The Reliability and Validity of an Instrumented Device for Tracking the Shoulder Range of Motion
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
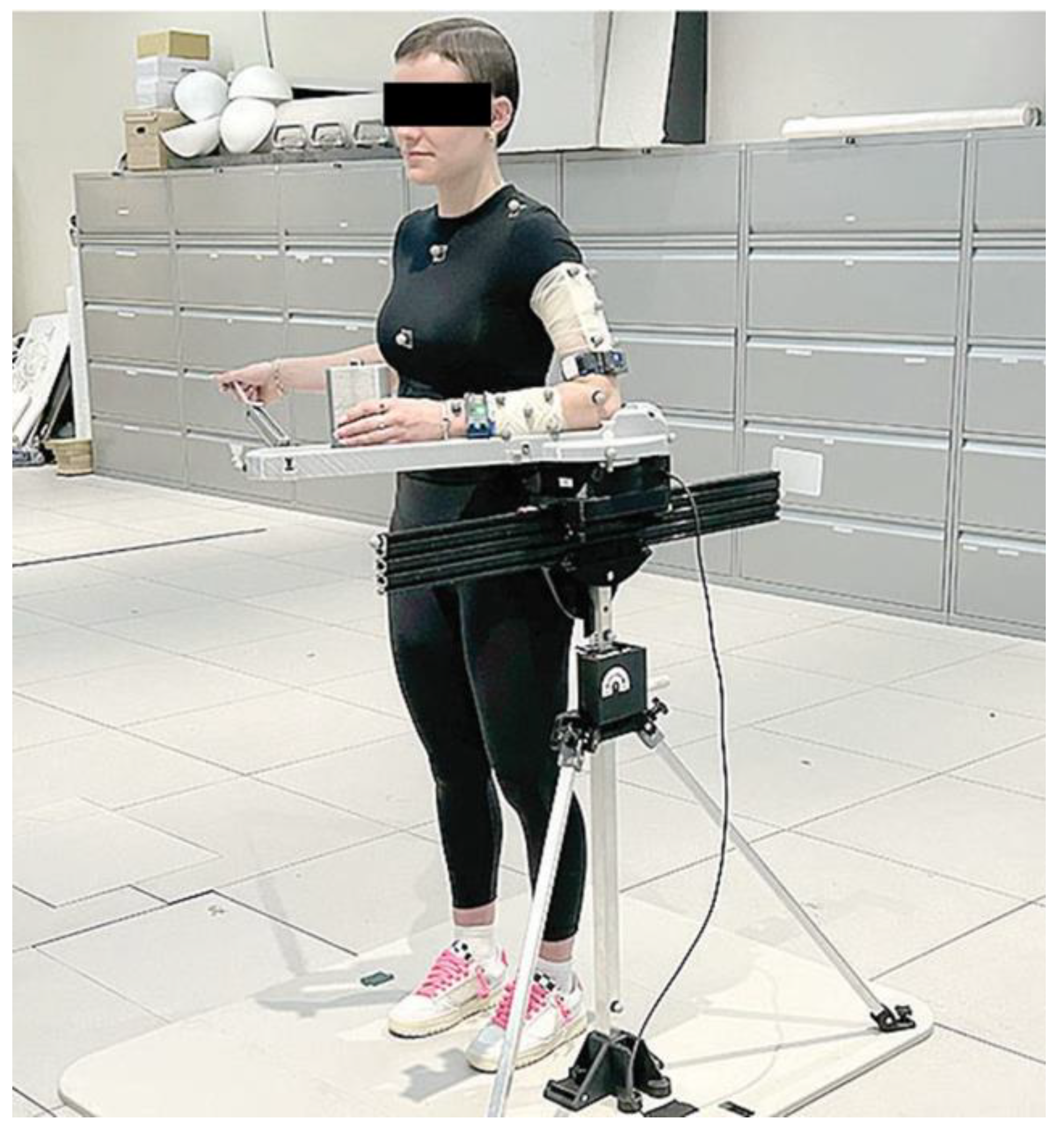
2.2. Instrumentation
2.3. Testing Procedures
- Internal rotation passive range of motion (IR PROM);
- External rotation passive range of motion (ER PROM);
- Internal rotation active range of motion (IR AROM);
- External rotation active range of motion (ER AROM);
- Retraction active range of motion;
- Protraction active range of motion;
- Forward elevation passive range of motion (FE PROM);
- Forward elevation active range of motion (FE AROM);
- Abduction passive range of motion (ABD PROM).
- Fifteen repetitions for IR PROM, ER PROM, IR AROM, ER AROM, and retraction/protraction AROM;
- Thirty repetitions for FE PROM, FE AROM, and ABD PROM.
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Participants and Missing Data
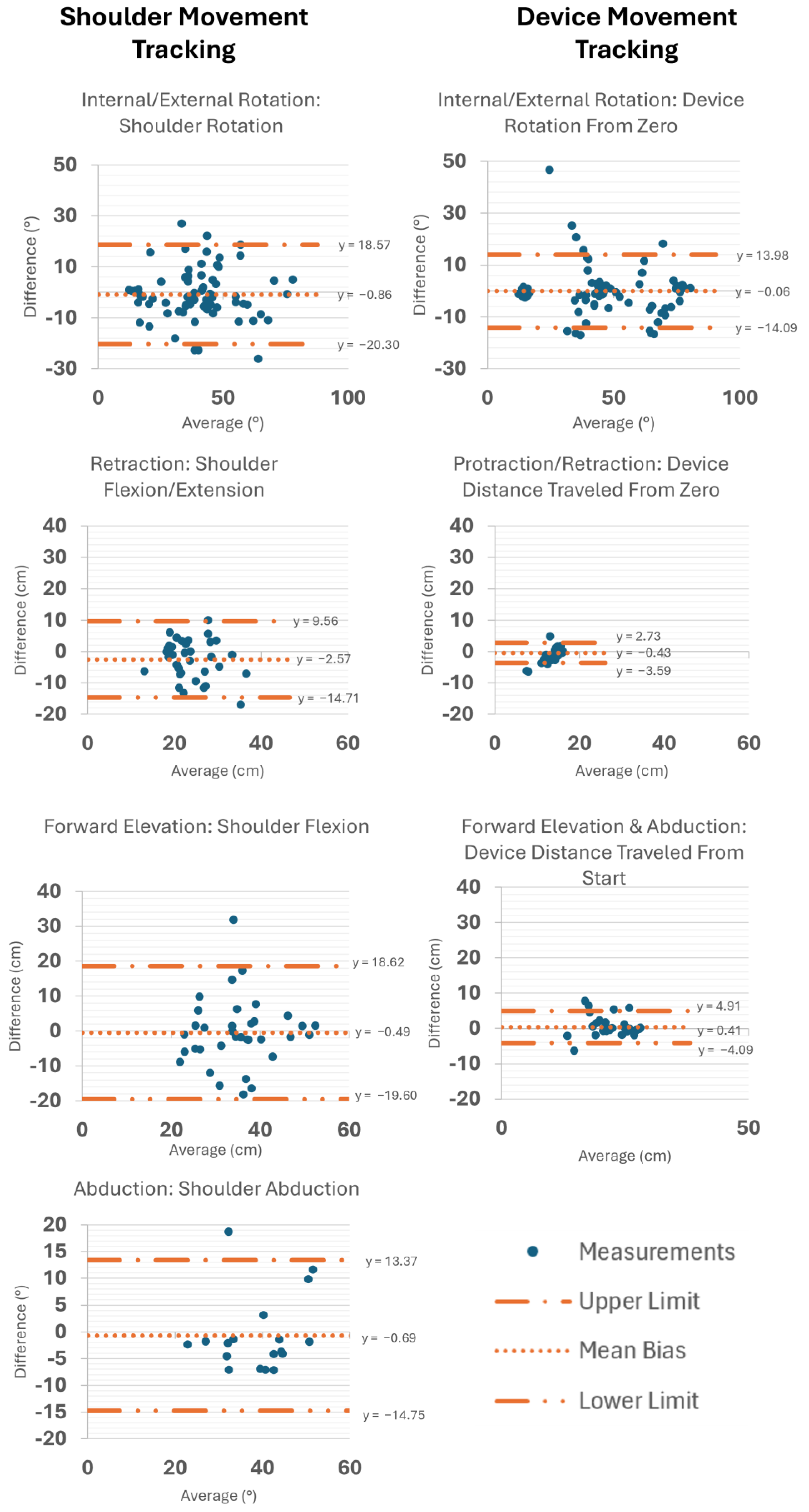
3.2. Concurrent Validity
3.3. Test–Retest Reliability
4. Discussion
4.1. Concurrent Validity
4.2. Test–Retest Reliability
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PT | Physical therapy |
| RCR | Rotator cuff repair |
| ROM | Range of motion |
| SSS | Strengthening and Stabilization System |
| IMU | Inertial measurement unit |
| STA | Soft tissue artifact |
| HMBL | Human Movement and Balance Laboratory |
| IR | Internal rotation |
| PROM | Passive range of motion |
| ER | External rotation |
| AROM | Active range of motion |
| ICC | Intraclass correlation coefficient |
| RMSE | Root mean square error |
References
- Kuhn, J. Prevalence, Natural History, and Nonoperative Treatment of Rotator Cuff Disease. Oper. Tech. Sports Med. 2023, 31, 150978. [Google Scholar] [CrossRef]
- Teunis, T.; Lubberts, B.; Reilly, B.T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J. Shoulder Elb. Surg. 2014, 23, 1913–1921. [Google Scholar] [CrossRef]
- Sciarretta, F.V.; Moya, D.; List, K. Current trends in rehabilitation of rotator cuff injuries. SICOT J. 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, C.A.; Shaffer, M.A.; Gaunt, B.W.; Leggin, B.G.; Williams, G.R.; Wilcox, R.B., III. The American Society of Shoulder and Elbow Therapists’ consensus statement on rehabilitation following arthroscopic rotator cuff repair. J. Shoulder Elb. Surg. 2016, 25, 521–535. [Google Scholar] [CrossRef]
- Gumina, S.; Izzo, R.; Pintabona, G.; Candela, V.; Savastano, R.; Santilli, V. Mobility recovery after arthroscopic rotator cuff repair. Eur. J. Phys. Rehabil. Med. 2017, 53, 49–56. [Google Scholar] [CrossRef]
- Rogers, M.J.; Penvose, I.; Curry, E.J.; Galvin, J.W.; Li, X. Insurance status affects access to physical therapy following rotator cuff repair surgery: A comparison of privately insured and Medicaid patients. Orthop. Rev. 2019, 11, 7989. [Google Scholar] [CrossRef]
- Arshi, A.; Kabir, N.; Cohen, J.R.; Lord, E.L.; Wang, J.C.; McAllister, D.R.; Petrigliano, F.A. Utilization and Costs of Postoperative Physical Therapy After Rotator Cuff Repair: A Comparison of Privately Insured and Medicare Patients. Arthroscopy 2015, 31, 2392–2399.e2391. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Indermuhle, T.; Curtis, D.; Perez, J.; Leland, J.M. Factors Affecting Postoperative Rehabilitation Therapy Utilization After Arthroscopic Rotator Cuff Repair: An Epidemiological Analysis. Cureus 2023, 15, e36740. [Google Scholar] [CrossRef]
- Thummak, S.; Uppor, W.; Wannarit, L.O. Patient compliance: A concept analysis. Belitung Nurs. J. 2023, 9, 421–427. [Google Scholar] [CrossRef]
- Kattan, A.E.; AlHemsi, H.B.; AlKhawashki, A.M.; AlFadel, F.B.; Almoosa, S.M.; Mokhtar, A.M.; Alasmari, B.A. Patient Compliance With Physical Therapy Following Orthopedic Surgery and Its Outcomes. Cureus 2023, 15, e37217. [Google Scholar] [CrossRef]
- Bender, M.; Jain, N.; Giron, A.; Harder, J.; Rounds, A.; Mackay, B. Factors Influencing Compliance to Follow-up Visits in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2024, 8, e23. [Google Scholar] [CrossRef] [PubMed]
- Ezeamii, V.C.; Okobi, O.E.; Wambai-Sani, H.; Perera, G.S.; Zaynieva, S.; Okonkwo, C.C.; Ohaiba, M.M.; William-Enemali, P.C.; Obodo, O.R.; Obiefuna, N.G. Revolutionizing Healthcare: How Telemedicine Is Improving Patient Outcomes and Expanding Access to Care. Cureus 2024, 16, e63881. [Google Scholar] [CrossRef]
- Gardner, E.C.; Podbielski, C.; Dunphy, E. Telerehabilitation to Address the Rehabilitation Gap in Anterior Cruciate Ligament Care: Survey of Physical Therapists/Care Providers. Telemed. Rep. 2024, 5, 18–35. [Google Scholar] [CrossRef]
- Agnihotri, S.; Gupta, N.; Sindwani, P.; Srivastava, A.; Ahmad, A.; Karki, M. Telerehabilitation: Exploring the Untapped Potential. Cureus 2024, 16, e57405. [Google Scholar] [CrossRef]
- Shambushankar, A.K.; Jose, J.; Gnanasekaran, S.; Kaur, G. Cost-Effectiveness of Telerehabilitation Compared to Traditional In-Person Rehabilitation: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e79028. [Google Scholar] [CrossRef]
- Simmich, J.; Ross, M.H.; Russell, T. Real-time video telerehabilitation shows comparable satisfaction and similar or better attendance and adherence compared with in-person physiotherapy: A systematic review. J. Physiother. 2024, 70, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Klinger, E.; Dorner, V.; Kadri, A.; Thierry, O.; Boumenir, Y.; Martin, W.; Poiraudeau, S.; Ville, I. Barriers to home-based exercise program adherence with chronic low back pain: Patient expectations regarding new technologies. Ann. Phys. Rehabil. Med. 2016, 59, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.W.; Oliveira, S.B.; Souza, L.M.D.N. Telerehabilitation from the perspective of patients and healthcare providers: A 3-year follow-up study. NeuroRehabilitation 2024, 55, 103–115. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
- Cain, S.M.; Morrow, M.M.B. Quantifying shoulder motion in the free-living environment using wearable inertial measurement units: Challenges and recommendations. J. Biomech. 2025, 182, 112589. [Google Scholar] [CrossRef]
- Tranquilli, C.; Bernetti, A.; Picerno, P. Ambulatory joint mobility and muscle strength assessment during rehabilitation using a single wearable inertial sensor. Med. Dello Sport 2013, 66, 583–597. [Google Scholar]
- Parel, I.; Candoli, V.; Filippi, M.V.; Padolino, A.; Merolla, G.; Sanniti, S.; Galassi, R.; Paladini, P.; Cutti, A.G. Shoulder Rehabilitation Exercises With Kinematic Biofeedback After Arthroscopic Rotator Cuff Repair: Protocol for a New Integrated Rehabilitation Program. JMIR Res. Protoc. 2023, 12, e35757. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.J.; Drain, N.P.; Lesniak, B.P.; Lin, A.; Musahl, V.; Irrgang, J.J.; Popchak, A.J. Self-Reported Outcomes in Early Postoperative Management After Shoulder Surgery Using a Home-Based Strengthening and Stabilization System With Telehealth. Sports Health 2023, 15, 599–605. [Google Scholar] [CrossRef]
- Riffitts, M.; Cook, H.; McClincy, M.; Bell, K. Evaluation of a Smart Knee Brace for Range of Motion and Velocity Monitoring during Rehabilitation Exercises and an Exergame. Sensors 2022, 22, 9965. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.M.; Onyeukwu, C.; McClincy, M.P.; Allen, M.; Bechard, L.; Mukherjee, A.; Hartman, R.A.; Smith, C.; Lynch, A.D.; Irrgang, J.J. Verification of a Portable Motion Tracking System for Remote Management of Physical Rehabilitation of the Knee. Sensors 2019, 19, 1021. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.M.; Onyeukwu, C.; Smith, C.N.; Oh, A.; Devito Dabbs, A.; Piva, S.R.; Popchak, A.J.; Lynch, A.D.; Irrgang, J.J.; McClincy, M.P. A Portable System for Remote Rehabilitation Following a Total Knee Replacement: A Pilot Randomized Controlled Clinical Study. Sensors 2020, 20, 6118. [Google Scholar] [CrossRef]
- Favata, A.; Gallart-Agut, R.; Pàmies-Vilà, R.; Torras, C.; Font-Llagunes, J.M. IMU-Based Systems for Upper-Limb Kinematic Analysis in Clinical Applications: A Systematic Review. IEEE Sens. J. 2024, 24, 28576–28594. [Google Scholar] [CrossRef]
- Iosa, M.; Picerno, P.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis. Expert Rev. Med. Devices 2016, 13, 641–659. [Google Scholar] [CrossRef]
- Cutti, A.G.; Giovanardi, A.; Rocchi, L.; Davalli, A.; Sacchetti, R. Ambulatory measurement of shoulder and elbow kinematics through inertial and magnetic sensors. Med. Biol. Eng. Comput. 2008, 46, 169–178. [Google Scholar] [CrossRef]
- Dellabiancia, F.; Parel, I.; Filippi, M.V.; Porcellini, G.; Merolla, G. Glenohumeral and scapulohumeral kinematic analysis of patients with traumatic anterior instability wearing a shoulder brace: A prospective laboratory study. Musculoskelet. Surg. 2017, 101 (Suppl. 2), 159–167. [Google Scholar] [CrossRef]
- Bruttel, H.; Spranz, D.M.; Wolf, S.I.; Maier, M.W. Scapulohumeral rhythm in patients after total shoulder arthroplasty compared to age-matched healthy individuals. Gait Posture 2020, 82, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.M.; Atkins, P.R.; Kutschke, M.J.; Goebel, J.M.; Foreman, K.B.; Anderson, A.E. Soft tissue artifact causes significant errors in the calculation of joint angles and range of motion at the hip. Gait Posture 2017, 55, 184–190. [Google Scholar] [CrossRef]
- Blache, Y.; Dumas, R.; Lundberg, A.; Begon, M. Main component of soft tissue artifact of the upper-limbs with respect to different functional, daily life and sports movements. J. Biomech. 2017, 62, 39–46. [Google Scholar] [CrossRef]
- Henschke, J.; Kaplick, H.; Wochatz, M.; Engel, T. Assessing the validity of inertial measurement units for shoulder kinematics using a commercial sensor-software system: A validation study. Health Sci. Rep. 2022, 5, e772. [Google Scholar] [CrossRef]
- Morrow, M.M.B.; Lowndes, B.; Fortune, E.; Kaufman, K.R.; Hallbeck, M.S. Validation of Inertial Measurement Units for Upper Body Kinematics. J. Appl. Biomech. 2017, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tao, K.; Chen, Q.; Tian, Y.; Sun, L. A Comprehensive Analysis of the Validity and Reliability of the Perception Neuron Studio for Upper-Body Motion Capture. Sensors 2022, 22, 6954. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.I.; Galán-Mercant, A.; Williams, J.M. The use of inertial sensors system for human motion analysis. Phys. Ther. Rev. 2010, 15, 462–473. [Google Scholar] [CrossRef]
- Álvarez, D.; Alvarez, J.C.; González, R.C.; López, A.M. Upper limb joint angle measurement in occupational health. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 159–170. [Google Scholar] [CrossRef]
- Choo, C.Z.Y.; Chow, J.Y.; Komar, J. Validation of the Perception Neuron system for full-body motion capture. PLoS ONE 2022, 17, e0262730. [Google Scholar] [CrossRef]
- Lavaill, M.; Martelli, S.; Kerr, G.K.; Pivonka, P. Statistical Quantification of the Effects of Marker Misplacement and Soft-Tissue Artifact on Shoulder Kinematics and Kinetics. Life 2022, 12, 819. [Google Scholar] [CrossRef]
- Picerno, P.; Iosa, M.; D’Souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis: A five-year update. Expert Rev. Med. Devices 2021, 18, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.M.; MacDermid, J.; Johnson, J.; Birmingham, T.B.; Shafiee, E. Applications of wearable sensors in upper extremity MSK conditions: A scoping review. J. Neuroeng. Rehabil. 2023, 20, 158. [Google Scholar] [CrossRef] [PubMed]




| Asymptomatic (N = 18) | |
| Age | 34.3 (17.5) |
| Sex | 12 Female, 6 Male |
| BMI | 26.6 (6.5) |
| Race | 83 (15) White, 11 (2) Black, 6 (1) Multiracial |
| Rotator cuff repair (N = 18) | |
| Age | 55.3 (7.4) |
| Sex | 8 Female, 10 Male |
| BMI | 30.2 (7.0) |
| Race | 89 (16) White, 11 (2) Black |
| Exercise Name(s) | System | Measurement | Unit | Rating | ICC (95% CI) | N | RMSE | SD |
|---|---|---|---|---|---|---|---|---|
| IR/ER | BioMech | Shoulder rotation | deg | Good | 0.81 (0.75–0.85) | 204 | 10.34 | ±9.28 |
| Forward Elevation | BioMech | Shoulder flexion | deg | Poor | 0.13 (−0.09–0.33) | 83 | 12.69 | ±11.76 |
| Abduction | BioMech | Shoulder abduction | deg | Poor | 0.21 (−0.07–0.47) | 48 | 10.96 | ±10.25 |
| IR/ER | CuffLink | Device rotation | deg | Excellent | 0.94 (0.92–0.95) | 412 | 6.02 | ±5.79 |
| Protraction/Retraction | CuffLink | Device distance from zero | cm | Good | 0.75 (0.68–0.85) | 204 | 2.41 | ±1.89 |
| Forward Elevation and Abduction | CuffLink | Device distance from start | cm | Good | 0.88 (0.83–0.91) | 132 | 2.02 | ±1.36 |
| Exercise Name(s) | System | Measurement | Unit | Rating | ICC (95% CI) | N | RMSE | SD |
|---|---|---|---|---|---|---|---|---|
| IR/ER | BioMech | Shoulder rotation | deg | Good | 0.81 (0.71–0.88) | 72 | 9.80 | ±9.74 |
| Protraction/Retraction | BioMech | Shoulder flexion/extension | cm | Poor | 0.44 (0.14–0.67) | 35 | 6.62 | ±6.10 |
| Forward Elevation | BioMech | Shoulder flexion | deg | Poor | 0.47 (0.16–0.69) | 35 | 9.62 | ±9.61 |
| Abduction | BioMech | Shoulder abduction | deg | Moderate | 0.69 (0.34–0.87) | 18 | 7.00 | ±6.97 |
| IR/ER | CuffLink | Device rotation | deg | Excellent | 0.94 (0.91–0.95) | 142 | 5.41 | ±5.35 |
| Protraction/Retraction | CuffLink | Device distance from zero | cm | Moderate | 0.57 (0.38–0.71) | 70 | 1.66 | ±1.60 |
| Forward Elevation and Abduction | CuffLink | Device distance from start | cm | Good | 0.79 (0.66–0.88) | 48 | 2.31 | ±2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roos, R.E.; Lambiase, J.; Riffitts, M.; Scholle, L.; Kulkarni, S.; Luck, C.L.; Parmanto, D.; Putraadinatha, V.; Yoga, M.D.; Lang, S.N.; et al. The Reliability and Validity of an Instrumented Device for Tracking the Shoulder Range of Motion. Sensors 2025, 25, 3818. https://doi.org/10.3390/s25123818
Roos RE, Lambiase J, Riffitts M, Scholle L, Kulkarni S, Luck CL, Parmanto D, Putraadinatha V, Yoga MD, Lang SN, et al. The Reliability and Validity of an Instrumented Device for Tracking the Shoulder Range of Motion. Sensors. 2025; 25(12):3818. https://doi.org/10.3390/s25123818
Chicago/Turabian StyleRoos, Rachel E., Jennifer Lambiase, Michelle Riffitts, Leslie Scholle, Simran Kulkarni, Connor L. Luck, Dharma Parmanto, Vayu Putraadinatha, Made D. Yoga, Stephany N. Lang, and et al. 2025. "The Reliability and Validity of an Instrumented Device for Tracking the Shoulder Range of Motion" Sensors 25, no. 12: 3818. https://doi.org/10.3390/s25123818
APA StyleRoos, R. E., Lambiase, J., Riffitts, M., Scholle, L., Kulkarni, S., Luck, C. L., Parmanto, D., Putraadinatha, V., Yoga, M. D., Lang, S. N., Tatko, E., Grant, J., Oakley, J. I., Disantis, A., Saptono, A., Parmanto, B., Popchak, A., McClincy, M. P., & Bell, K. M. (2025). The Reliability and Validity of an Instrumented Device for Tracking the Shoulder Range of Motion. Sensors, 25(12), 3818. https://doi.org/10.3390/s25123818







