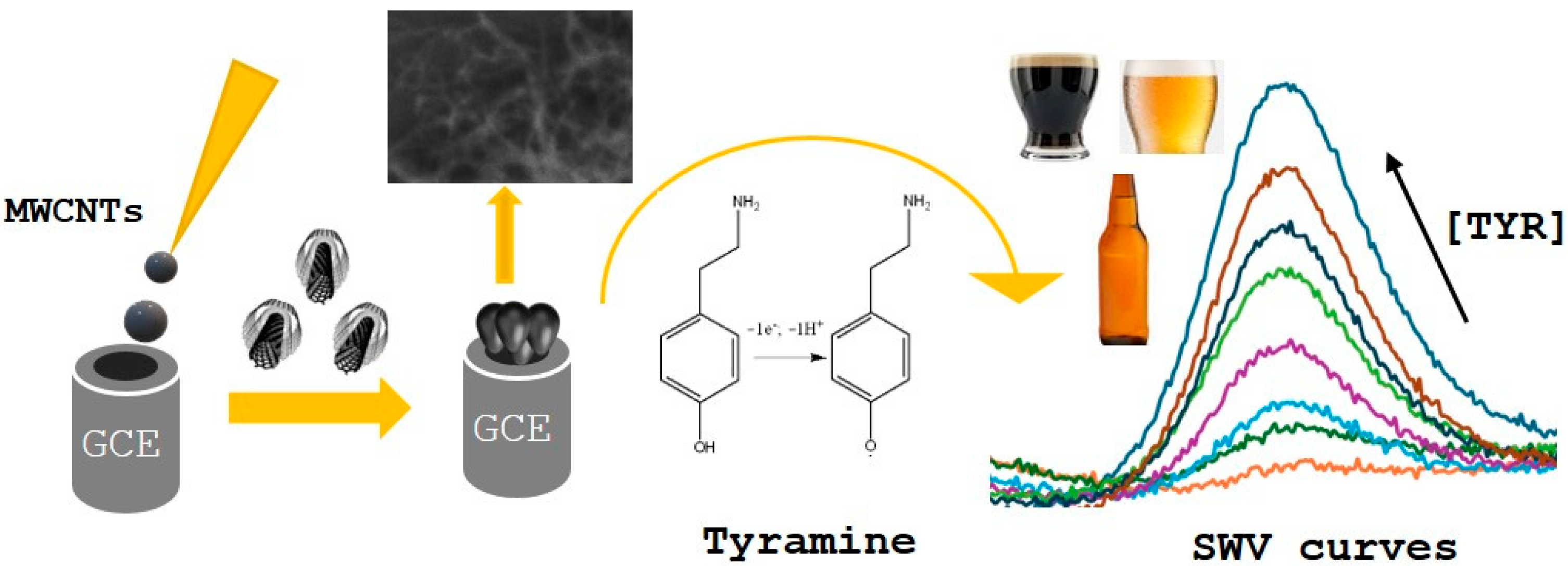
Direct Electrochemical Detection of Tyramine in Beer Samples Using a MWCNTs Modified GCE
Abstract
Highlights
- Direct determination of tyramine (TYR) in different beer samples, using an electrochemical method with a GCE electrode modified with MWCNTs. By using SWV and controlled pH conditions, TYR was assessed in beers with basic sample pretreatments to provide an easy and rapid method. The developed method was compared with a standard technique to validate its reliability.
- Portable electrochemical method for TYR detection with simple sample pretreatment.
- Direct TYR determination in beer samples.
- Rapid on-site TYR assessment.
- Quality control and corrective measures implementation.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Standard Solutions
2.2. Electrode Preparation
2.3. Surface Characterization
2.4. Electrochemical Analysis
2.5. Beer Samples Preparation
2.6. Tyramine Analysis by HPLC-FLD
3. Results
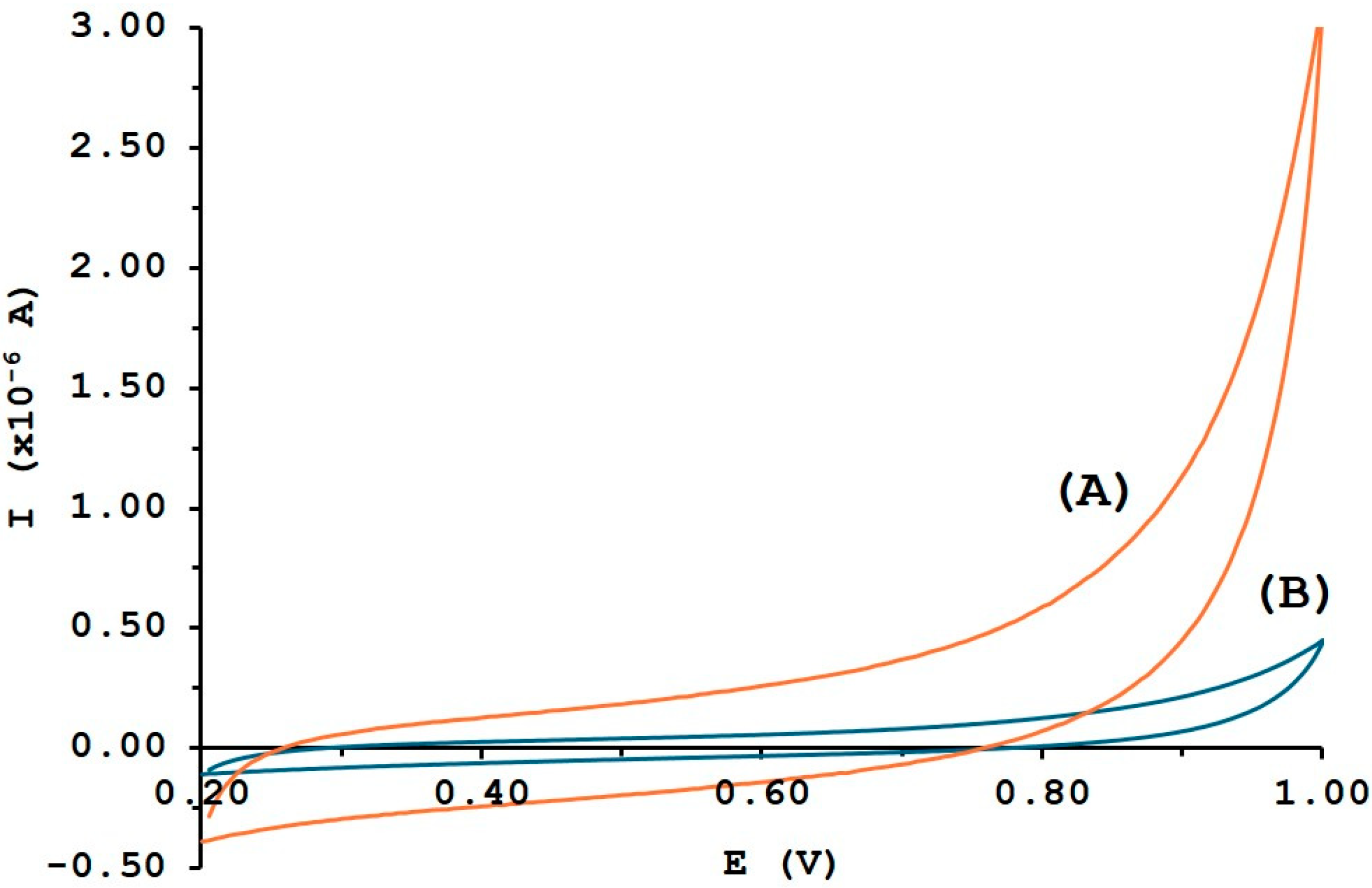
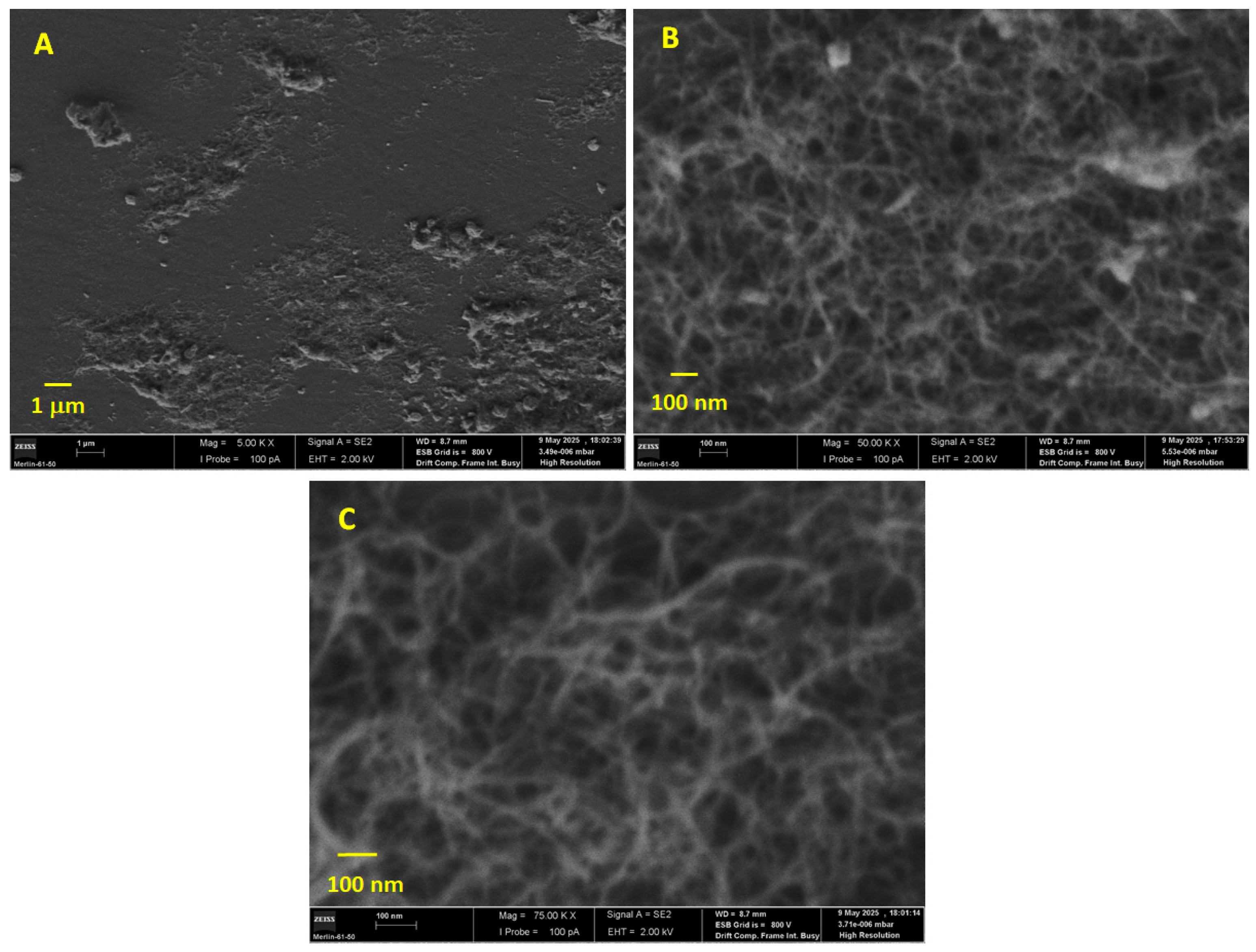
3.1. Surface Characterization of MWCNTs/GCE by CV, SEM and EIS
3.2. Evaluation of the Electrode Surface Area
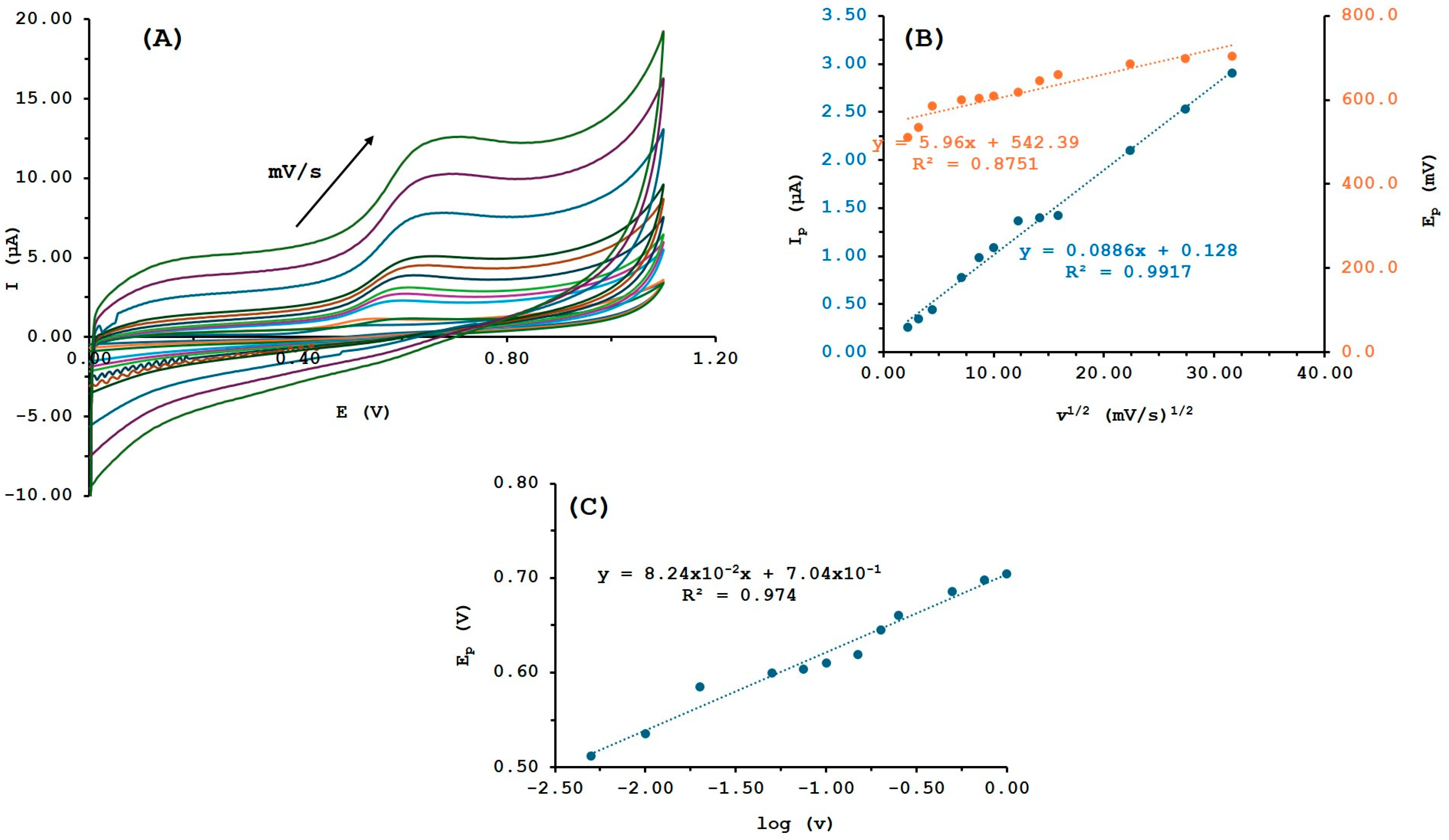
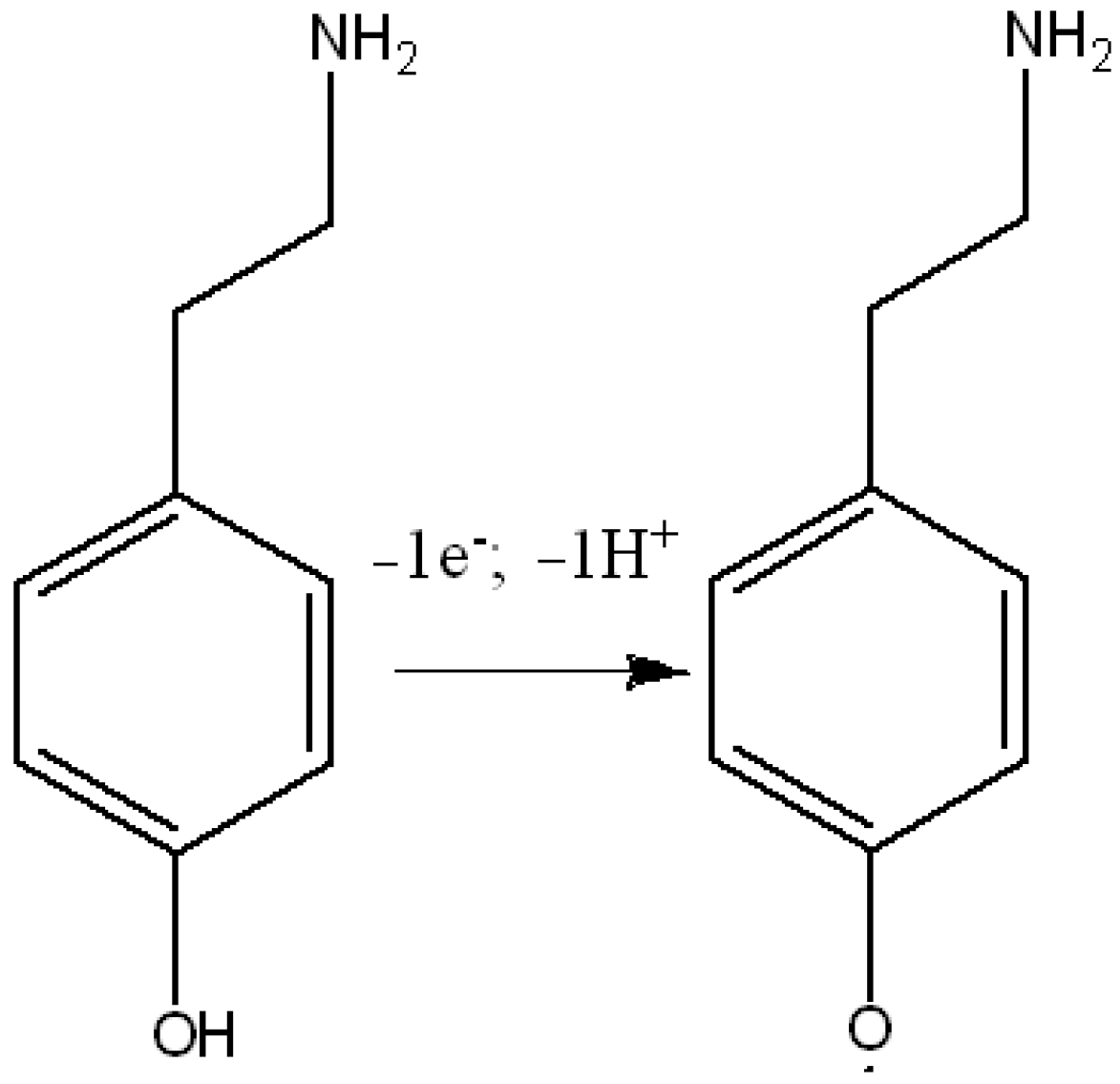
3.3. Electrochemical Behavior of Tyramine
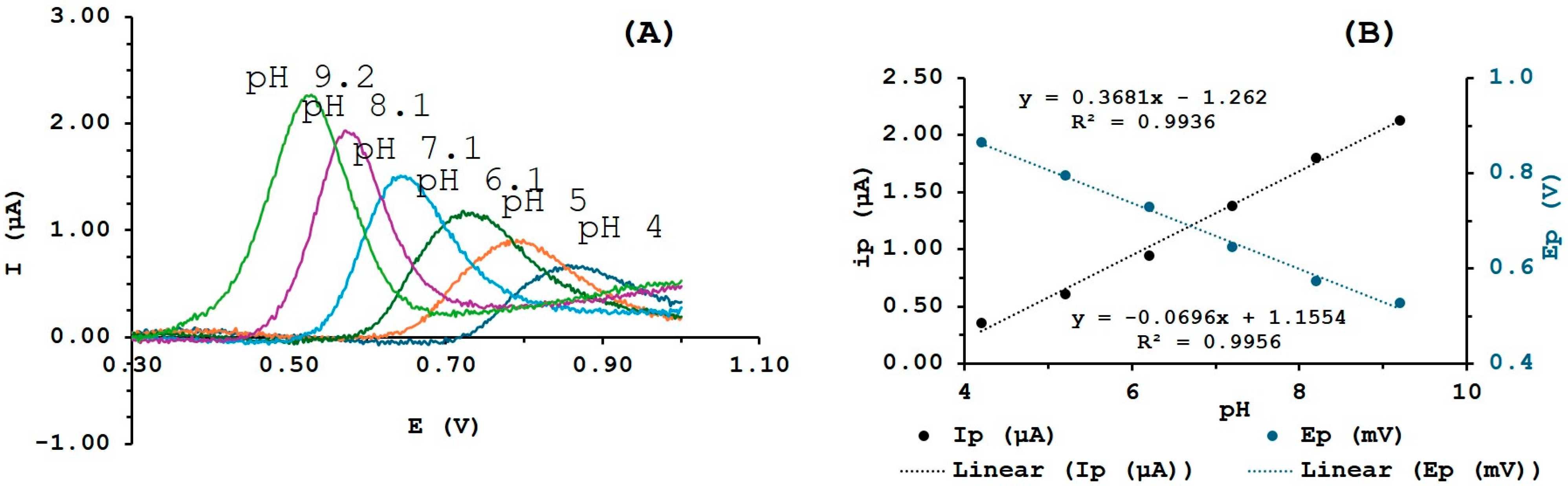
3.4. Effect of pH
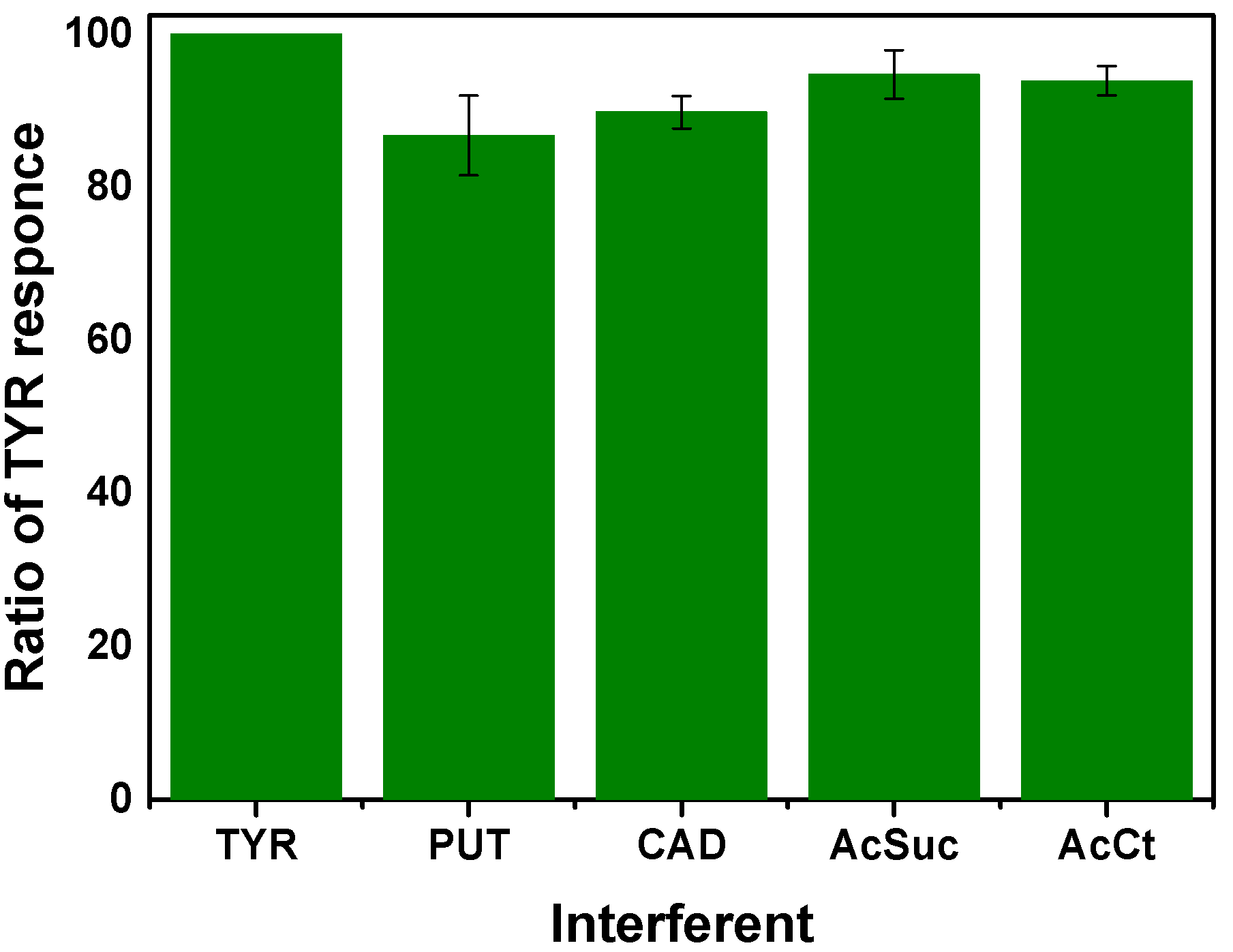
3.5. Interference Studies
3.6. Stability Studies
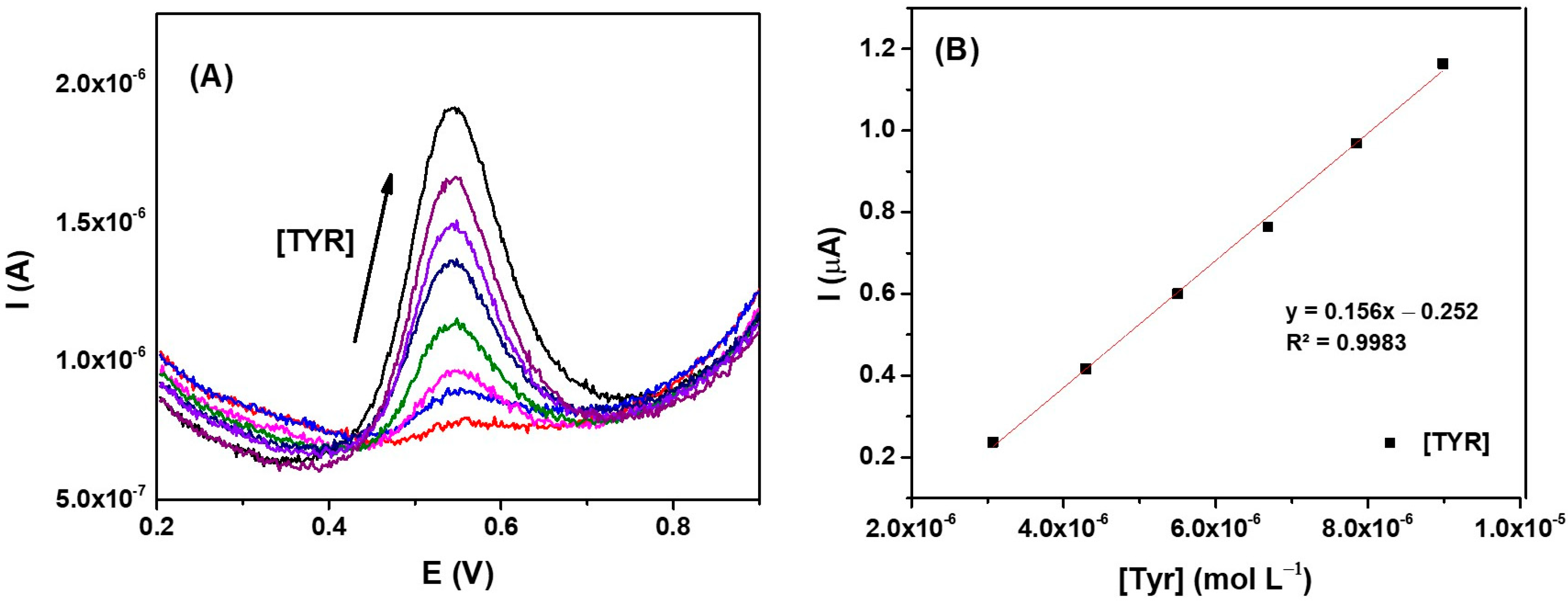
3.7. Analytical Applications
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khwanchuea, R.; Mulvany, M.J.; Jansakul, C. Cardiovascular effects of tyramine: Adrenergic and cholinergic interactions. Eur. J. Pharmacol. 2008, 579, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Gillman, P.K. A reassessment of the safety profile of monoamine oxidase inhibitors: Elucidating tired old tyramine myths. J. Neural Transm. 2018, 125, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Mohammed, G.; Al-Eryani, D.; Saigl, Z.; Alyoubi, A.; Alwael, H.; Bashammakh, A.; O’Sullivan, C.; El-Shahawi, M. Biogenic amines formation mechanism and determination strategies: Future challenges and limitations. Crit. Rev. Anal. Chem. 2020, 50, 485–500. [Google Scholar] [CrossRef]
- Erdag, D.; Merhan, O.; Yildiz, B. Biochemical and pharmacological properties of biogenic amines. In Biogenic Amines; InTechOpen: London, UK, 2018; Volume 8, pp. 1–14. [Google Scholar]
- Izquierdo-Pulido, M.; Carceller-Rosa, J.-M.; Mariné-Font, A.; Vidal-Carou, M.C. Tyramine formation by Pediococcus spp. during beer fermentation. J. Food Prot. 1997, 60, 831–836. [Google Scholar] [CrossRef]
- Izquierdo-Pulido, M.; Mariné-Font, A.; Vidal-Carou, M.C. Effect of tyrosine on tyramine formation during beer fermentation. Food Chem. 2000, 70, 329–332. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Noworyta, K.R.; Gajda, M.; Ziminska, A.; Sosnowska, M.; Piechowska, J.; Borowicz, P.; Lisowski, W.; Shao, S. Electrochemical sensor for selective tyramine determination, amplified by a molecularly imprinted polymer film. Bioelectrochemistry 2021, 138, 107695. [Google Scholar] [CrossRef]
- da Silva, A.G.; Franco, D.L.; Santos, L.D. A simple, fast, and direct electrochemical determination of tyramine in Brazilian wines using low-cost electrodes. Food Control 2021, 130, 108369. [Google Scholar] [CrossRef]
- Givanoudi, S.; Heyndrickx, M.; Depuydt, T.; Khorshid, M.; Robbens, J.; Wagner, P. A review on bio-and chemosensors for the detection of biogenic amines in food safety applications: The status in 2022. Sensors 2023, 23, 613. [Google Scholar] [CrossRef]
- Almeida, C.M.; Magalhães, J.M.; Barroso, M.F.; Durães, L. Biogenic Amines Detection in Food: Emerging Trends in Electrochemical Sensors. Talanta 2025, 292, 127918. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Baghayeri, M.; Amiri-Aref, M. Application of a glassy carbon electrode modified with functionalized multi-walled carbon nanotubes as a sensor device for simultaneous determination of acetaminophen and tyramine. Anal. Methods 2012, 4, 1579–1587. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Amiri-Aref, M.; Baghayeri, M. Electrodeposition of quercetin at a multi-walled carbon nanotubes modified glassy carbon electrode as a novel and efficient voltammetric sensor for simultaneous determination of levodopa, uric acid and tyramine. Sens. Actuators B Chem. 2012, 166, 508–518. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Wei, Q.; Gao, Y.; Guo, L.; Al-Ghanim, K.A.; Mahboob, S.; Zhang, X. An easily fabricated electrochemical sensor based on a graphene-modified glassy carbon electrode for determination of octopamine and tyramine. Sensors 2016, 16, 535. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Lin, M. An electroanalytical sensor based on overoxidized polypyrrole-gold composite for the detection of tyramine in fermented food. Int. J. Electrochem. Sci. 2023, 18, 100399. [Google Scholar] [CrossRef]
- Eteya, M.M.; Rounaghi, G.H.; Deiminiat, B. Electrochemical Determination of Tyramine Using a Carbon Ionic Liquid Paste Electrode Modified with f-MWCNTs/Graphene Nanocomposite Film. Electrocatalysis 2023, 14, 688–696. [Google Scholar] [CrossRef]
- Erdogan, Z.O.; Kursunlu, A.N.; Kucukkolbasi, S. Pillar(5)arene based non-enzymatic and enzymatic tyramine sensor. IEEE Sens. J. 2020, 21, 5728–5735. [Google Scholar] [CrossRef]
- Cragg, P.J.; Sharma, K. Pillar(5)arenes: Fascinating cyclophanes with a bright future. Chem. Soc. Rev. 2012, 41, 597–607. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, L.; Wang, C.; Xian, Y.; Zeng, X.; Bai, W. Simple and sensitive electrochemical sensor for tyramine determination based on overoxidized poly (o-aminophenol) film modified electrode. Int. J. Electrochem. Sci. 2018, 13, 10289–10301. [Google Scholar] [CrossRef]
- Chakkarapani, L.D.; Brandl, M. Highly sensitive electrochemical detection of tyramine using a poly (toluidine blue)-modified carbon screen-printed electrode. IEEE Sens. J. 2021, 22, 2974–2983. [Google Scholar] [CrossRef]
- Angulo, M.F.; Flores, M.; Aranda, M.; Henriquez-Aedo, K. Fast and selective method for biogenic amines determination in wines and beers by ultra high-performance liquid chromatography. Food Chem. 2020, 309, 125689. [Google Scholar] [CrossRef]
- Herrero, A.; Sanllorente, S.; Reguera, C.; Ortiz, M.C.; Sarabia, L.A. A new multiresponse optimization approach in combination with a D-Optimal experimental design for the determination of biogenic amines in fish by HPLC-FLD. Anal. Chim. Acta 2016, 945, 31–38. [Google Scholar] [CrossRef]
- Chrzescijanska, E.; Wudarska, E.; Kusmierek, E.; Rynkowski, J. Study of acetylsalicylic acid electroreduction behavior at platinum electrode. J. Electroanal. Chem. 2014, 713, 17–21. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanisms; VCH: New York, NY, USA, 1993; Volume 43. [Google Scholar]
- Masood, Z.; Muhammad, H.; Tahiri, I.A. Comparison of different electrochemical methodologies for electrode reactions: A case study of paracetamol. Electrochem 2024, 5, 57–69. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Adenier, A.; Chehimi, M.M.; Gallardo, I.; Pinson, J.; Vila, N. Electrochemical oxidation of aliphatic amines and their attachment to carbon and metal surfaces. Langmuir 2004, 20, 8243–8253. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Sahudin, M.A.; Tan, L.L.; Su’ait, M.S.; Abd Karim, N.H.; Mackeen, M.M. Regenerable and selective histamine impedimetric sensor based on hydroxyl functionalised Schiff base complex electrode. Electrochim. Acta 2021, 379, 138186. [Google Scholar] [CrossRef]
- Poveda, J. Biogenic amines and free amino acids in craft beers from the Spanish market: A statistical approach. Food Control 2019, 96, 227–233. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and chemometrics for analytical chemistry. Signal 1998, 100, 95. [Google Scholar]
- Izquierdo-Pulido, M.; Vidal-Carou, M.d.C.; Marine-Font, A. Histamine and tyramine in beers: Contents and relationships with other analytical data. J. Food Compos. Anal. 1989, 2, 219–227. [Google Scholar] [CrossRef]




| System | Detection Technique | LOD (µM)/LOQ (µM) | Linear Range (μM) | Ref. |
|---|---|---|---|---|
| fMWCNT/GCE | DPV | 0.8/n.a. | Up to 85 | [11] |
| fMWCNTs/GCE | DPV | 0.647/n.a. | 0.70–75.0 | [12] |
| rGO/GCE | DPV | 0.03/n.a. | 0.1–25 | [13] |
| Au/PPyox/GCE | ChrAmp | 0.027/n.a. | 0.2–1200 | [14] |
| fMWCNTs/graphene film/CPE | SWV | 0.5/n.a. | 1–1000 | [15] |
| Overoxidized Poly(o-aminophenol)/GCE | SWV | 0.054/n.a. | 0.1–10 | [18] |
| Poly(Toluidine Blue)/CarbonSPE | DPV | 0.007/n.a. | 0.02–270.5 | [19] |
| MWCNTs/GCE | SWV | 0.34/1 | 3–9 | This Work |
| Beer Sample | HPLC (M) | RD (%) | MWCNTs/GCE (M) | RSD (%) |
| black | 1.65 × 10−4 ± 0.04 × 10−4 | 0.05 | 1.58 × 10−4 ± 0.34 × 10−4 | 18.3 |
| blond | 5.27 × 10−5 ± 0.28 × 10−5 | 0.02 | 9.02 × 10−5 ± 0.88 × 10−5 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.M.R.; Barroso, M.F.; Moreira, M.M.; Magalhães, J.M.C.S.; Durães, L. Direct Electrochemical Detection of Tyramine in Beer Samples Using a MWCNTs Modified GCE. Sensors 2025, 25, 3322. https://doi.org/10.3390/s25113322
Almeida CMR, Barroso MF, Moreira MM, Magalhães JMCS, Durães L. Direct Electrochemical Detection of Tyramine in Beer Samples Using a MWCNTs Modified GCE. Sensors. 2025; 25(11):3322. https://doi.org/10.3390/s25113322
Chicago/Turabian StyleAlmeida, Cláudio M. R., Maria Fátima Barroso, Manuela M. Moreira, Júlia M. C. S. Magalhães, and Luisa Durães. 2025. "Direct Electrochemical Detection of Tyramine in Beer Samples Using a MWCNTs Modified GCE" Sensors 25, no. 11: 3322. https://doi.org/10.3390/s25113322
APA StyleAlmeida, C. M. R., Barroso, M. F., Moreira, M. M., Magalhães, J. M. C. S., & Durães, L. (2025). Direct Electrochemical Detection of Tyramine in Beer Samples Using a MWCNTs Modified GCE. Sensors, 25(11), 3322. https://doi.org/10.3390/s25113322










