Skin Hydration Monitoring Using a Microwave Sensor: Design, Fabrication, and In Vivo Analysis
Abstract
1. Introduction
2. Sensor Design and Circuit Analysis
3. Experimental Setup and Measurements
3.1. Experiment Setup
3.2. Measured Results
3.3. Future Work and Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Liaqat, S.; Dashtipour, K.; Arshad, K.; Ramzan, N. Non invasive skin hydration level detection using machine learning. Electronics 2020, 9, 1086. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A. Diet and skin health: The good and the bad. Nutrition 2024, 119, 1123. [Google Scholar] [CrossRef] [PubMed]
- Camilion, J.V.; Khanna, S.; Anasseri, S.; Laney, C.; Mayrovitz, H.N. Physiological, pathological, and circadian factors impacting skin hydration. Cureus 2022, 14, e27666. [Google Scholar] [CrossRef]
- Chang, C.; Keen, C.L.; Gershwin, M.E. Treatment of eczema. Clin. Rev. Allergy Immunol. 2007, 33, 204–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Son, S.W.; Cho, S.H. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol. Res. 2016, 8, 181–190. [Google Scholar] [CrossRef]
- Huang, T.H.; Chou, J.C.; Sun, T.P.; Hsiung, S.K. A device for skin moisture and environment humidity detection. Sens. Actuators B Chem. 2008, 134, 206–212. [Google Scholar] [CrossRef]
- Li, S.; Wan, T.; Wei, H.; Wang, S.; Wang, B.; Cheng, B. Flexible highly-sensitive humidity sensor based on CGO/SMPLAF for wearable human skin humidity detection. Sens. Actuators B Chem. 2022, 362, 131806. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B Chem. 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- Cui, Y.; An, Y.; Jin, T.; Zhang, F.; He, P. Real-time monitoring of skin wound healing on nano-grooves topography using electric cell-substrate impedance sensing (ECIS). Sens. Actuators B Chem. 2017, 250, 461–468. [Google Scholar] [CrossRef]
- Humbert, P.; Dréno, B.; Krutmann, J.; Luger, T.A.; Triller, R.; Meaume, S.; Seité, S. Recommendations for managing cutaneous disorders associated with advancing age. Clin. Interv. Aging 2016, 11, 141–148. [Google Scholar] [PubMed]
- Flour, M. Dermatological Issues in Lymphoedema and Chronic Oedema; The International Lymphoedema Framework: London, UK, 2013; p. 49. [Google Scholar]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Epidermal barrier function and skin homeostasis in atopic dermatitis: The impact of age. Life 2022, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Meyers, C.L.; Subramanyan, K.; Hancewicz, T.M. Near infrared imaging for measuring and visualizing skin hydration. A comparison with visual assessment and electrical methods. J. Biomed. Opt. 2005, 10, 031107. [Google Scholar] [CrossRef]
- Kislevitz, M.; Akgul, Y.; Wamsley, C.; Hoopman, J.; Kenkel, J. Use of optical coherence tomography (OCT) in aesthetic skin assessment—A short review. Lasers Surg. Med. 2020, 52, 699–704. [Google Scholar] [CrossRef]
- Madhvapathy, S.R.; Ma, Y.; Patel, M.; Krishnan, S.; Wei, C.; Li, Y.; Xu, S.; Feng, X.; Huang, Y.; Rogers, J.A. Epidermal electronic systems for measuring the thermal properties of human skin at depths of up to several millimeters. Adv. Funct. Mater. 2018, 28, 1802083. [Google Scholar] [CrossRef]
- Letertre, M.P.; Giraudeau, P.; De Tullio, P. Nuclear magnetic resonance spectroscopy in clinical metabolomics and personalized medicine: Current challenges and perspectives. Front. Mol. Biosci. 2021, 8, 698337. [Google Scholar] [CrossRef]
- Elsner, P.; Berardesca, E.; Maibach, H.I. Bioengineering of the Skin: Water and the Stratum Corneum; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Novak, P. Electrochemical skin conductance: A systematic review. Clin. Auton. Res. 2019, 29, 17–29. [Google Scholar] [CrossRef]
- Qassem, M.; Kyriacou, P. Review of modern techniques for the assessment of skin hydration. Cosmetics 2019, 6, 19. [Google Scholar] [CrossRef]
- Song, G.; Jelly, E.T.; Chu, K.K.; Kendall, W.Y.; Wax, A. A review of low-cost and portable optical coherence tomography. Prog. Biomed. Eng. 2021, 3, 032002. [Google Scholar] [CrossRef]
- Bracciano, A.G.; Bracciano, E. Thermal Modalities Therapeutic Heat. In Physical Agent Modalities; Routledge: London, UK, 2024; pp. 149–186. [Google Scholar]
- Luo, Y.; Yan, T.; Yu, J. Integrated analytical modeling of transient heat transfer inside and outside U-tube ground heat exchanger: A new angle from composite-medium method. Int. J. Heat Mass Transf. 2020, 162, 120373. [Google Scholar] [CrossRef]
- Bernatchez, S.F.; Bichel, J. The science of skin: Measuring damage and assessing risk. Adv. Wound Care 2023, 12, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Kammeyer, A.; Calkoen, F.; Fluhr, J.; Bos, J. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: Evaluation of minimally invasive methods. Br. J. Dermatol. 2009, 161, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.; De Benedetto, E.; Schiavoni, R.; Monti, G.; Tedesco, A.; Masciullo, A.; Piuzzi, E.; Tarricone, L. Portable microwave reflectometry system for skin sensing. IEEE Trans. Instrum. Meas. 2022, 71, 1–8. [Google Scholar] [CrossRef]
- Jadoul, A.; Bouwstra, J.; Preat, V. Effects of iontophoresis and electroporation on the stratum corneum: Review of the biophysical studies. Adv. Drug Deliv. Rev. 1999, 35, 89–105. [Google Scholar] [CrossRef]
- Laura Moretti, A.; Vergílio, M.M.; Silva, S.A.M.; Ricci, G. Evaluation of operational parameters for clinical evaluation of skin hydration by corneometry method. Braz. J. Pharm. Sci. 2025, 61, e23182. [Google Scholar]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Ávila-Navarro, E.; Sabater-Navarro, J.M. Concentration measurement of microliter-volume water–glucose solutions using Q factor of microwave sensors. IEEE Trans. Instrum. Meas. 2018, 68, 2621–2634. [Google Scholar] [CrossRef]
- Piekarz, I.; Wincza, K.; Gruszczynski, S.; Sorocki, J. Detection of methanol contamination in ethyl alcohol employing a purpose-designed high-sensitivity microwave sensor. Measurement 2021, 174, 108993. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Beziuk, G.; Ghorbani, K. Amplitude Variation Microwave Sensors for High-Sensitivity Detection of Solid and Microfluidic Dielectrics. IEEE Trans. Microw. Theory Tech. 2024, 72, 6460–6472. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Peytral-Rieu, O.; Velez, P.; Dubuc, D.; Grenier, K.; Martin, F. Characterization of the denaturation of bovine serum albumin (BSA) protein by means of a differential-mode microwave microfluidic sensor based on slot resonators. IEEE Sens. J. 2022, 22, 14075–14083. [Google Scholar] [CrossRef]
- Calvel, A.; Peytral-Rieu, O.; Rols, M.P.; Dubuc, D.; Grenier, K. Extension to In Situ Single-Cell Electroporation of a Microwave Biosensor. IEEE Microw. Wirel. Technol. Lett. 2024, 34, 849–852. [Google Scholar] [CrossRef]
- Calvel, A.; de Caro, A.; Peytral-Rieu, O.; Gironde, C.; Furger, C.; Dubuc, D.; Grenier, K.; Rols, M.P. Analysis of In Vitro Cell Viability Approaches to Provide Early Efficacy Prediction of Electrochemotherapy Treatments. IEEE J. Electromagn. Microwaves Med. Biol. 2024, 8, 229–237. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Ghorbani, K. High-sensitivity detection of solid and liquid dielectrics using a branch line coupler sensor. IEEE Trans. Microw. Theory Tech. 2023, 71, 5233–5245. [Google Scholar] [CrossRef]
- Piekarz, I.; Sorocki, J.; Górska, S.; Bartsch, H.; Rydosz, A.; Smolarz, R.; Wincza, K.; Gruszczynski, S. High sensitivity and selectivity microwave biosensor using biofunctionalized differential resonant array implemented in LTCC for Escherichia coli detection. Measurement 2023, 208, 112473. [Google Scholar] [CrossRef]
- Juan, C.G.; Potelon, B.; Quendo, C.; García-Martínez, H.; Ávila-Navarro, E.; Bronchalo, E.; Sabater-Navarro, J.M. Study of Qu-based resonant microwave sensors and design of 3-D-printed devices dedicated to glucose monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Tovar-Lopez, F.J.; Scott, J.; Ghorbani, K. Differential microwave sensor for characterization of glycerol–water solutions. Sens. Actuators B Chem. 2020, 321, 128561. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Microwave reflective biosensor for glucose level detection in aqueous solutions. Sens. Actuators A Phys. 2020, 301, 111662. [Google Scholar] [CrossRef]
- Velez, P.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Highly-sensitive microwave sensors based on open complementary split ring resonators (OCSRRs) for dielectric characterization and solute concentration measurement in liquids. IEEE Access 2018, 6, 48324–48338. [Google Scholar] [CrossRef]
- Jain, M.C.; Nadaraja, A.V.; Mohammadi, S.; Vizcaino, B.M.; Zarifi, M.H. Passive microwave biosensor for real-time monitoring of subsurface bacterial growth. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 122–132. [Google Scholar] [CrossRef]
- Kazemi, N.; Abdolrazzaghi, M.; Musilek, P.; Daneshmand, M. A temperature-compensated high-resolution microwave sensor using artificial neural network. IEEE Microw. Wirel. Components Lett. 2020, 30, 919–922. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nadaraja, A.V.; Luckasavitch, K.; Jain, M.C.; Roberts, D.J.; Zarifi, M.H. A label-free, non-intrusive, and rapid monitoring of bacterial growth on solid medium using microwave biosensor. IEEE Trans. Biomed. Circuits Syst. 2019, 14, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Vélez, P.; Su, L.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Microwave microfluidic sensor based on a microstrip splitter/combiner configuration and split ring resonators (SRRs) for dielectric characterization of liquids. IEEE Sens. J. 2017, 17, 6589–6598. [Google Scholar] [CrossRef]
- Kazemi, N.; Abdolrazzaghi, M.; Light, P.E.; Musilek, P. In–human testing of a non-invasive continuous low–energy microwave glucose sensor with advanced machine learning capabilities. Biosens. Bioelectron. 2023, 241, 115668. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Mobashsher, A.T.; Mohammed, B.; Foong, D.; Abbosh, A. Handheld Microwave System for In-vivo Skin Cancer Detection: Development and Clinical Validation. IEEE Trans. Instrum. Meas. 2024, 73, 6006816. [Google Scholar] [CrossRef]
- Awal, M.A.; Naqvi, S.A.R.; Foong, D.; Abbosh, A. Adaptive Weighted Vector Means Optimization for Healthy and Malignant Skin Modeling at Microwave Frequencies Using Clinical Data. IEEE J. Electromagn. Microwaves Med. Biol. 2024, 8, 170–181. [Google Scholar] [CrossRef]
- Kilpijärvi, J.; Tolvanen, J.; Juuti, J.; Halonen, N.; Hannu, J. A non-invasive method for hydration status measurement with a microwave sensor using skin phantoms. IEEE Sens. J. 2019, 20, 1095–1104. [Google Scholar] [CrossRef]
- Baghelani, M.; Abbasi, Z.; Daneshmand, M.; Light, P.E. Non-Invasive Microwave Sensor for Real-Time Continuous Dehydration Monitoring. IEEE Sens. J. 2024, 24, 9959–9969. [Google Scholar] [CrossRef]
- Bing, S.; Chawang, K.; Chiao, J.C. A flexible tuned radio-frequency planar resonant loop for noninvasive hydration sensing. IEEE J. Microwaves 2022, 3, 181–192. [Google Scholar] [CrossRef]
- Masi, A.; Brizi, D.; Monorchio, A. Millimetric inclusion detection through a contactless microwave spiral sensor for biomedical applications. IEEE Sens. J. 2023, 23, 12796–12807. [Google Scholar] [CrossRef]
- Lazzoni, V.; Brizi, D.; Stagliano, N.; Giordano, C.; Pecoraro, E.; Anichini, M.; Ugolini, F.; Bindi, M.; Argenti, G.; Monorchio, A.; et al. Development of a microwave sensor for the non-invasive detection of plant responses to water stress: A practical application on maize (Zea mays L.). Biosyst. Eng. 2024, 246, 191–203. [Google Scholar] [CrossRef]
- Brizi, D.; Fontana, N.; Costa, F.; Monorchio, A. Accurate extraction of equivalent circuit parameters of spiral resonators for the design of metamaterials. IEEE Trans. Microw. Theory Tech. 2018, 67, 626–633. [Google Scholar] [CrossRef]
- Gabriel, C. Dielectric properties of biological materials. In Bioengineering and Biophysical Aspects of Electromagnetic Fields; CRC Press: Boca Raton, FL, USA, 2018; pp. 87–136. [Google Scholar]
- Huclova, S.; Erni, D.; Fröhlich, J. Modelling and validation of dielectric properties of human skin in the MHz region focusing on skin layer morphology and material composition. J. Phys. Appl. Phys. 2011, 45, 025301. [Google Scholar] [CrossRef]
- Chemical Book. Petrolatum Properties. 2024. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9854422.htm (accessed on 15 March 2025).
- Chaudhary, P.; Maurya, D.K.; Pandey, A.; Verma, A.; Tripathi, R.K.; Kumar, S.; Yadav, B. Design and development of flexible humidity sensor for baby diaper alarm: Experimental and theoretical study. Sens. Actuators B Chem. 2022, 350, 130818. [Google Scholar] [CrossRef]
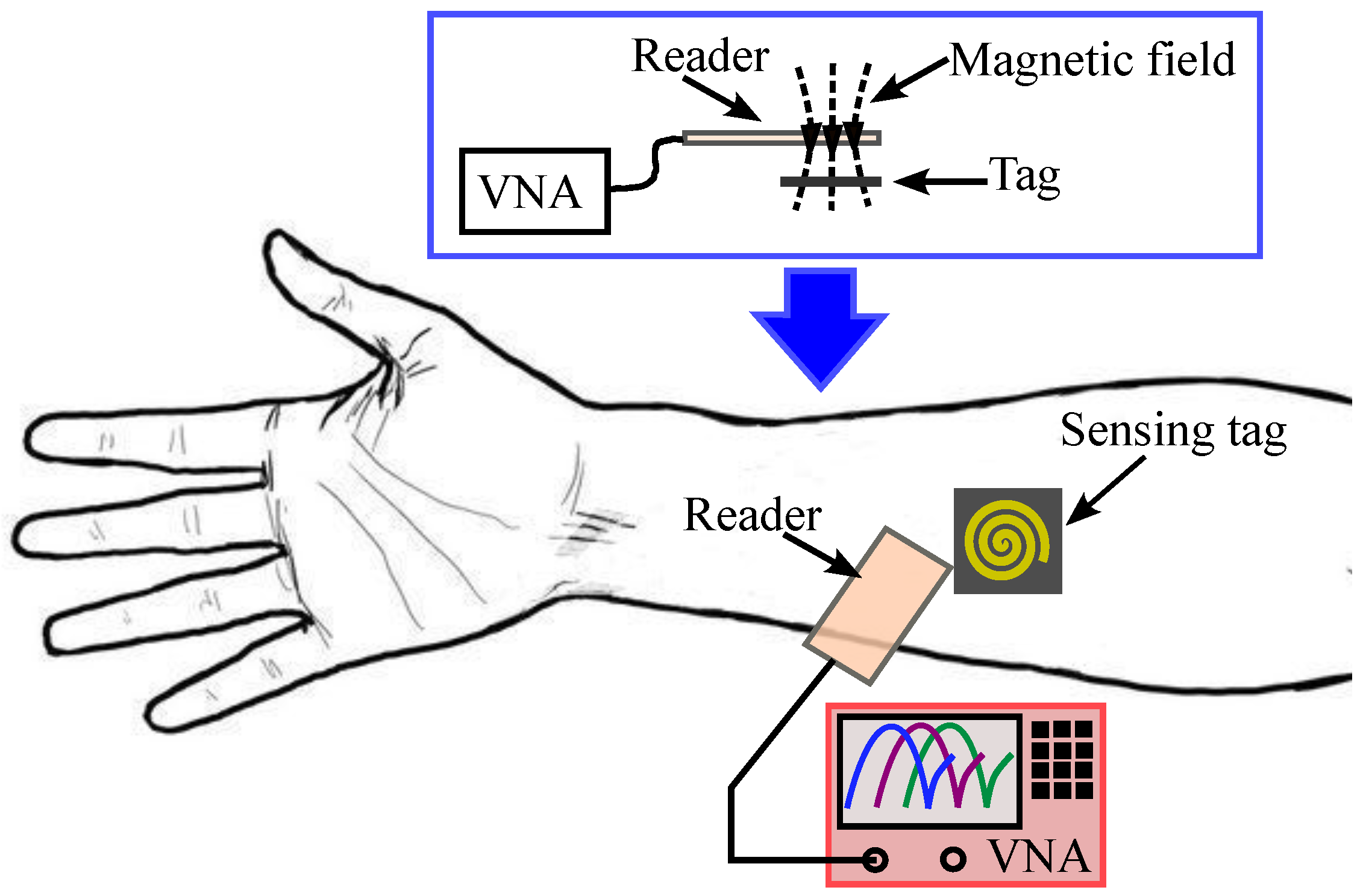
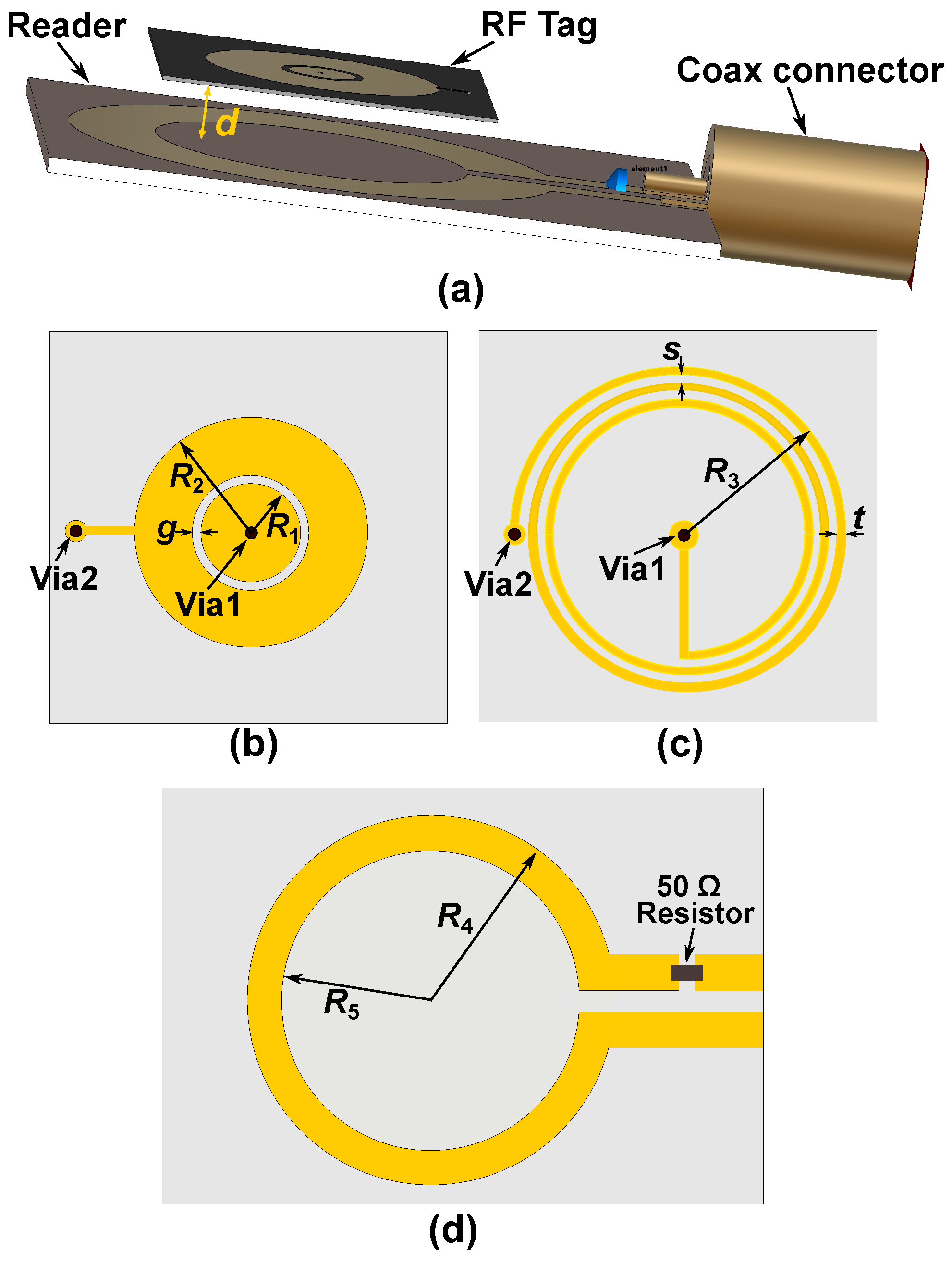
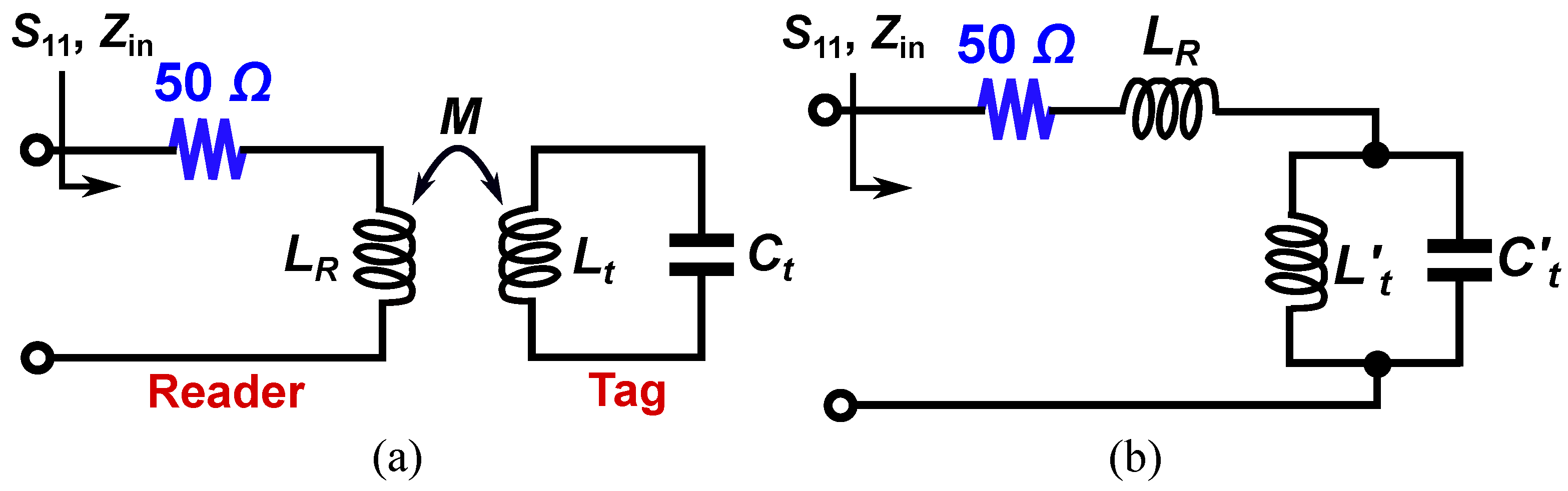


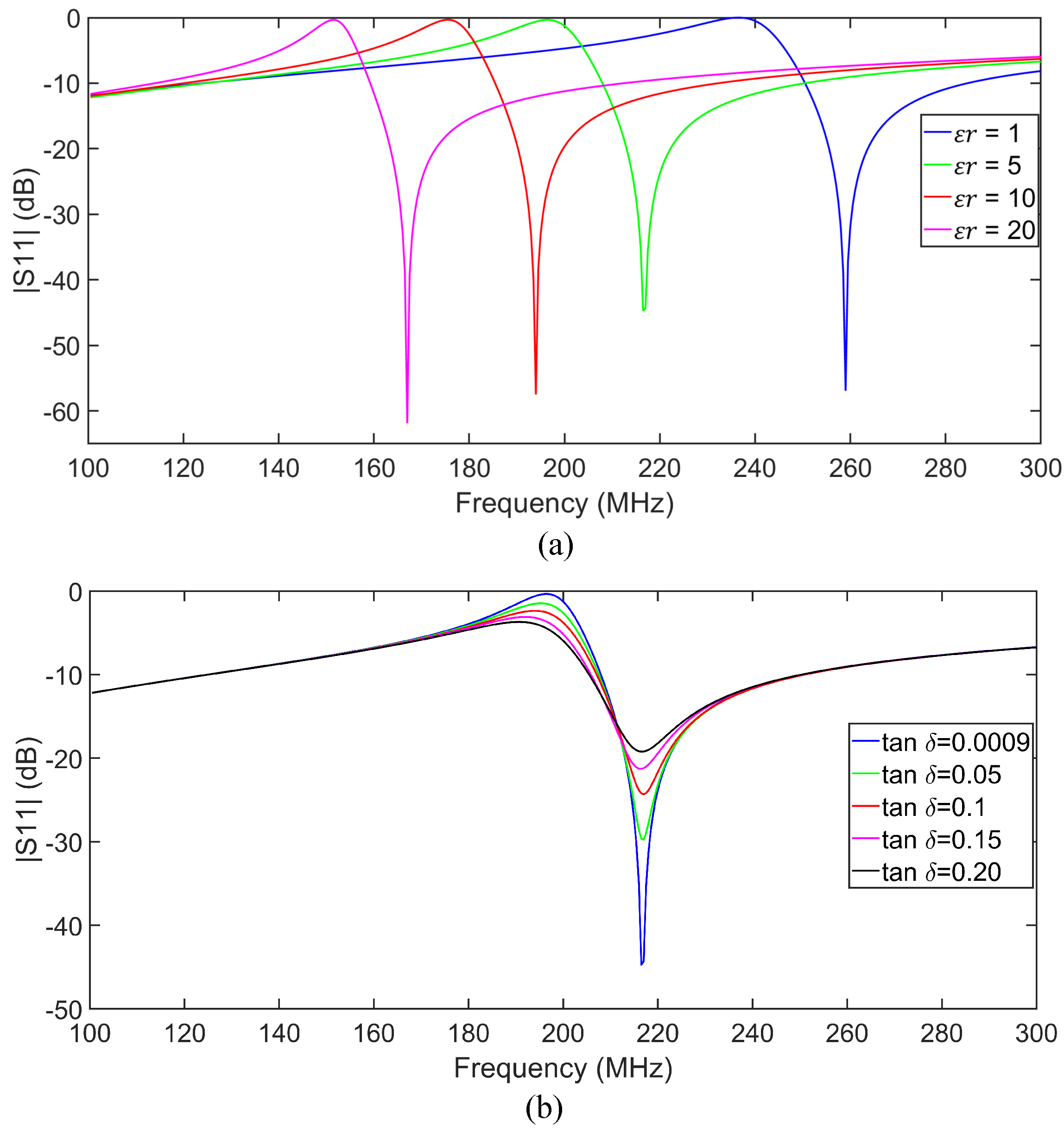



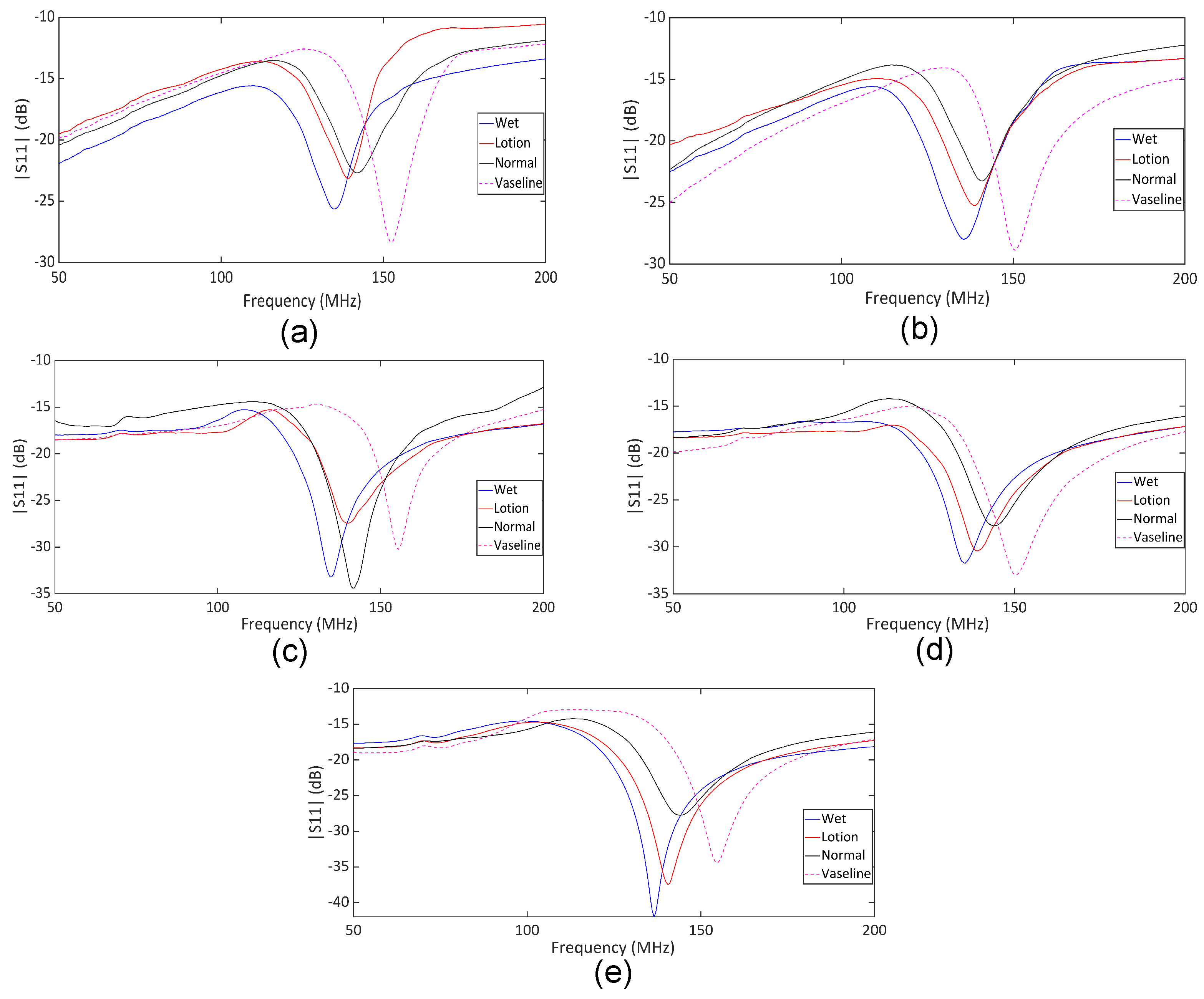
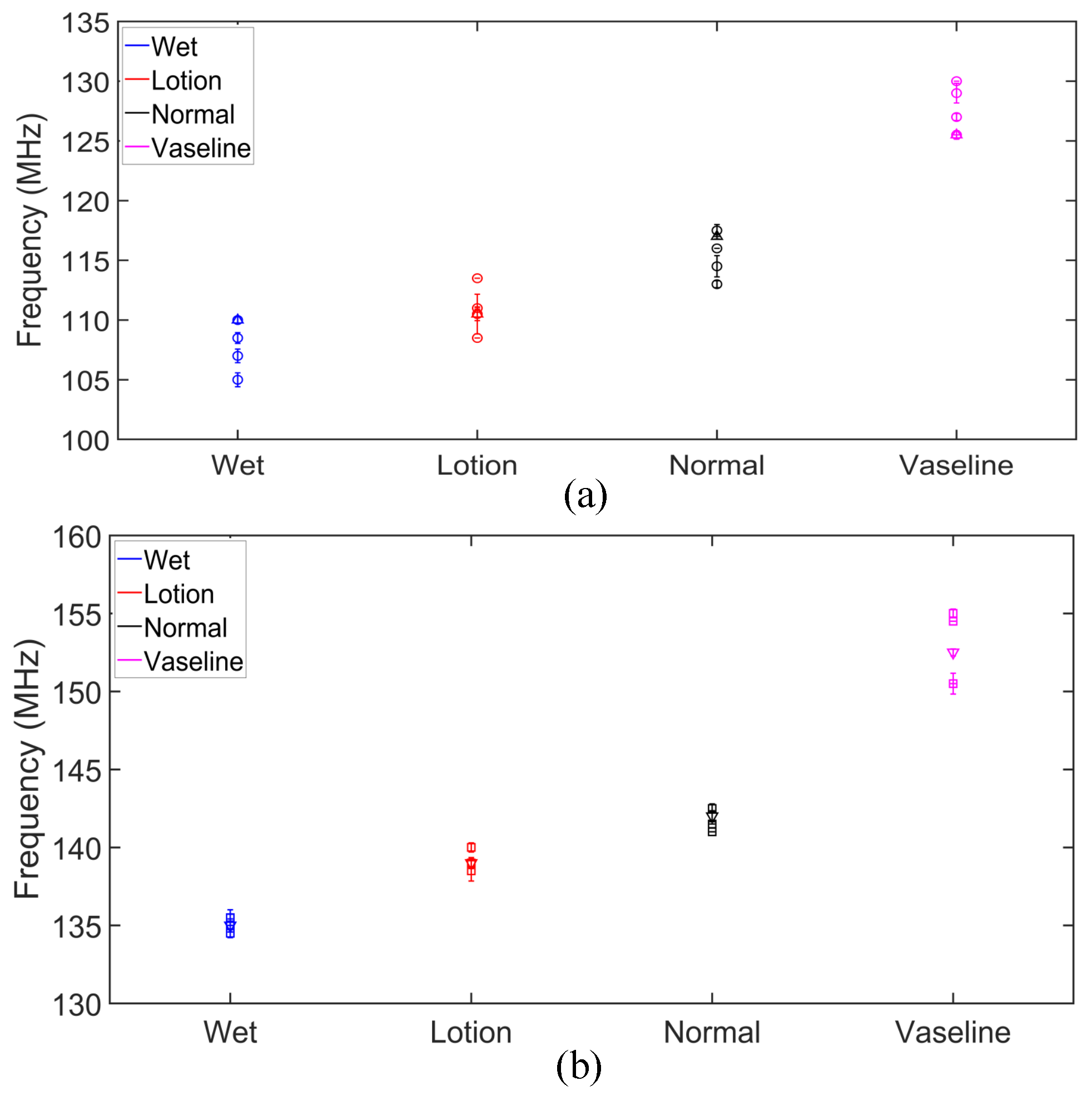
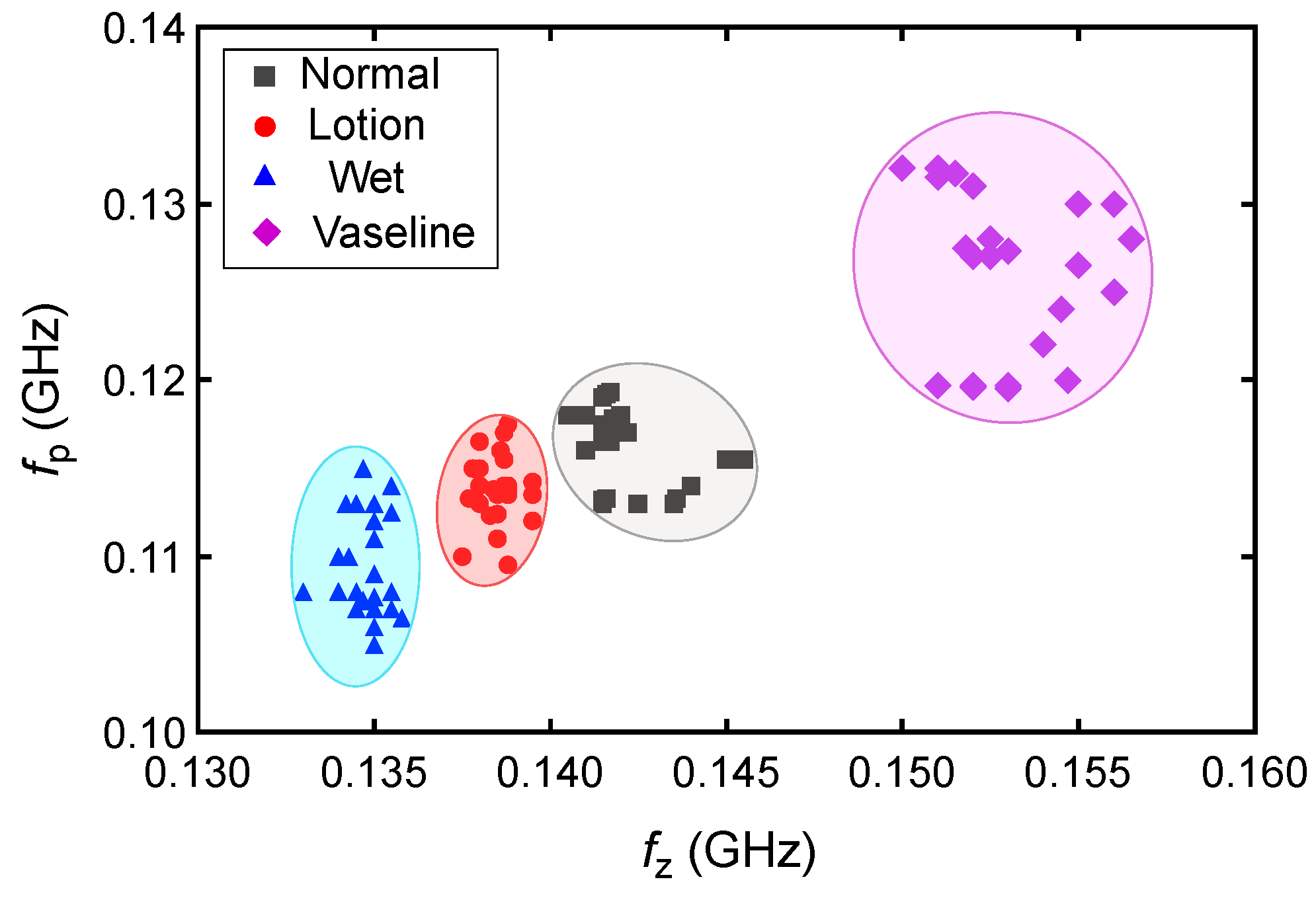
| Subj. | Conditions | Pole Freq. (MHz) | Pole SD (±MHz) | Zero Freq. (MHz) | Zero SD (±MHz) |
|---|---|---|---|---|---|
| 1 | Wet | 110.0 | 0.27 | 135.0 | 0.42 |
| Lotion | 110.5 | 1.66 | 139.0 | 0.35 | |
| Normal | 117.0 | 0.22 | 142.0 | 0.35 | |
| Vaseline | 125.5 | 0.35 | 152.5 | 0.22 | |
| 2 | Wet | 108.5 | 0.45 | 135.5 | 0.22 |
| Lotion | 110.5 | 0.55 | 138.5 | 0.65 | |
| Normal | 114.5 | 0.89 | 141.0 | 0.00 | |
| Vaseline | 129.0 | 0.82 | 150.5 | 0.67 | |
| 3 | Wet | 107.0 | 0.57 | 134.5 | 0.29 |
| Lotion | 111.0 | 0.10 | 139.0 | 0.28 | |
| Normal | 116.0 | 0.00 | 141.5 | 0.00 | |
| Vaseline | 130.0 | 0.00 | 155.0 | 0.28 | |
| 4 | Wet | 110.0 | 0.29 | 135.0 | 0.00 |
| Lotion | 113.5 | 0.00 | 139.0 | 0.29 | |
| Normal | 117.5 | 0.50 | 141.5 | 0.00 | |
| Vaseline | 125.5 | 0.00 | 150.5 | 0.00 | |
| 5 | Wet | 105.0 | 0.58 | 135.5 | 0.50 |
| Lotion | 108.5 | 0.29 | 140.0 | 0.29 | |
| Normal | 113.0 | 0.29 | 142.5 | 0.29 | |
| Vaseline | 127.0 | 0.29 | 154.5 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, S.; Ebrahimi, A.; Ghorbani, K.; Tovar-Lopez, F. Skin Hydration Monitoring Using a Microwave Sensor: Design, Fabrication, and In Vivo Analysis. Sensors 2025, 25, 3445. https://doi.org/10.3390/s25113445
Chowdhury S, Ebrahimi A, Ghorbani K, Tovar-Lopez F. Skin Hydration Monitoring Using a Microwave Sensor: Design, Fabrication, and In Vivo Analysis. Sensors. 2025; 25(11):3445. https://doi.org/10.3390/s25113445
Chicago/Turabian StyleChowdhury, Shabbir, Amir Ebrahimi, Kamran Ghorbani, and Francisco Tovar-Lopez. 2025. "Skin Hydration Monitoring Using a Microwave Sensor: Design, Fabrication, and In Vivo Analysis" Sensors 25, no. 11: 3445. https://doi.org/10.3390/s25113445
APA StyleChowdhury, S., Ebrahimi, A., Ghorbani, K., & Tovar-Lopez, F. (2025). Skin Hydration Monitoring Using a Microwave Sensor: Design, Fabrication, and In Vivo Analysis. Sensors, 25(11), 3445. https://doi.org/10.3390/s25113445







