Performance Fatigability and Neuromuscular Patterns of Response. Sensors, 25(13), 4013. https://doi.org/10.3390/s25134013





