Sensor Technologies for Non-Invasive Blood Glucose Monitoring
Abstract
1. Introduction
2. Common Sensor Technologies for Blood Glucose Monitoring
2.1. Invasive Sensors
2.1.1. Self-Monitoring Blood Glucose
2.1.2. Continuous Glucose Monitoring
2.2. Non-Invasive Sensors
2.2.1. Optical Sensor Technologies
Near-Infrared/Mid-Infrared Spectroscopy
Fluorescence Spectroscopy
Raman Spectroscopy
Optical Coherence Tomography
2.2.2. Non-Optical Sensor Technologies
Bioimpedance Spectroscopy
Reverse Iontophoresis
Blood Substitute Measurement
Metabolic Balance
Electromagnetic Techniques
3. Antenna-Sensors
3.1. Rigid Antenna-Sensor
3.1.1. Substrate Materials
3.1.2. Conductive Materials
3.1.3. Fabrication Methods
3.1.4. Applications
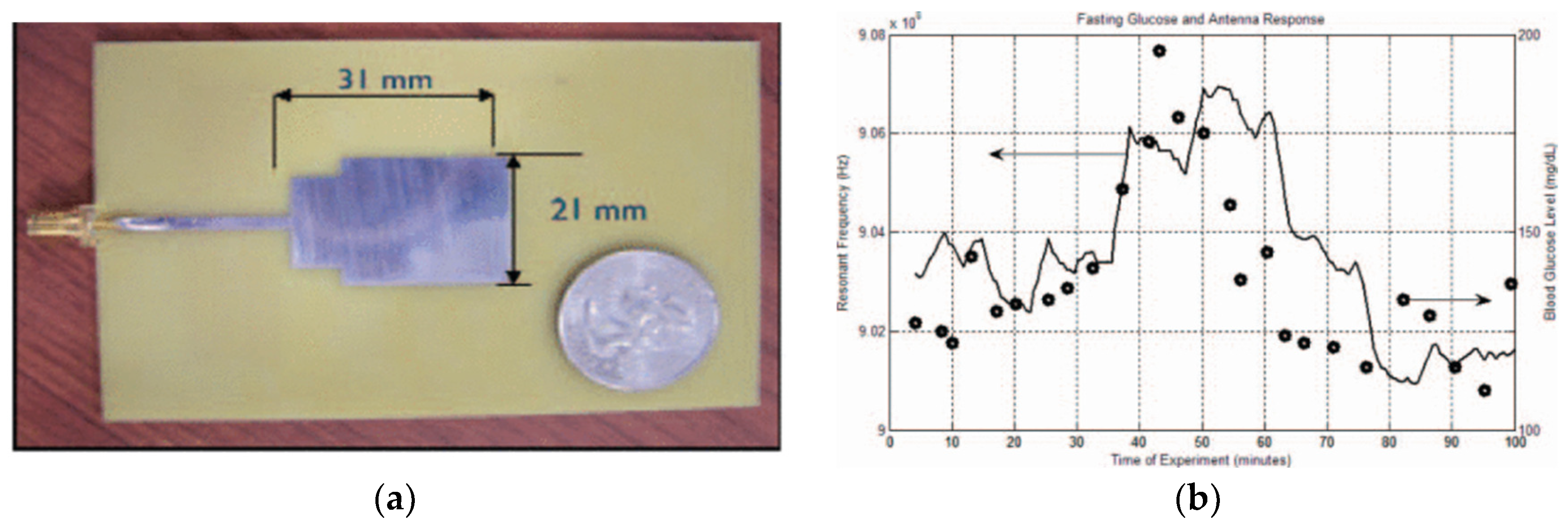
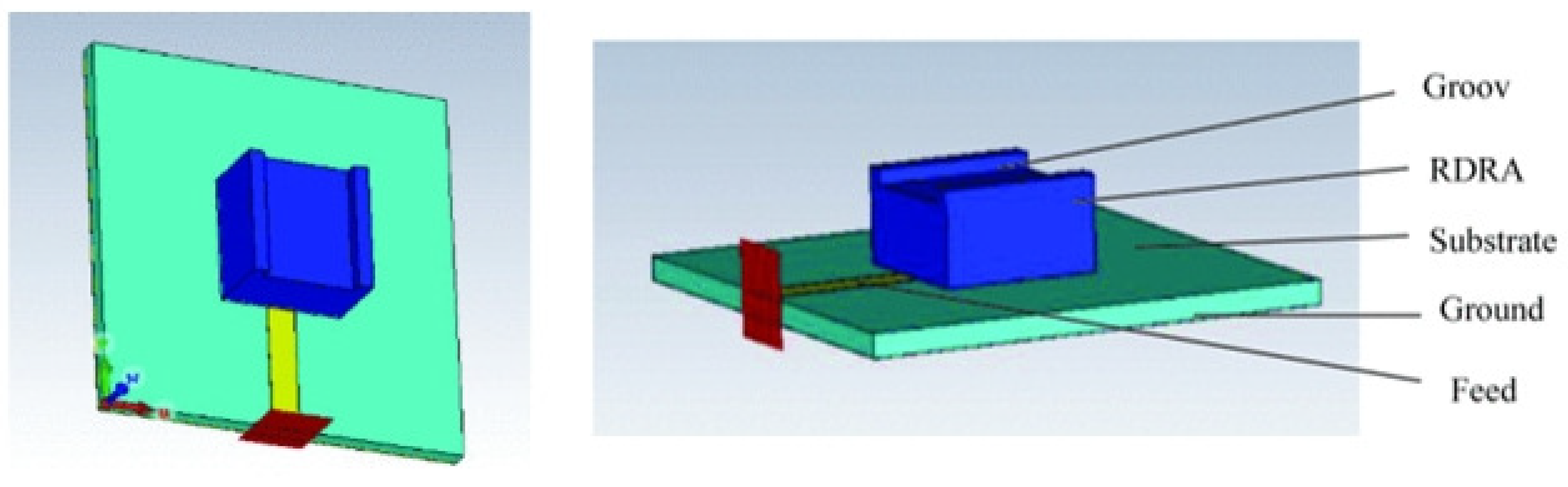
Patch Antenna-Sensors
Metamaterial Antenna-Sensors
UWB Antenna-Sensors
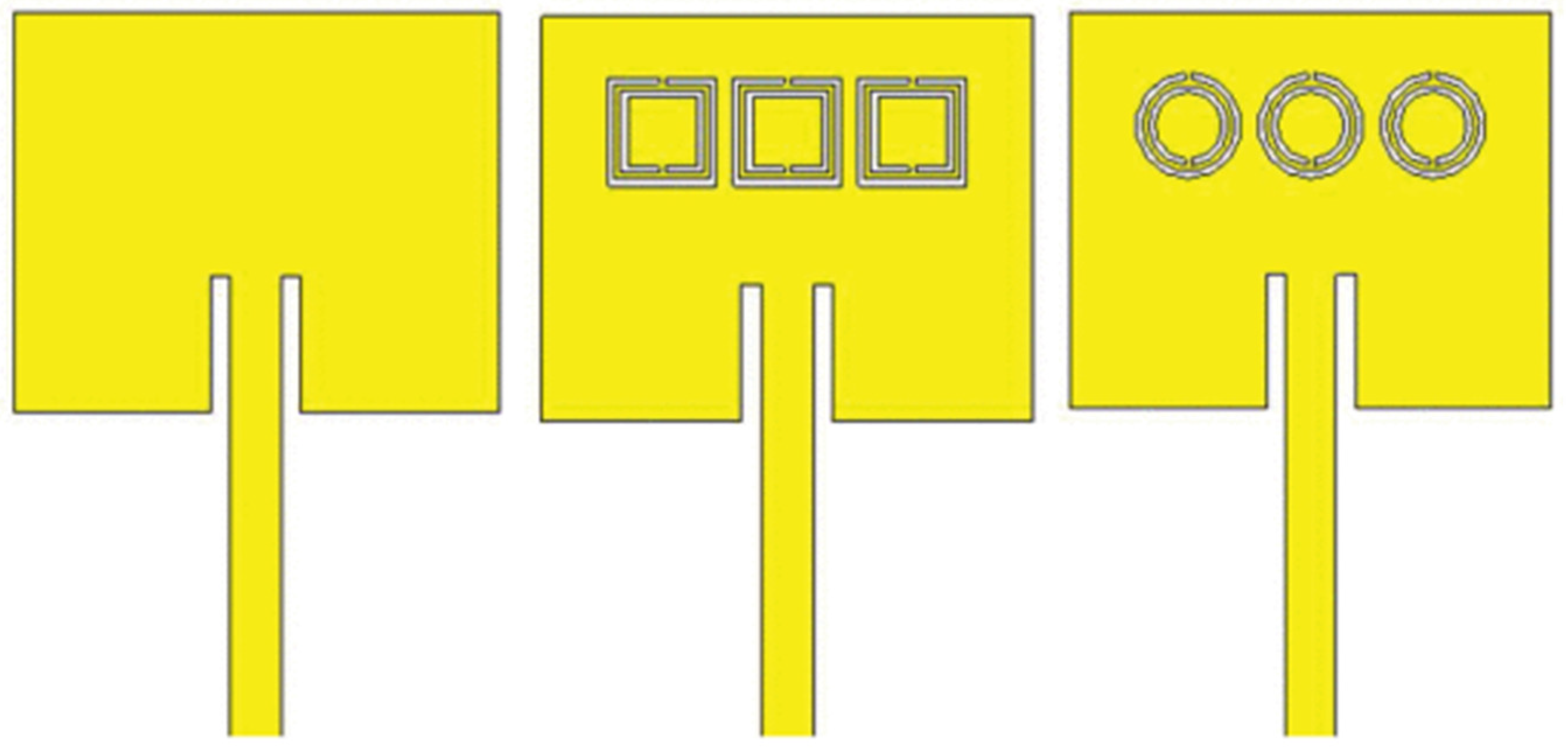
Resonator Antenna-Sensors
3.2. Flexible Antenna-Sensors
3.2.1. Substrate Materials
| Reference | Substrates | Dielectric Constant | Dielectric Loss | Thickness |
|---|---|---|---|---|
| [60] | Kapton | 3.5 | 0.002 | 100 μm |
| [62] | PET | 2.63 | 0.004 | 0.5 mm |
| [64] | PDMS | 2.65 | 0.02 | 2 mm |
| [66] | LCP | 3.16 | 0.025 | 4 mm |
| [67] | Paper | 1.9 | 0.025 | 100 μm |
3.2.2. Conductive Materials
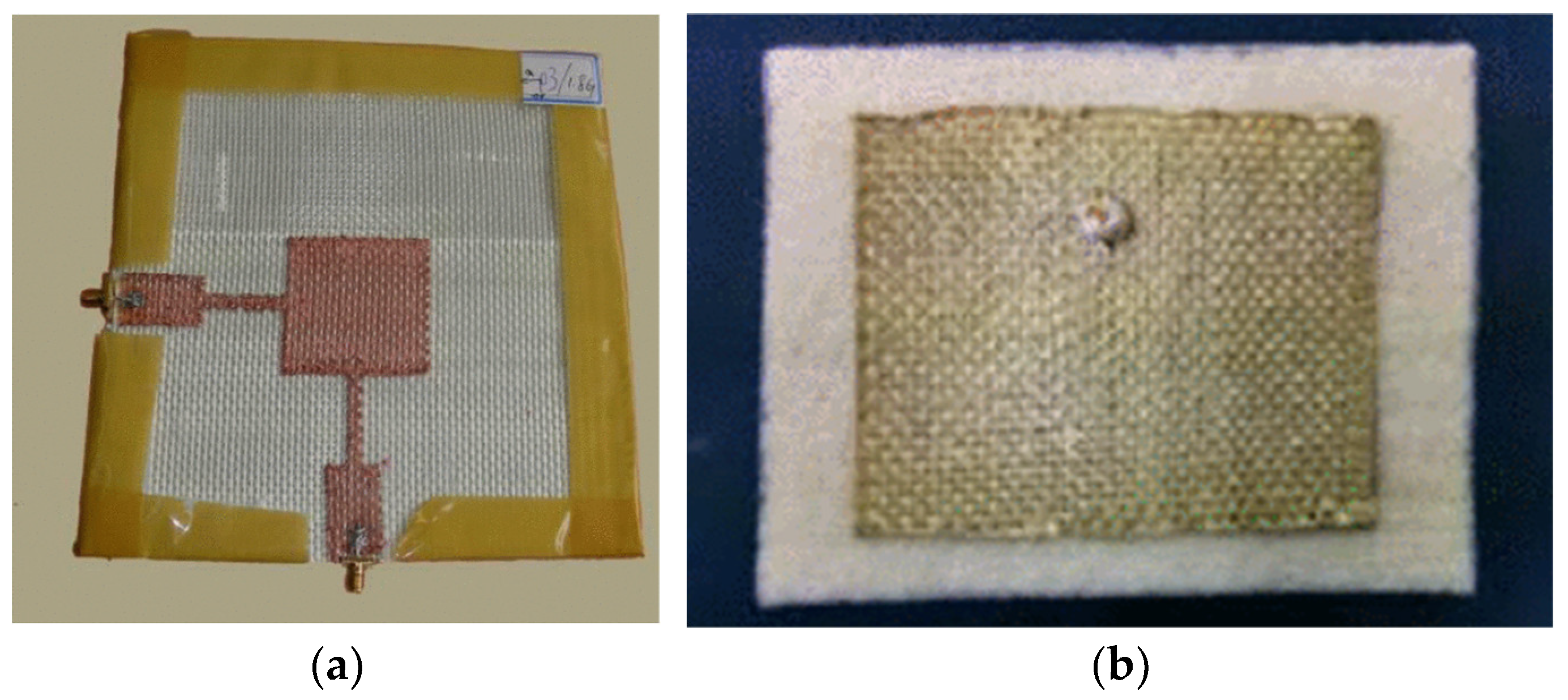
3.2.3. Textile Antenna-Sensors
3.2.4. Fabrication Methods
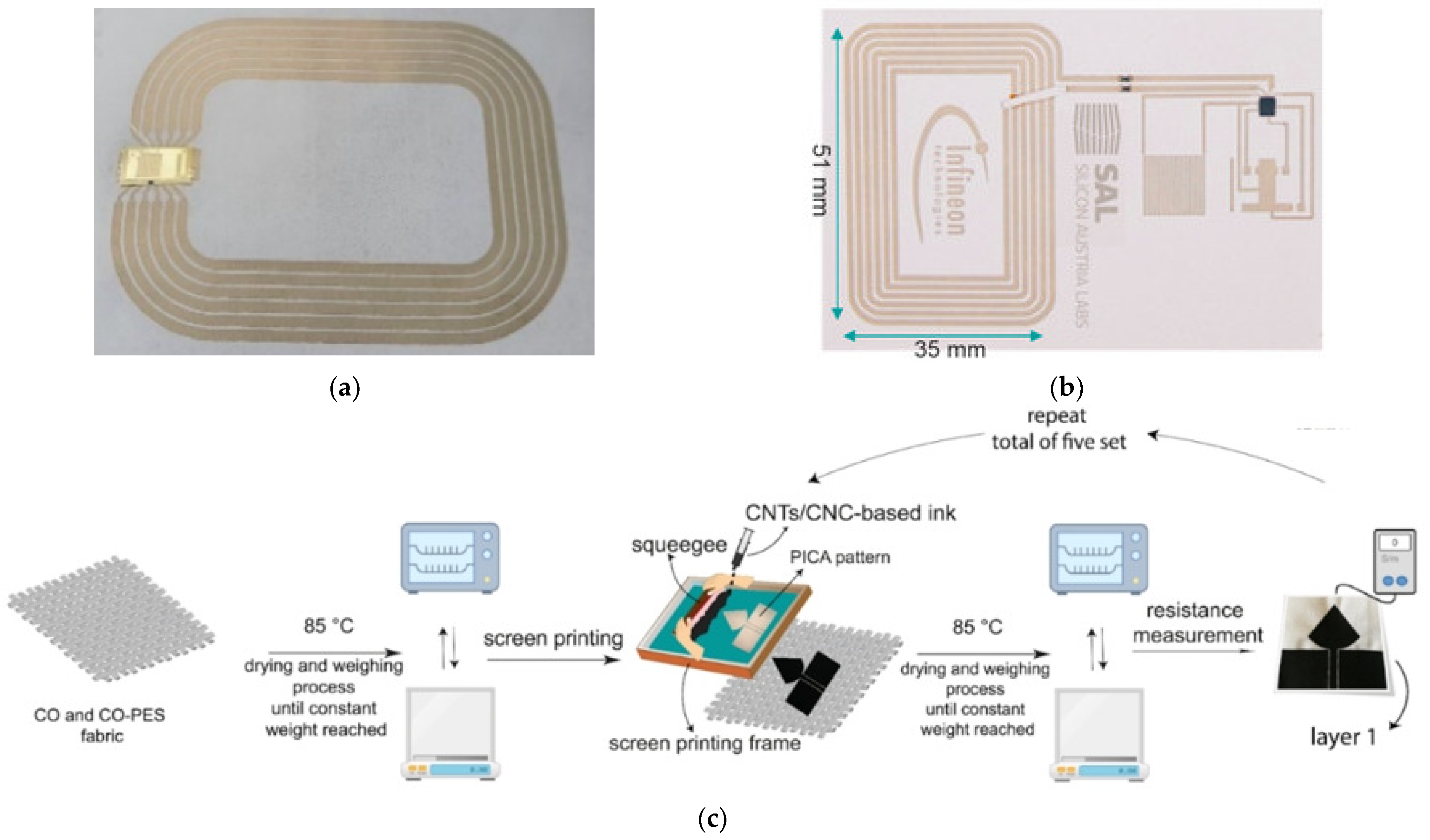
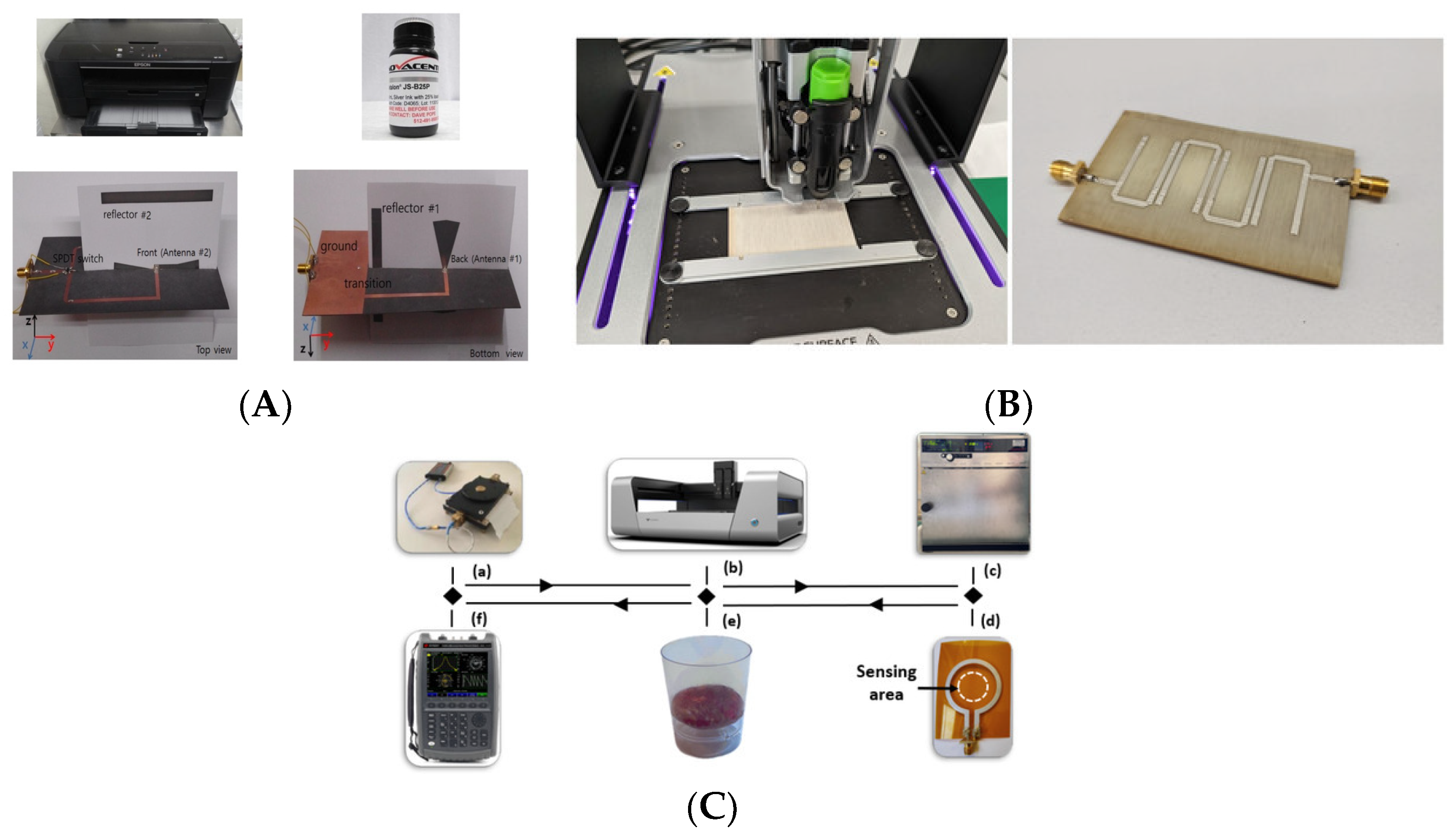
Screen Printing
Inkjet Printing
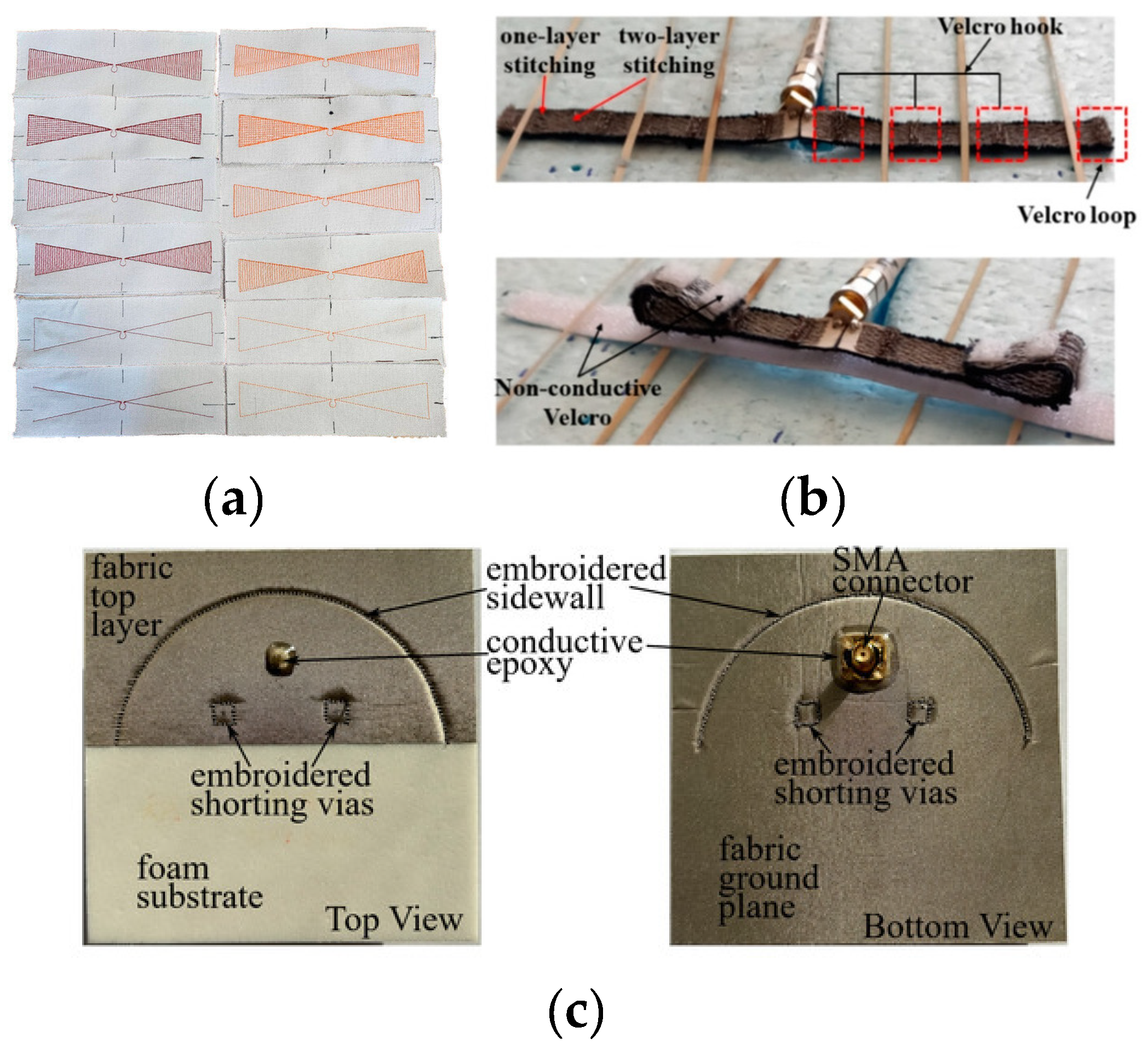
Embroidering
Weaving
3.2.5. Human Impact
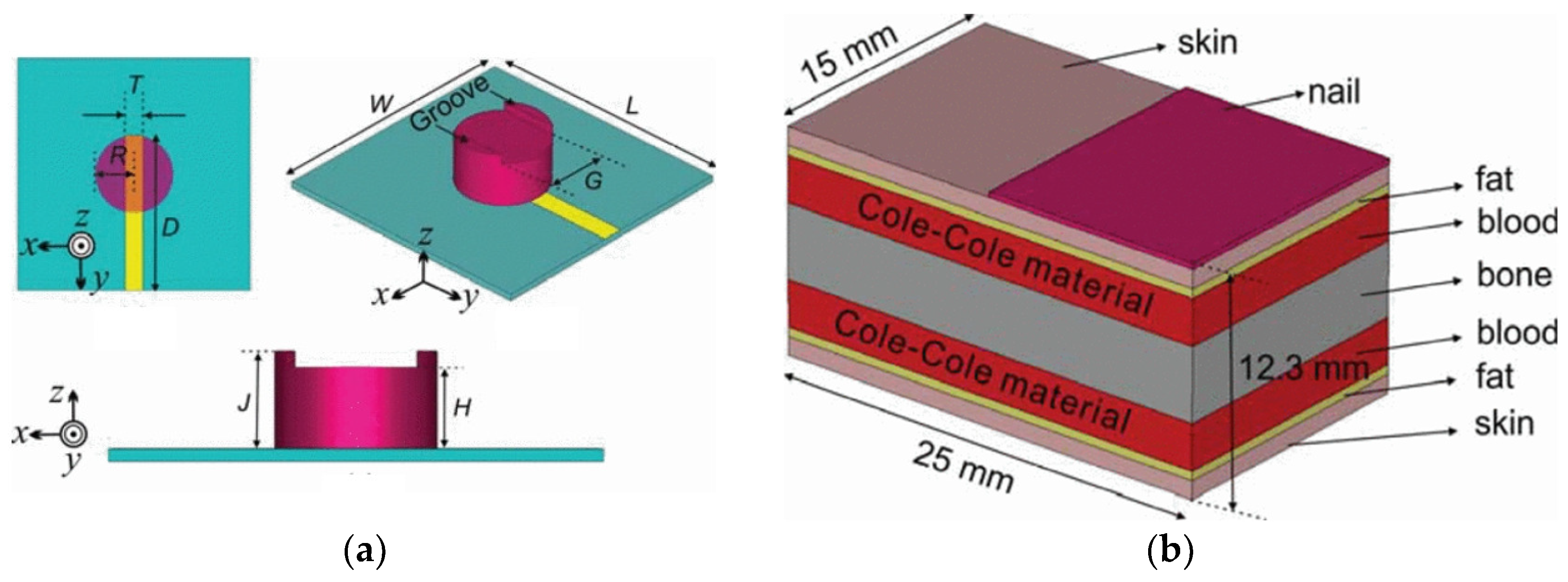
Phantom
SAR
Blood Glucose Measurement Regulation
3.2.6. Data Processing and Modeling
- Non-uniqueness: Distinct dielectric distributions can produce similar antenna responses.
- Instability: Minor variations in skin–sensor coupling, motion, or environmental humidity may induce disproportionate shifts in signal.
- High dimensionality: Measured features span multiple domains (e.g., frequency, amplitude, phase), yet the features that are truly related to glucose levels are limited.
- Gradient Boosting Decision Trees: Effective for tabular feature sets, capable of modeling complex interactions while maintaining interpretability.
- Support Vector Machines: Well-suited for high-dimensional feature spaces with limited training data.
- Artificial Neural Networks and deep convolutional models: Particularly useful when raw spectral inputs are available, though requiring substantial data volume and careful regularization.
3.2.7. Blood Glucose Monitoring Application
4. Summary, Challenges, and Conclusions
4.1. Summary
4.2. Challenges
- Mitigating the influence of environmental factors (e.g., humidity, temperature fluctuations) and human factors (e.g., mechanical stress from washing, sweat-induced corrosion) on antenna-sensor performance.
- Enhancing the precision and efficiency of the current fabrication techniques.
- Incorporating flexible antenna-sensors into everyday clothing while ensuring biocompatibility and long-term durability.
- Investigating new yarns and conductive textiles with lower resistivity or higher conductivity to improve antenna-sensor performance.
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. Physiology, Glucose Metabolism; StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General Aspects of Diabetes Mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [PubMed]
- Zajec, A.; Trebušak Podkrajšek, K.; Tesovnik, T.; Bratina, N.; Debeljak, M.; Battelino, T.; Bratanic, N. Pathogenesis of Type 1 Diabetes: Established Facts and New Insights. Genes 2022, 13, 706. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 Diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and Phenotype of Type 1 Diabetes in the First Six Decades of Life: A Cross-Sectional, Genetically Stratified Survival Analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, R.; Adeli, K. Hepatic Insulin Resistance, Metabolic Syndrome and Cardiovascular Disease. Clin. Biochem. 2009, 42, 1331–1346. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Simonson, D.; Ferrannini, E. Hepatic and Peripheral Insulin Resistance: A Common Feature of Type 2 (Non-Insulin-Dependent) and Type 1 (Insulin-Dependent) Diabetes Mellitus. Diabetologia 1982, 23, 313–319. [Google Scholar] [CrossRef]
- Farmaki, P.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Savvanis, S.; Diamantis, E. Complications of the Type 2 Diabetes Mellitus. Curr. Cardiol. Rev. 2020, 16, 249–251. [Google Scholar] [CrossRef]
- Shah, N.S.; Wang, M.C.; Freaney, P.M.; Perak, A.M.; Carnethon, M.R.; Kandula, N.R.; Gunderson, E.P.; Bullard, K.M.; Grobman, W.A.; O’brien, M.J.; et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011–2019. JAMA 2021, 326, 660–669. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; 2021; Available online: https://www.idf.org (accessed on 3 February 2025).
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of Diet on Type 2 Diabetes Mellitus: A Review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Church, T.S.; Blair, S.N.; Cocreham, S.; Johannsen, N.; Johnson, W.; Kramer, K.; Mikus, C.R.; Myers, V.; Nauta, M.; Rodarte, R.Q.; et al. Effects of Aerobic and Resistance Training on Hemoglobin A1c Levels in Patients with Type 2 Diabetes: A Randomized Controlled Trial. JAMA 2010, 304, 2253–2262, Correction in JAMA 2011, 305, 892. [Google Scholar] [CrossRef]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003, 26 (Suppl. S1), S5–S20. [Google Scholar] [CrossRef]
- Benjamin, E.M. Self-Monitoring of Blood Glucose: The Basics. Clin. Diabetes 2002, 20, 45–47. [Google Scholar] [CrossRef]
- Abbott. FreeStyle® Precision Neo. (n.d.). Available online: https://www.freestyle.abbott/us-en/home.html (accessed on 3 February 2025).
- Freckmann, G.; Pleus, S.; Grady, M.; Setford, S.; Levy, B. Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. J. Diabetes Sci. Technol. 2019, 13, 575–583. [Google Scholar] [CrossRef]
- Dexcom. Dexcom G6. (n.d.). Available online: https://www.dexcom.com/ (accessed on 3 February 2025).
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Marius, I.; Sever, P. Measuring Glucose Blood with Spectroscopy Skin in Near Infrared. In Proceedings of the 2019 11th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Pitesti, Romania, 27–29 June 2019; pp. 1–6. [Google Scholar]
- Li, Q.B.; Li, L.N.; Zhang, G.J. A Nonlinear Model for Calibration of Blood Glucose Noninvasive Measurement Using Near Infrared Spectroscopy. Infrared Phys. Technol. 2010, 53, 410–417. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hahn, S.; Yoon, G. Determination of Glucose in Whole Blood Samples by Mid-Infrared Spectroscopy. Appl. Opt. 2003, 42, 745–749. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kino, S.; Matsuura, Y. Noninvasive Measurement of Blood Glucose Level Using Mid-Infrared Quantum Cascade Lasers. In Proceedings of the Biomedical Imaging and Sensing Conference, Yokohama, Japan, 19–21 April 2017; Volume 10251, pp. 168–170. [Google Scholar]
- Delbeck, S.; Heise, H.M. Evaluation of Opportunities and Limitations of Mid-Infrared Skin Spectroscopy for Noninvasive Blood Glucose Monitoring. J. Diabetes Sci. Technol. 2021, 15, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Hussain, F.; Evans, N.D.; Rolinski, O.J.; Birch, D.J.S. Fluorescence-Based Glucose Sensors. Biosens. Bioelectron. 2005, 20, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lin, M.; Li, Y.; Li, X.; Liu, J.; Liang, J.; Yao, H. In Vivo Blood Glucose Quantification Using Raman Spectroscopy. PLoS ONE 2012, 7, e48127. [Google Scholar] [CrossRef] [PubMed]
- Scholtes-Timmerman, M.J.; Bijlsma, S.; Fokkert, M.J.; Slingerland, R.; van Veen, S.J.F. Raman Spectroscopy as a Promising Tool for Noninvasive Point-of-Care Glucose Monitoring. J. Diabetes Sci. Technol. 2014, 8, 974–979. [Google Scholar] [CrossRef]
- Larin, K.V.; Eledrisi, M.S.; Motamedi, M.; Esenaliev, R.O. Noninvasive Blood Glucose Monitoring with Optical Coherence Tomography: A Pilot Study in Human Subjects. Diabetes Care 2002, 25, 2263–2267. [Google Scholar] [CrossRef]
- Ensaliev, R.O.; Larin, K.V.; Larina, I.V.; Motamedi, M. Noninvasive Monitoring of Glucose Concentration with Optical Coherence Tomography. Opt. Lett. 2001, 26, 992–994. [Google Scholar]
- Kamat, D.K.; Bagul, D.; Patil, P.M. Blood Glucose Measurement Using Bioimpedance Technique. Adv. Electron. 2014, 2014, 406257. [Google Scholar] [CrossRef]
- Caduff, A.; Hirt, E.; Feldman, Y.; Ali, Z.; Heinemann, L. First Human Experiments with a Novel Non-Invasive, Non-Optical Continuous Glucose Monitoring System. Biosens. Bioelectron. 2003, 19, 209–217. [Google Scholar] [CrossRef]
- McCormick, C.; Heath, D.; Connolly, P. Towards Blood Free Measurement of Glucose and Potassium in Humans Using Reverse Iontophoresis. Sens. Actuators B Chem. 2012, 166, 593–600. [Google Scholar] [CrossRef]
- Vashist, S.K. Non-Invasive Glucose Monitoring Technology in Diabetes Management: A Review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Wang, M.L. Noninvasive Glucose Monitoring Using Saliva Nano-Biosensor. Sens. Bio-Sens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef]
- Cho, O.K.; Kim, Y.O.; Mitsumaki, H.; Kuwa, K. Noninvasive Measurement of Glucose by Metabolic Heat Conformation Method. Clin. Chem. 2004, 50, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Costantine, J.; Kanj, R.; Eid, A.; Tawk, Y.; Ramadan, A.H. Electromagnetic Based Devices for Non-Invasive Glucose Monitoring. In Proceedings of the 2018 IEEE Conference on Antenna Measurements & Applications (CAMA), Västerås, Sweden, 3–6 September 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- Sharma, S.; Tripathi, C.C.; Rishi, R. Impedance Matching Techniques for Microstrip Patch Antenna. Indian J. Sci. Technol. 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Balanis, C.A. Modern Antenna Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Qian, Z.; Tang, Q.; Li, J.; Zhao, H.; Zhang, W. Analysis and Design of a Strain Sensor Based on a Microstrip Patch Antenna. In Proceedings of the 2012 International Conference on Microwave and Millimeter Wave Technology (ICMMT), Shenzhen, China, 5–8 May 2012; pp. 1–3. [Google Scholar]
- Sanders, J.W.; Yao, J.; Huang, H. Microstrip Patch Antenna Temperature Sensor. IEEE Sens. J. 2015, 15, 5312–5319. [Google Scholar] [CrossRef]
- Lin, X.; Seet, B.-C.; Joseph, F. Wearable Humidity Sensing Antenna for BAN Applications over 5G Networks. In Proceedings of the 2018 IEEE 19th Wireless and Microwave Technology Conference (WAMICON), Sand Key, FL, USA, 9–10 April 2018; pp. 1–4. [Google Scholar]
- El Gharbi, M.; Fernández-García, R.; Ahyoud, S.; Gil, I. A Review of Flexible Wearable Antenna Sensors: Design, Fabrication Methods, and Applications. Materials 2020, 13, 3781. [Google Scholar] [CrossRef]
- Freer, B.; Venkataraman, J. Feasibility Study for Non-Invasive Blood Glucose Monitoring. In Proceedings of the 2010 IEEE Antennas and Propagation Society International Symposium, Toronto, ON, Canada, 11–17 July 2010; pp. 1–4. [Google Scholar]
- Khan, A.; Nema, R. Analysis of Five Different Dielectric Substrates on Microstrip Patch Antenna. Int. J. Comput. Appl. 2012, 55, 40–47. [Google Scholar] [CrossRef]
- Shoaib, H.M.; Snigdho, M.K.; Islam, A.K.M.E. Comparative Study on the Performance of Microstrip Patch Antenna Using Gold, Silver, and Copper as Radiating Surfaces. In Proceedings of the 2024 Second International Conference on Emerging Trends in Information Technology and Engineering (ICETITE), Vellore, India, 22–23 February 2024; pp. 1–5. [Google Scholar]
- Sricharani, G.; Singh, U.; Mukherjee, J. In-Silico Non-Invasive Glucose Monitoring Using Patch Antenna. In Proceedings of the 2023 IEEE Microwaves, Antennas, and Propagation Conference (MAPCON), Ahmedabad, India, 11–14 December 2023; pp. 1–4. [Google Scholar]
- Syamala, N.; Kumar, B.K.; Raju, K.P. A Planar ɸ-Shaped Microwave Antenna Sensor for Glucose Concentration Monitoring. In Proceedings of the 2024 IEEE Wireless Antenna and Microwave Symposium (WAMS), Visakhapatnam, India, 29 February–3 March 2024; pp. 1–5. [Google Scholar]
- Aminuzzaman, M.M.; Hossam-E-Haider, M. A Novel Non-Invasive Method to Measure Glucose Concentration Using Triple Pole CSRR Based Sensor. In Proceedings of the 2022 12th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 21–23 December 2022; pp. 16–19. [Google Scholar]
- Lv, Z.; Wang, J.; Li, G. An Antenna Loaded with Complementary Split Ring Resonator for Non-Invasive Blood Glucose Measurement. In Proceedings of the 2024 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Glasgow, UK, 20–23 May 2024; pp. 1–6. [Google Scholar]
- Venkataraman, J.; Freer, B. Feasibility of Non-Invasive Blood Glucose Monitoring: In-Vitro Measurements and Phantom Models. In Proceedings of the 2011 IEEE International Symposium on Antennas and Propagation (APSURSI), Spokane, WA, USA, 3–8 July 2011; pp. 603–606. [Google Scholar]
- Xiao, X.; Li, Q. A Noninvasive Measurement of Blood Glucose Concentration by UWB Microwave Spectrum. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1040–1043. [Google Scholar] [CrossRef]
- Hasan, M.N.; Tamanna, S.; Singh, P.; Nadeem, M.D.; Rudramuni, M. Cylindrical Dielectric Resonator Antenna Sensor for Non-Invasive Glucose Sensing Application. In Proceedings of the 2019 6th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 7–8 March 2019; pp. 961–964. [Google Scholar]
- Mittal, V.; Yaduvanshi, R.S. Blood Glucose Sensor Design for Biomedical Application. In Proceedings of the 2022 2nd Asian Conference on Innovation in Technology (ASIANCON), Ravet, India, 26–28 August 2022; pp. 1–4. [Google Scholar]
- Çelenk, E.; Türker Tokan, N. All-Textile on-Body Metasurface Antenna. Prog. Electromagn. Res. M 2022, 110, 119–131. [Google Scholar] [CrossRef]
- Wagih, M.; Wei, Y.; Beeby, S. Flexible 2.4 GHz Node for Body Area Networks with a Compact High-Gain Planar Antenna. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 49–53. [Google Scholar] [CrossRef]
- Ahmad, W.; Budimir, D. Inkjet-Printed Antennas for 28 GHz 5G Applications. In Proceedings of the 2016 Asia-Pacific Microwave Conference (APMC), New Delhi, India, 5–9 December 2016; pp. 1–4. [Google Scholar]
- Faridani, M.; Xiao, G.; Amaya, R.E.; Javanbakht, N.; Yagoub, M.C.E. A Kapton-Based Flexible Wideband Antenna with Metamaterial Resonators for Millimeter-Wave Wireless Applications. In Proceedings of the 2021 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (APS/URSI), Singapore, 4–10 December 2021; pp. 1055–1056. [Google Scholar]
- Wang, L.; Guo, Y.-X.; Salam, B.; Lu, C.W.A. A Flexible Modified Dipole Antenna Printed on PET Film. In Proceedings of the 2012 IEEE Asia-Pacific Conference on Antennas and Propagation, Singapore, 27–29 August 2012; pp. 239–240. [Google Scholar]
- Maithani, S.; Saini, G.; Dhaliwal, B.S.; Gangwar, R.K. A Conformal, Planar Inverted-F Antenna Using Low-Cost Multilayer PET Sheet for Vehicular Application. In Proceedings of the 2023 IEEE Wireless Antenna and Microwave Symposium (WAMS), Ahmedabad, India, 7–10 June 2023; pp. 1–5. [Google Scholar]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of Microfluidic Systems in Polydimethylsiloxane. Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Rahman, H.A.; Rahim, S.K.A. Dual-Band PDMS-Based Flexible Antenna for Wearable Application. In Proceedings of the 2015 IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-BIO), Taipei, Taiwan, 21–23 September 2015; pp. 193–194. [Google Scholar]
- Yun, W.; Sundaram, V.; Swaminathan, M. High-Q Embedded Passives on Large Panel Multilayer Liquid Crystalline Polymer-Based Substrate. IEEE Trans. Adv. Packag. 2007, 30, 580–591. [Google Scholar] [CrossRef]
- Wahiba, B.; Miloud, B.; Mohammed, M.S. Microstrip Antenna Based on Crystal Polymer Liquid (LCP) Textile for RFID Medical Application. In Proceedings of the 2022 2nd International Conference on Advanced Electrical Engineering (ICAEE), Constantine, Algeria, 29–31 October 2022; pp. 1–6. [Google Scholar]
- Eldamak, A.R.; Fear, E.C. Flexible Patch Antennas on Filter Paper Substrate for Biosensing Applications. In Proceedings of the 2020 37th National Radio Science Conference (NRSC), Cairo, Egypt, 8–10 September 2020; pp. 41–47. [Google Scholar]
- Baytöre, C.; Zoral, E.Y.; Göçen, C.; Palandöken, M.; Kaya, A. Coplanar Flexible Antenna Design Using Conductive Silver Nano Ink on Paper Substrate for Wearable Antenna Applications. In Proceedings of the 2018 28th International Conference Radioelektronika (RADIOELEKTRONIKA), Prague, Czech Republic, 19–20 April 2018; pp. 1–6. [Google Scholar]
- Farooq, U.; Iftikhar, A.; Fida, A.; Shafique, M.F.; Asif, S.M.; Braaten, B.D. UWB Antenna Printing on Glass Substrate Through Cost-Effective Copper Foils. In Proceedings of the 2019 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Atlanta, GA, USA, 7–12 July 2019; pp. 1741–1742. [Google Scholar]
- Masihi, S.; Arshad, W.; Khan, M.Z.; Abbasi, M.I.; Hussain, Z.; Haq, F.U.; Shaheen, M.; Khan, T.; Qasim, M. Rapid Prototyping of a Tunable and Compact Microstrip Antenna by Laser Machining Flexible Copper Tape. In Proceedings of the 2019 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Glasgow, UK, 7–10 July 2019; pp. 1–3. [Google Scholar]
- Ameen, M.; Mouthaan, K. Flexible and Conformal Antennas Based on Corrugated Copper Clad Polyimide Films. In Proceedings of the 2024 IEEE International Workshop on Antenna Technology (iWAT), Sendai, Japan, 15–18 April 2024; pp. 88–91. [Google Scholar]
- Björninen, T.; Virkki, J.; Sydänheimo, L.; Ukkonen, L. Manufacturing of Antennas for Passive UHF RFID Tags by Direct Write Dispensing of Copper and Silver Inks on Textiles. In Proceedings of the 2015 International Conference on Electromagnetics in Advanced Applications (ICEAA), Turin, Italy, 7–11 September 2015; pp. 589–592. [Google Scholar]
- Hamouda, Z.; Wojkiewicz, J.-L.; Pud, A.A.; Koné, L.; Bergheul, S.; Lasri, T. Magnetodielectric Nanocomposite Polymer-Based Dual-Band Flexible Antenna for Wearable Applications. IEEE Trans. Antennas Propag. 2018, 66, 3271–3277. [Google Scholar] [CrossRef]
- Verma, A.; Fumeaux, C.; Truong, V.-T.; Bates, B.D. A 2 GHz Polypyrrole Microstrip Patch Antenna on Plexiglas™ Substrate. In Proceedings of the 2009 Asia Pacific Microwave Conference, Singapore, 7–10 December 2009; pp. 36–39. [Google Scholar]
- Hamouda, Z.; Wojkiewicz, J.-L.; Pud, A.A.; Koné, L.; Bergheul, S.; Lasri, T. Dual-Band Elliptical Planar Conductive Polymer Antenna Printed on a Flexible Substrate. IEEE Trans. Antennas Propag. 2015, 63, 5864–5867. [Google Scholar] [CrossRef]
- Hamouda, Z.; Wojkiewicz, J.-L.; Pud, A.A.; Belaabed, B.; Bergheul, S.; Lasri, T. Polyaniline-Carbon Nanotubes Composites-Based Patch Antenna. In Proceedings of the 8th European Conference on Antennas and Propagation (EuCAP 2014), The Hague, The Netherlands, 6–11 April 2014; pp. 2197–2200. [Google Scholar]
- Sankaralingam, S.; Gupta, B. Determination of Dielectric Constant of Fabric Materials and Their Use as Substrates for Design and Development of Antennas for Wearable Applications. IEEE Trans. Instrum. Meas. 2010, 59, 3122–3130. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chappell, W.J. High Frequency Properties of Electro-Textiles for Wearable Antenna Applications. IEEE Trans. Antennas Propag. 2008, 56, 381–389. [Google Scholar] [CrossRef]
- Shawl, R.K.; Longj, B.R.; Werner, D.H.; Gavrin, A. The Characterization of Conductive Textile Materials Intended for Radio Frequency Applications. IEEE Antennas Propag. Mag. 2007, 49, 28–40. [Google Scholar] [CrossRef]
- Harmer, S.W.; Rezgui, N.; Bowring, N.; Luklinska, Z.; Ren, G. Determination of the Complex Permittivity of Textiles and Leather in the 14–40 GHz Millimeter-Wave Band Using a Free-Wave Transmittance-Only Method. IET Microw. Antennas Propag. 2008, 2, 606–614. [Google Scholar] [CrossRef]
- Declercq, F.; Rogier, H.; Hertleer, C. Permittivity and Loss Tangent Characterization for Garment Antennas Based on a New Matrix-Pencil Two-Line Method. IEEE Trans. Antennas Propag. 2008, 6, 2548–2554. [Google Scholar] [CrossRef]
- Cicchetti, R.; Miozzi, E.; Testa, O. Wideband and UWB Antennas for Wireless Applications: A Comprehensive Review. Int. J. Antennas Propag. 2017, 2017, 1–45. [Google Scholar] [CrossRef]
- Jalil, M.E.; Rahim, M.K.A.; Samsuri, N.A.; Abdullah, M.A.; Kamardin, K. Wetness Experiment for Compact CPW-Fed Ultra-Wideband Antenna Using Textile Material. In Proceedings of the 2012 IEEE Asia-Pacific Conference on Applied Electromagnetics (APACE), Melaka, Malaysia, 11–13 December 2012; pp. 298–301. [Google Scholar]
- Salvado, R.; Loss, C.; Gonçalves, R.; Pinho, P. Textile Materials for the Design of Wearable Antennas: A Survey. Sensors 2012, 12, 15841–15857. [Google Scholar] [CrossRef]
- Lamminen, A.; Tuovinen, T.; Björninen, T.; Virkki, J.; Sydänheimo, L.; Ukkonen, L. Graphene-Flakes Printed Wideband Elliptical Dipole Antenna for Low-Cost Wireless Communications Applications. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1883–1886. [Google Scholar] [CrossRef]
- Machiels, J.; Appeltans, R.; Bauer, D.K.; Segers, E.; Henckens, Z.; Van Rompaey, W.; Adons, D.; Peeters, R.; Geiβler, M.; Kuehnoel, K.; et al. Screen Printed Antennas on Fiber-Based Substrates for Sustainable HF RFID Assisted E-Fulfilment Smart Packaging. Materials 2021, 14, 5500. [Google Scholar] [CrossRef] [PubMed]
- Kordzadeh, A.; Holzmann, D.; Binder, A.; Moldaschl, T.; Sturm, J.; Roshanghias, A. Miniaturized On-Chip NFC Antenna versus Screen-Printed Antenna for the Flexible Disposable Sensor Strips. IoT 2020, 1, 309–319. [Google Scholar] [CrossRef]
- Ibanez Labiano, I.; Arslan, D.; Ozden Yenigun, E.; Asadi, A.; Cebeci, H.; Alomainy, A. Screen Printing Carbon Nanotubes Textiles Antennas for Smart Wearables. Sensors 2021, 21, 4934. [Google Scholar] [CrossRef]
- Xiao, G.G.; Zhang, Z.; Lang, S.; Tao, Y. Screen Printing RF Antennas. In Proceedings of the 17th International Symposium on Antenna Technology and Applied Electromagnetics (ANTEM), Montreal, QC, Canada, 10–13 July 2016; pp. 1–2. [Google Scholar]
- Merilampi, S.L.; Björninen, T.; Vuorimäki, A.; Ukkonen, L.; Ruuskanen, P.; Sydänheimo, L. The Effect of Conductive Ink Layer Thickness on the Functioning of Printed UHF RFID Antennas. Proc. IEEE 2010, 98, 1610–1619. [Google Scholar] [CrossRef]
- Lakafosis, V.; Rida, A.; Vyas, R.; Yang, L.; Nikolaou, S.; Tentzeris, M.M. Progress Towards the First Wireless Sensor Networks Consisting of Inkjet-Printed, Paper-Based RFID-Enabled Sensor Tags. Proc. IEEE 2010, 98, 1601–1609. [Google Scholar] [CrossRef]
- Wiklund, J.; Karlsen, M.; Sørensen, R.; Bakke, T.; Nilsson, H.E.; Sandström, A. A Review on Printed Electronics: Fabrication Methods, Inks, Substrates, Applications and Environmental Impacts. J. Manuf. Mater. Process. 2021, 5, 89. [Google Scholar] [CrossRef]
- Eom, S.-H.; Seo, Y.; Lim, S. Pattern Switchable Antenna System Using Inkjet-Printed Directional Bow-Tie for Bi-Direction Sensing Applications. Sensors 2015, 15, 31171–31179. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, G.; Quattrocchi, A.; Campobello, G.; Crupi, G.; Donato, N. On the Development of Inkjet-Printed Band Pass Filters Based on the Microstrip Hairpin Structure. Instruments 2024, 8, 23. [Google Scholar] [CrossRef]
- Abounasr, J.; Gharbi, M.E.; García, R.F.; Gil, I. A High-Sensitivity Inkjet-Printed Flexible Resonator for Monitoring Dielectric Changes in Meat. Sensors 2025, 25, 1338. [Google Scholar] [CrossRef]
- Jiang, Y.; Leng, T.; Fang, Y.; Hu, Z.; Xu, L. Machine Embroidered Wearable e-Textile Wideband UHF RFID Tag Antenna. In Proceedings of the IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Atlanta, GA, USA, 7–12 July 2019; pp. 643–644. [Google Scholar]
- Liu, Y.; Xu, L.; Li, Y.; Ye, T.T. Textile Based Embroidery-Friendly RFID Antenna Design Techniques. In Proceedings of the IEEE International Conference on RFID (RFID), Phoenix, AZ, USA, 2–4 April 2019; pp. 1–6. [Google Scholar]
- Nizioł, M.; Jankowski-Mihułowicz, P.; Węglarski, M. Effect of Embroidery Style on the Bandwidth of Textronic RFID UHF Transponder Antenna. Sensors 2025, 25, 371. [Google Scholar] [CrossRef] [PubMed]
- Bakogianni, S.; Tsolis, A.; Angelaki, C.; Alexandridis, A.A. On the Development of Embroidered Reconfigurable Dipole Antennas: A Textile Approach to Mechanical Reconfiguration. Electronics 2024, 13, 3649. [Google Scholar] [CrossRef]
- Liu, F.-X.; Meng, F.-Y.; Chen, Y.-J.; Gao, Z.-H.; Cui, J.; Zhang, L. Textile Bandwidth-Enhanced Half-Mode Substrate-Integrated Cavity Antenna Based on Embroidered Shorting Vias. Micromachines 2024, 15, 1081. [Google Scholar] [CrossRef]
- Du, C.-Z.; Yang, F.-H.; Zhong, S.-S. Dual-Polarized Textile Antenna Integrated in the Three-Dimensional Orthogonal Woven Fabrics. In Proceedings of the 2015 IEEE MTT-S International Microwave Workshop Series on Advanced Materials and Processes for RF and THz Applications (IMWS-AMP), Suzhou, China, 1–3 July 2015; pp. 1–3. [Google Scholar]
- Nguyen, T.M.; Chung, J.-Y.; Lee, B. Radiation Characteristics of Woven Patch Antennas Composed of Conductive Threads. IEEE Trans. Antennas Propag. 2015, 63, 2796–2801. [Google Scholar] [CrossRef]
- Boulerbah, R.; Chaabane, A.; Attia, H.; Aissaoui, D. Breast Cancer Detection Using Circularly Polarized Printed UWB Antenna. In Proceedings of the 2nd International Conference on Electronics, Energy and Measurement (IC2EM), Medea, Algeria, 28–30 November 2023; pp. 1–5. [Google Scholar]
- Raj, S.; Tripathi, S.; Upadhyay, G.; Tripathi, S.S.; Tripathi, V.S. An Electromagnetic Band Gap-Based Complementary Split Ring Resonator Loaded Patch Antenna for Glucose Level Measurement. IEEE Sens. J. 2021, 21, 22679–22687. [Google Scholar] [CrossRef]
- Yilmaz, T.; Foster, R.; Hao, Y. Broadband Tissue Mimicking Phantoms and a Patch Resonator for Evaluating Noninvasive Monitoring of Blood Glucose Levels. IEEE Trans. Antennas Propag. 2014, 62, 3064–3075. [Google Scholar] [CrossRef]
- Garcia-Pardo, C.; Antonino-Daviu, E.; Castelló-Palacios, S.; Vila-Jimenez, A.; Vallés-Lluch, A.; Cardona, N. 60 GHz Wearable Flexible Antenna in a Customized Multilayer Body Phantom. In Proceedings of the 33rd Annual International Symposium on Personal, Indoor and Mobile Radio Communications (PIMRC), Kyoto, Japan, 12–15 September 2022; pp. 1–5. [Google Scholar]
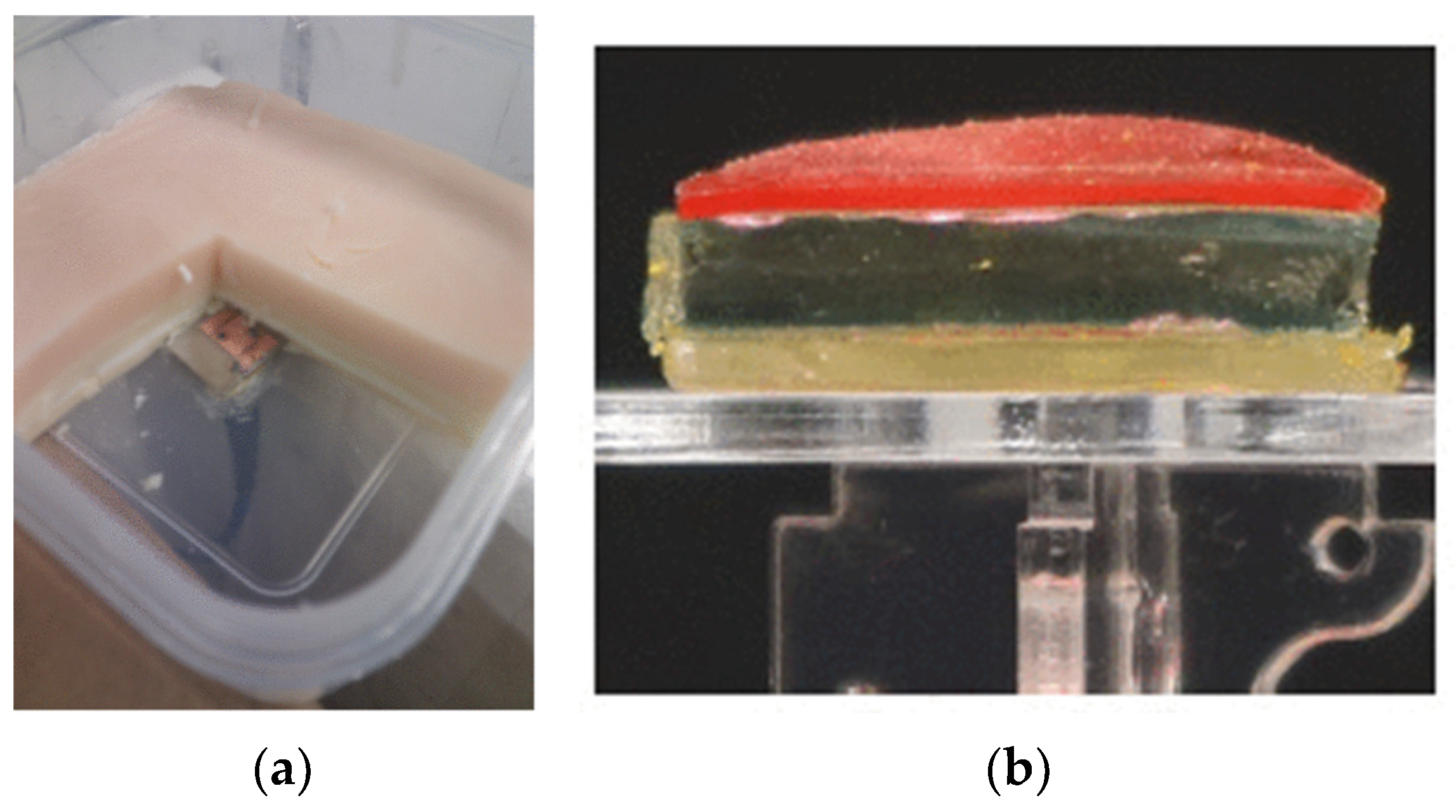
- Castelló-Palacios, S.; Garcia-Pardo, C.; Alloza-Pascual, M.; Fornes-Leal, A.; Cardona, N.; Vallés-Lluch, A. Gel Phantoms for Body Microwave Propagation in the (2 to 26.5) GHz Frequency Band. IEEE Trans. Antennas Propag. 2019, 67, 6564–6573. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz); International Commission on Non-Ionizing Radiation Protection: Munich, Germany, 2020. [Google Scholar]
- ISO 15197:2015; In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. International Organization for Standardization: Geneva, Switzerland, 2015.
- Gharbi, M.E.; Fernández-García, R.; Gil, I. Textile Antenna-Sensor for In Vitro Diagnostics of Diabetes. Electronics 2021, 10, 1570. [Google Scholar] [CrossRef]
- Hanna, J.; Costantine, J.; Kanj, R.; Tawk, Y.; Ramadan, A.H.; Eid, A.A. A Vasculature Anatomy Inspired Flexible Slot Antenna for Continuous Non-invasive Glucose Monitoring. In Proceedings of the IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (APS/URSI), Singapore, 4–10 December 2021; pp. 803–804. [Google Scholar]
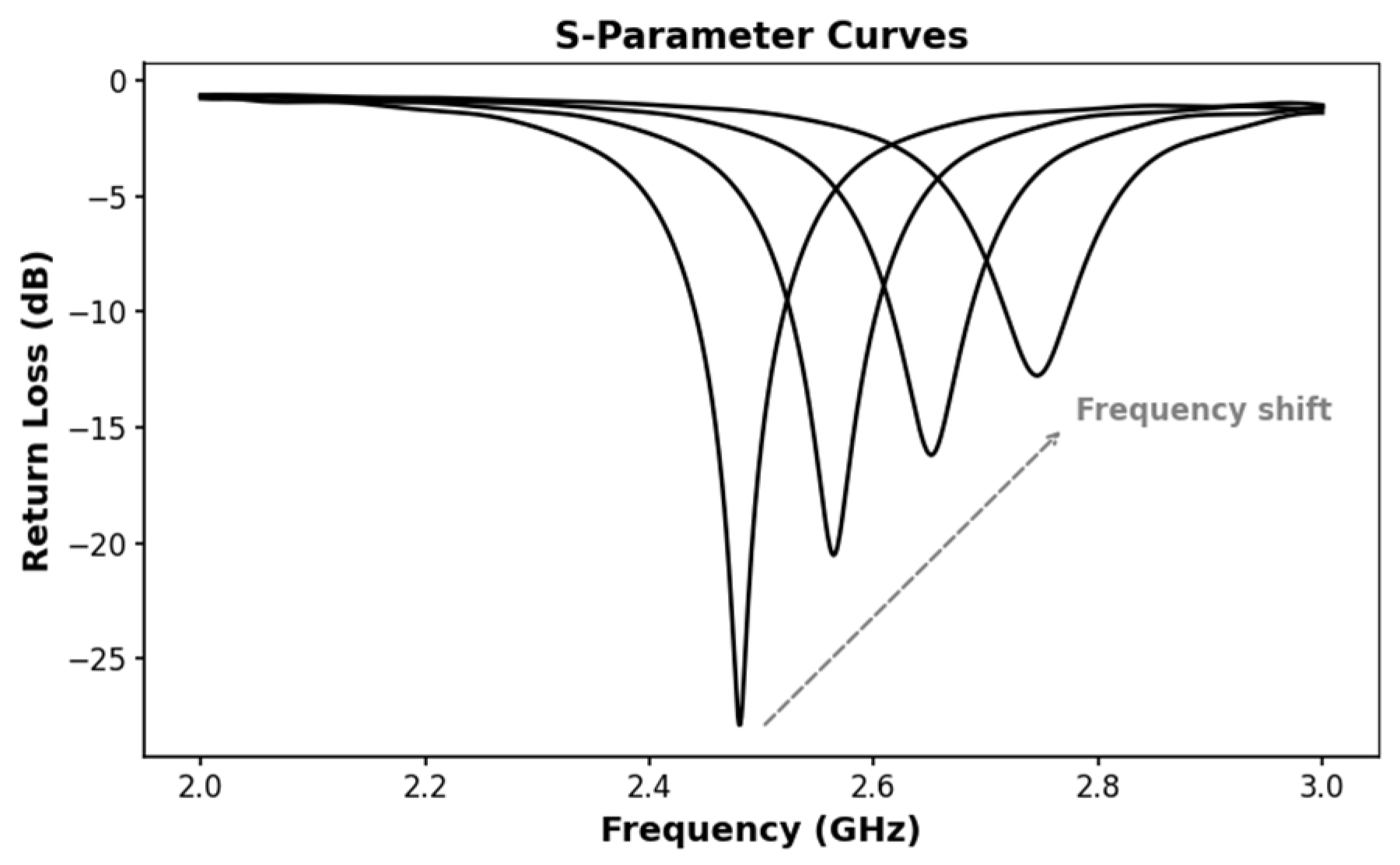
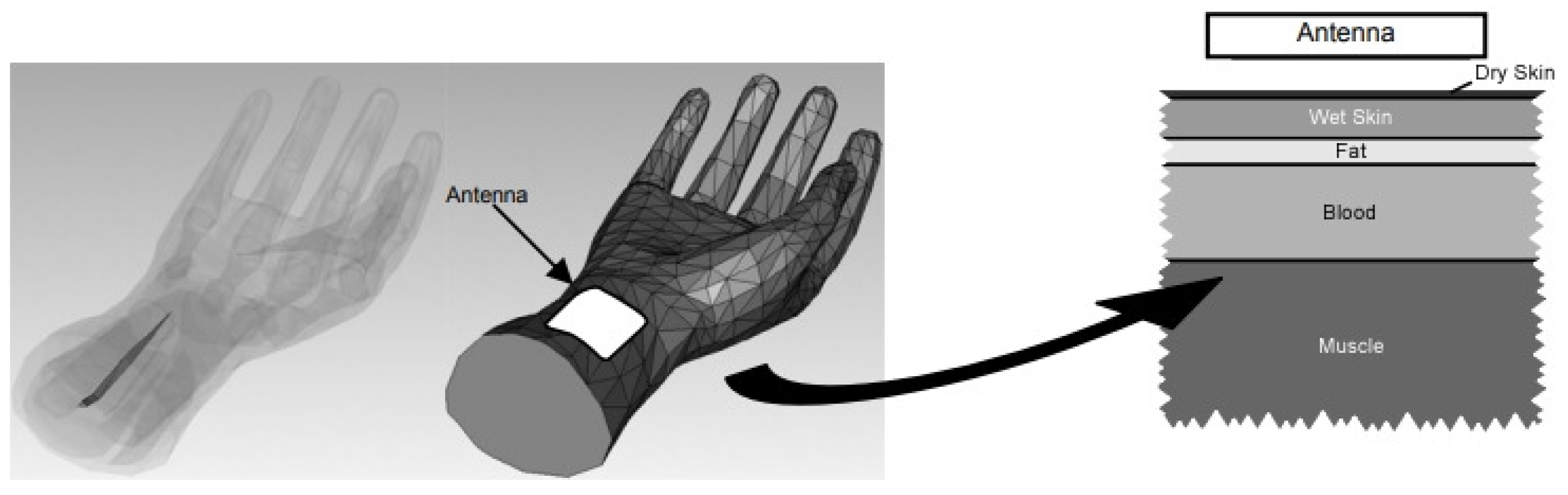
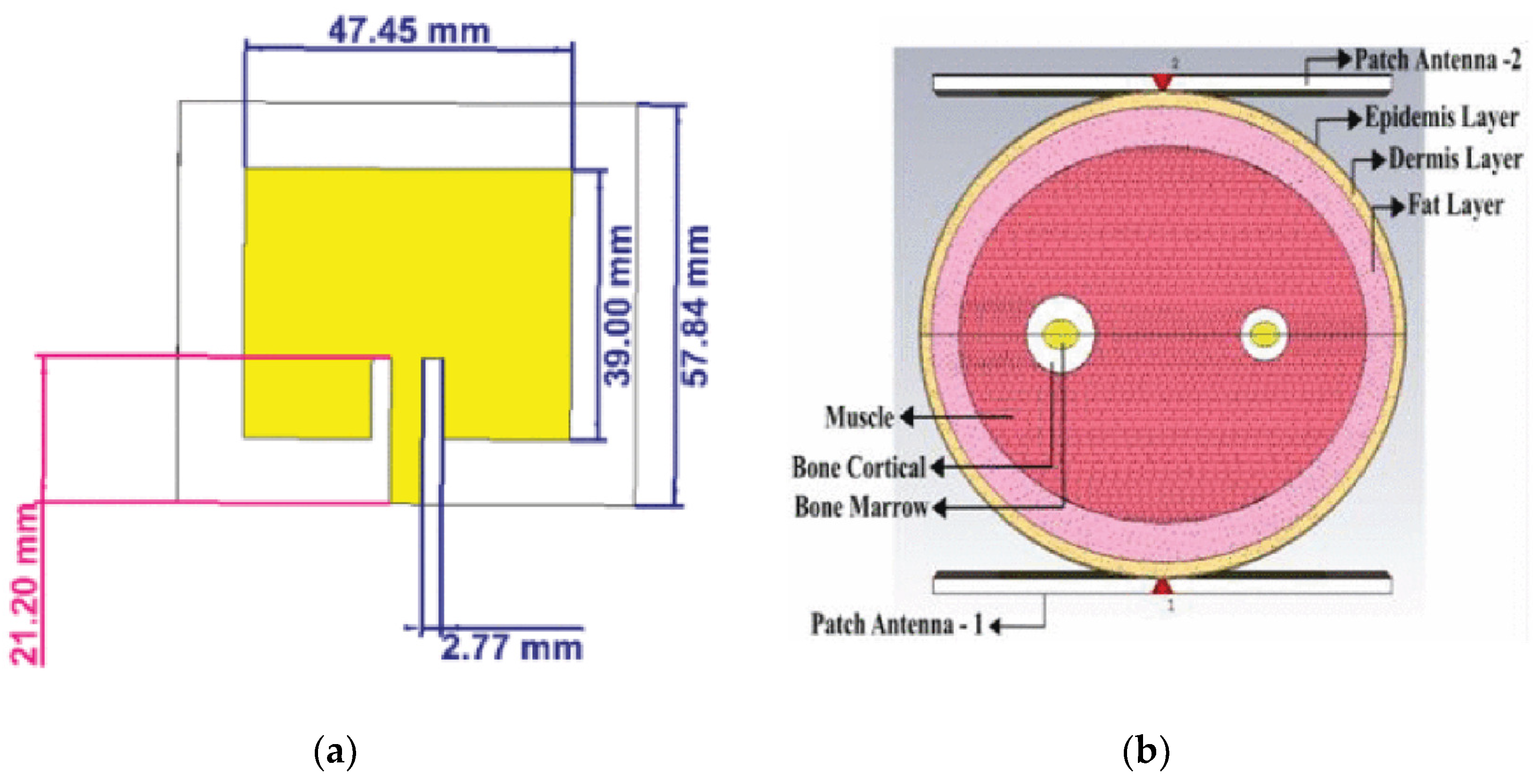
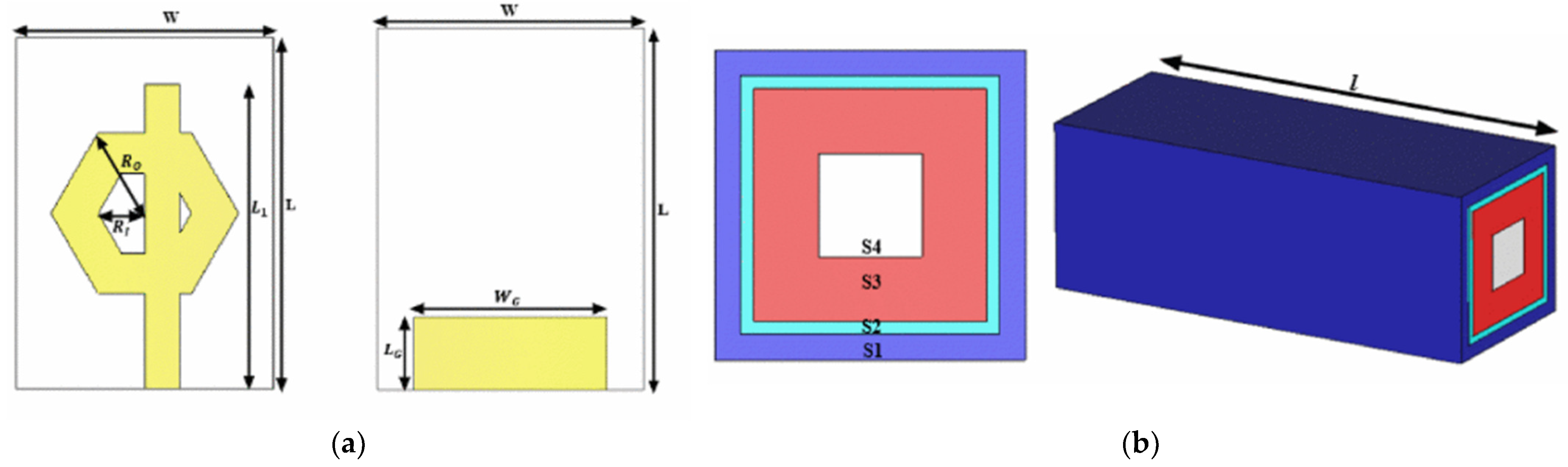




| Region | 2000 | 2021 | 2030 | 2045 |
|---|---|---|---|---|
| Africa | 2532.9 | 23,633.9 | 33,446.0 | 55,254.4 |
| Europe | 22,373.1 | 61,425.1 | 67,000.0 | 69,000.0 |
| Middle East and North Africa | 17,007.6 | 72,671.9 | 95,000.0 | 135,700.0 |
| North America and Caribbean | 21,375.1 | 50,547.0 | 57,000.0 | 63,000.0 |
| South and Central America | 8553.3 | 32,497.1 | 40,000.0 | 49,000.0 |
| South-East Asia | 34,882.2 | 90,204.5 | 113,300.0 | 151,500.0 |
| Western Pacific | 44,097.9 | 205,640.2 | 238,300.0 | 260,200.0 |
| Material | Relative Permittivity | Advantages | Limitations |
|---|---|---|---|
| FR-4 (Flame Retardant 4) | 4.4 | Cost-effective and widely available; mechanically robust; compatible with standard PCB fabrication processes. | High dielectric loss; limited suitability for microwave and millimeter-wave applications. |
| Rogers Laminates (e.g., RO4003C, RO4350B) | 2.2–10 | Low dielectric loss; stable dielectric constant; superior thermal and mechanical stability. | Higher cost; requires specialized manufacturing processes; slightly lower mechanical strength. |
| Ceramic (e.g., Alumina, LTCC) | >10 | Extremely low dielectric loss; stable high dielectric constant; high thermal stability; ideal for compact, high-frequency applications. | High fabrication costs; mechanically fragile; limited availability for rapid prototyping. |
| PTFE (Polytetrafluoroethylene) | 2.1 | Low dielectric loss; stable dielectric constant; excellent chemical and thermal resistance; preferred for precision RF applications. | High manufacturing cost; complex processing requirements; poor mechanical rigidity; susceptible to dimensional instability. |
| Material | Conductivity (S/m) | Advantages | Limitations |
|---|---|---|---|
| Copper | 5.96 × 107 | High electrical conductivity; cost-effective; good mechanical strength and ductility. | Susceptible to oxidation; requires protective coatings or encapsulation in harsh environments. |
| Silver | 6.3 × 107 | Highest electrical conductivity; excellent reflectivity and thermal conductivity; low contact resistance. | High cost; susceptible to tarnishing; softer and less mechanically robust. |
| Aluminum | 3.5 × 107 | Lightweight; cost-effective; natural corrosion resistance. | Lower electrical conductivity; oxide affects electrical connections; brittle. |
| Gold | 4.1 × 107 | Resistant to oxidation and corrosion; excellent biocompatibility. | Expensive; soft and prone to mechanical wear; higher density. |
| Material | Conductivity (S/m) | Advantages | Limitations |
|---|---|---|---|
| Adhesive copper, copper tape, and copper cladding | 5.96 × 107 | Excellent electrical conductivity; high mechanical strength; stable under environmental conditions. | High density; prone to oxidation; limited stretchability; additional processes required. |
| Silver nanoparticle inks | 105 − 106 | Superior conductivity; low-temperature sintering; oxidation-resistant. | High cost; complex sintering process; limited stretchability. |
| Copper nanoparticle inks | 104 − 105 | Lower cost compared to silver; suitable for large-area printed electronics. | Prone to oxidation, requires protective coatings or reducing agents. |
| PANI and PPy | 100 − 102 | Lightweight, flexible; chemically tunable, environmentally stable. | lower conductivity than metals; mechanical fragility; conductivity varies with the doping level. |
| Nonconductive Fabric | Dielectric Constant | Dielectric Loss |
|---|---|---|
| Cotton | 1.6 | 0.04 |
| Felt | 1.215 | 0.016 |
| Silk | 1.75 | 0.012 |
| Jeans | 1.7 | 0.025 |
| Fleece | 1.17 | 0.0035 |
| Denim | 1.6 | 0.05 |
| Zelt | Pure Copper Taffeta Fabric | Shieldit Super | |
|---|---|---|---|
| Conductivity (S/m) | 1.749 × 105 | 2.5 × 105 | 6.67 × 105 |
| Surface Resistance (Ω) | 0.05 | 0.05 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Fernández-García, R.; Gil, I. Sensor Technologies for Non-Invasive Blood Glucose Monitoring. Sensors 2025, 25, 3591. https://doi.org/10.3390/s25123591
Shi J, Fernández-García R, Gil I. Sensor Technologies for Non-Invasive Blood Glucose Monitoring. Sensors. 2025; 25(12):3591. https://doi.org/10.3390/s25123591
Chicago/Turabian StyleShi, Jiale, Raúl Fernández-García, and Ignacio Gil. 2025. "Sensor Technologies for Non-Invasive Blood Glucose Monitoring" Sensors 25, no. 12: 3591. https://doi.org/10.3390/s25123591
APA StyleShi, J., Fernández-García, R., & Gil, I. (2025). Sensor Technologies for Non-Invasive Blood Glucose Monitoring. Sensors, 25(12), 3591. https://doi.org/10.3390/s25123591








