Fabrication of Oxygen Vacancy-Rich WO3 Porous Thin Film by Sputter Deposition for Ultrasensitive Mustard-Gas Simulants Sensor
Abstract
1. Introduction
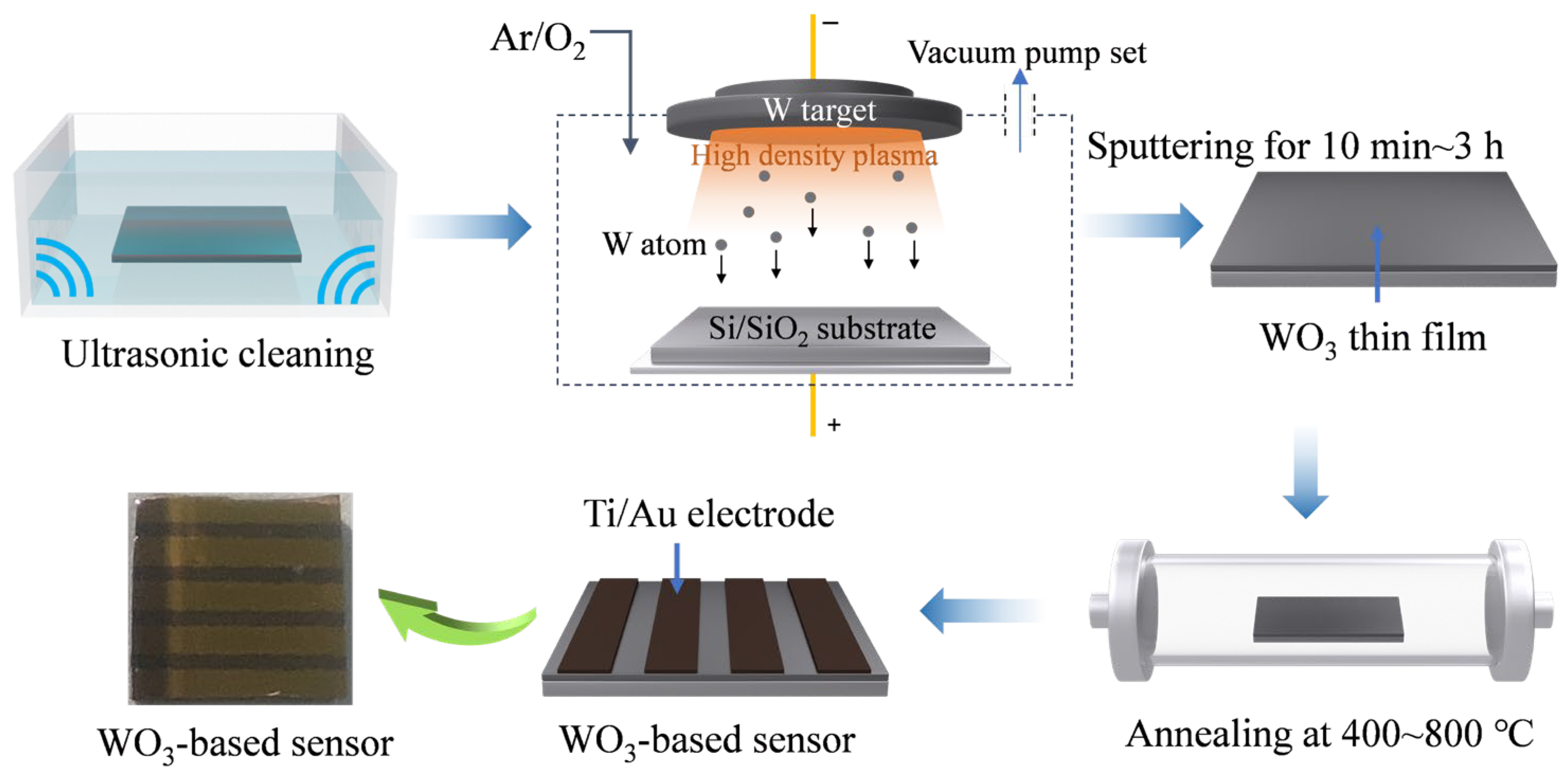
2. Materials and Methods
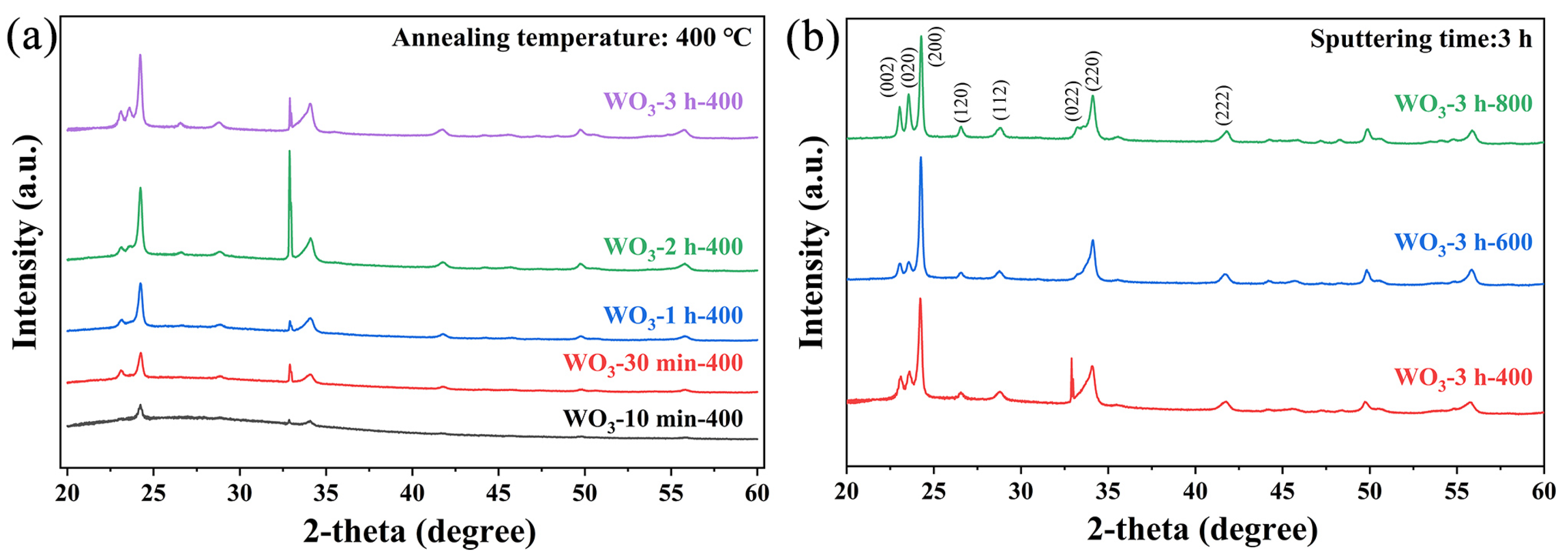
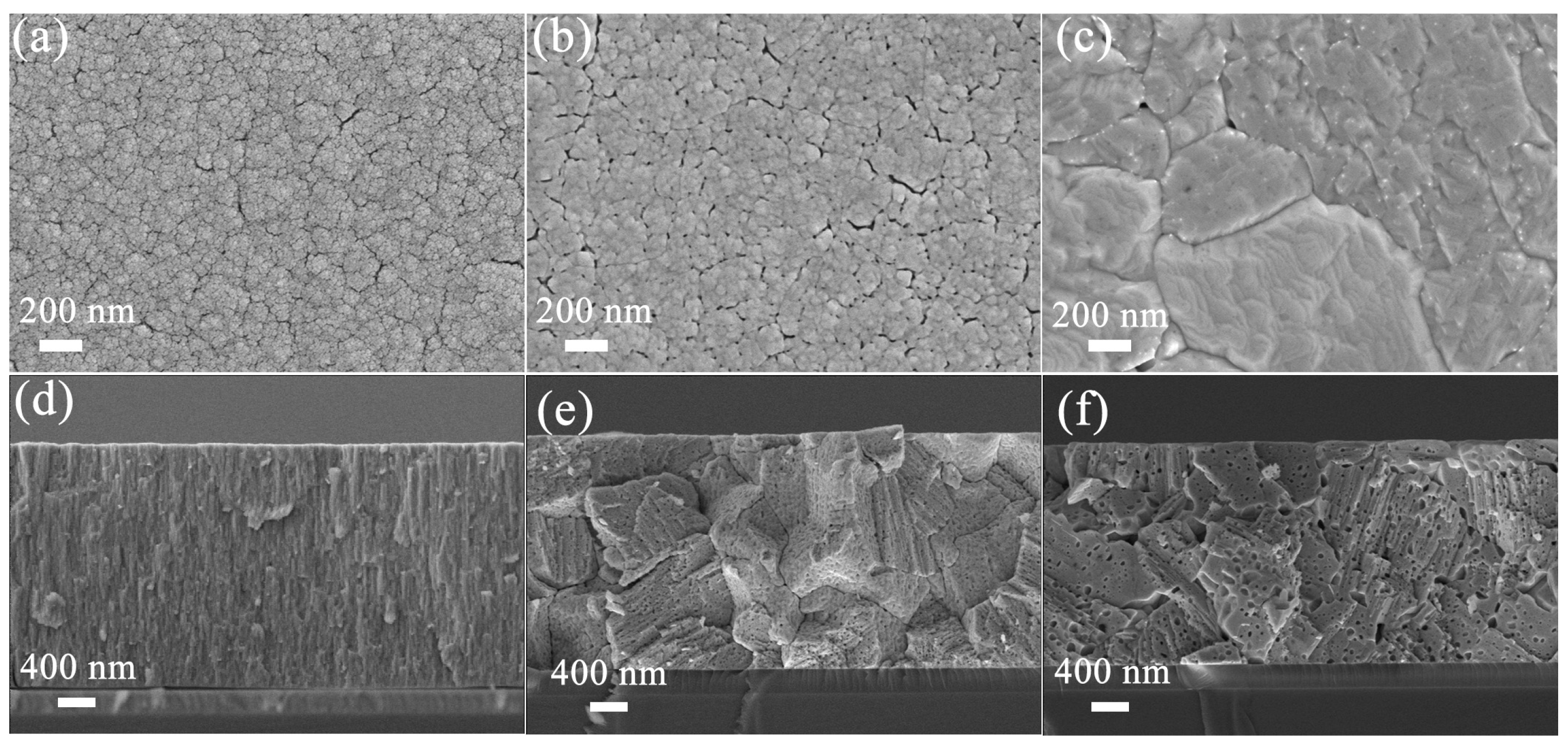
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and detection of chemical warfare agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.-Z.; Xue, H.; Gao, X.-Y.; Wang, Y.; Shen, J.; Li, X.-B.; Zheng, Y.-C.; Wang, H. Room Temperature Chemiresistive Sensing of Sulfur Mustard Simulant by 2D Conjugated MOF Surface Modification. ACS Mater. Lett. 2024, 6, 5058–5065. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, B.; Gao, R.; Zhang, X.; Cheng, X.; Huo, L.; Major, Z.; Xu, Y. Crosslinked WO3 nanonet for rapid detection of sulfur mustard gas simulant: Mechanism insights and sensing application. Sens. Actuators B 2023, 385, 133704. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.; Yoo, R.; Park, Y.; Lee, H.-s.; Choe, Y.-S.; Lee, W. Real-time selective detection of 2-chloroethyl ethyl sulfide (2-CEES) using an Al-doped ZnO quantum dot sensor coupled with a packed column for gas chromatography. Sens. Actuators B 2019, 284, 444–450. [Google Scholar] [CrossRef]
- Yoo, R.; Lee, D.; Cho, S.; Lee, W. Doping effect on the sensing properties of ZnO nanoparticles for detection of 2-chloroethyl ethylsulfide as a mustard simulant. Sens. Actuators B 2018, 254, 1242–1248. [Google Scholar] [CrossRef]
- Aliha, H.M.; Khodadadi, A.A.; Mortazavi, Y. The sensing behaviour of metal oxides (ZnO, CuO and Sm2O3) doped-SnO2 for detection of low concentrations of chlorinated volatile organic compounds. Sens. Actuators B 2013, 181, 637–643. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Xin, Y.; Major, Z.; Zhang, X.; Wang, T.; Huo, L.; Cheng, X.; Xu, Y. In situ construction of hierarchical Fe2O3 nanotube arrays for real-time detection and degradation of 2-CEES gas. Sens. Actuators B 2023, 383, 133590. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Wu, J.; Shen, J.; Chen, L.; Zhang, J.; Mao, Y.; Cheng, H.; Zhang, M.; Ma, Q.; et al. Ultrathin WO3 Nanosheets/Pd with Strong Metal–Support Interactions for Highly Sensitive and Selective Detection of Mustard-Gas Simulants. ACS Sens. 2024, 9, 3773–3782. [Google Scholar] [CrossRef]
- Oros, C.; Horprathum, M.; Wisitsoraat, A.; Srichaiyaperk, T.; Samransuksamer, B.; Limwichean, S.; Eiamchai, P.; Phokharatkul, D.; Nuntawong, N.; Chananonnawathorn, C.; et al. Ultra-sensitive NO2 sensor based on vertically aligned SnO2 nanorods deposited by DC reactive magnetron sputtering with glancing angle deposition technique. Sens. Actuators B 2016, 223, 936–945. [Google Scholar] [CrossRef]
- Horprathum, M.; Srichaiyaperk, T.; Samransuksamer, B.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Chananonnawathorn, C.; Aiempanakit, K.; Nuntawong, N.; Patthanasettakul, V.; et al. Ultrasensitive hydrogen sensor based on Pt-decorated WO(3) nanorods prepared by glancing-angle dc magnetron sputtering. ACS Appl. Mater. Interfaces 2014, 6, 22051–22060. [Google Scholar] [CrossRef]
- Merie, V.; Pustan, M.; Negrea, G.; Bîrleanu, C. Research on titanium nitride thin films deposited by reactive magnetron sputtering for MEMS applications. Appl. Surf. Sci. 2015, 358, 525–532. [Google Scholar] [CrossRef]
- Preiß, E.M.; Rogge, T.; Krauß, A.; Seidel, H. Tin oxide-based thin films prepared by pulsed laser deposition for gas sensing. Sens. Actuators B 2016, 236, 865–873. [Google Scholar] [CrossRef]
- Izydorczyk, W.; Izydorczyk, J. Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO(2) Thin Films in an Oxidizing Atmosphere. Sensors 2021, 21, 5741. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.; Hsu, B.; Stan, L.; Stroscio, M.; Dutta, M. Optimized oxygen deprived low temperature sputtered WO3 thin films for crystalline structures. Nanotechnology 2020, 31, 95706. [Google Scholar] [CrossRef]
- Wang, K.; Chang, Y.; Lv, L.; Long, Y. Effect of annealing temperature on oxygen vacancy concentrations of nanocrystalline CeO2 film. Applied Surface Science 2015, 351, 164–168. [Google Scholar] [CrossRef]
- Saritas, S. Crystal structure effects on the Co-sputtered p-type Fe2-xSnxO3 hydrogen gas sensors. Int. J. Hydrogen Energy 2023, 48, 35809–35817. [Google Scholar] [CrossRef]
- Hemberg, A.; Konstantinidis, S.; Viville, P.; Renaux, F.; Dauchot, J.P.; Llobet, E.; Snyders, R. Effect of the thickness of reactively sputtered WO3 submicron thin films used for NO2 detection. Sens. Actuators B 2012, 171–172, 18–24. [Google Scholar] [CrossRef]
- Shaji, K.; Haviar, S.; Zeman, P.; Kos, Š.; Čerstvý, R.; Čapek, J. Controlled sputter deposition of oxide nanoparticles-based composite thin films. Surf. Coat. Technol. 2024, 477, 130325. [Google Scholar] [CrossRef]
- Mathankumar, G.; Bharathi, P.; Mohan, M.K.; Archana, J.; Harish, S.; Navaneethan, M. Defect manipulation of WO3 nanostructures by yttrium for ultra-sensitive and highly selective NO2 detection. Sens. Actuators B 2022, 353, 131057. [Google Scholar] [CrossRef]
- Adilakshmi, G.; Reddy, R.S.; Reddy, A.S.; Reddy, P.S.; Reddy, C.S. Ag-doped WO3 nanostructure films for organic volatile gas sensor application. J. Mater. Sci. Mater. Electron. 2020, 31, 12158–12168. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Marsman, M.; Kresse, G. Relaxed core projector-augmented-wave method. J. Chem. Phys. 2006, 125, 104101. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Chadi, D.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1977, 16, 1746–1747. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- He, K.; Jin, Z.; Chu, X.; Bi, W.; Wang, W.; Wang, C.; Liu, S. Fast response-recovery time toward acetone by a sensor prepared with Pd doped WO3 nanosheets. RSC Adv. 2019, 9, 28439–28450. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, J.-H.; Kim, S.S. Crystallinity dependent gas-sensing abilities of ZnO hollow fibers. Met. Mater. Int. 2016, 22, 942–946. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Guo, L.; Wang, Y.; Sun, J.; Fu, Q. Effect of temperature-dependent nano SiC on the ablation resistance of ZrC coating. J. Eur. Ceram. Soc. 2024, 44, 6875–6888. [Google Scholar] [CrossRef]
- Punginsang, M.; Zappa, D.; Comini, E.; Wisitsoraat, A.; Sberveglieri, G.; Ponzoni, A.; Liewhiran, C. Selective H2S gas sensors based on ohmic hetero-interface of Au-functionalized WO3 nanowires. Appl. Surf. Sci. 2022, 571, 151262. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.Y.; Yoo, H.S.; Lee, W. Pd-WO3 chemiresistive sensor with reinforced self-assembly for hydrogen detection at room temperature. Sens. Actuators B 2022, 368, 132236. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Zhao, L.; Fei, T.; Liu, S.; Zhang, T. The synergistic effects of oxygen vacancy engineering and surface gold decoration on commercial SnO2 for ppb-level DMMP sensing. J. Colloid Interface Sci. 2022, 608 Pt 3, 2703–2717. [Google Scholar] [CrossRef] [PubMed]
- Patil, L.A.; Deo, V.V.; Shinde, M.D.; Bari, A.R.; Kaushik, M.P. Sensing of 2-chloroethyl ethyl sulfide (2-CEES)—A CWA simulant—Using pure and platinum doped nanostructured CdSnO3 thin films prepared from ultrasonic spray pyrolysis technique. Sens. Actuators B 2011, 160, 234–243. [Google Scholar] [CrossRef]
- Lee, J.-H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Panahi, N.; Hosseinnejad, M.T.; Shirazi, M.; Ghoranneviss, M. Optimization of Gas Sensing Performance of Nanocrystalline SnO2 Thin Films Synthesized by Magnetron Sputtering. Chin. Phys. Lett. 2016, 6, 66802. [Google Scholar]
- Godbole, R.; Godbole, V.P.; Alegaonkar, P.S.; Bhagwat, S. Effect of film thickness on gas sensing properties of sprayed WO3 thin films. New J. Chem. 2017, 41, 11807–11816. [Google Scholar] [CrossRef]
- Mariappan, R.; Ponnuswamy, V.; Suresh, P.; Ashok, N.; Jayamurugan, P.; Chandra Bose, A. Influence of film thickness on the properties of sprayed ZnO thin films for gas sensor applications. Superlattices Microstruct. 2014, 71, 238–249. [Google Scholar] [CrossRef]
- Yang, W.; Chen, H.; Li, C.; Meng, H. Surface porosity-rich In2O3 microcubes as efficient channel for trace NO2 detection. Mater. Lett. 2020, 271, 127782. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Gong, F.; Jiu, B.; Li, F. Large scale synthesis of hexagonal simonkolleit nanosheets for ZnO gas sensors with enhanced performances. Mater. Lett. 2017, 186, 7–11. [Google Scholar] [CrossRef]
- Geng, W.; Ma, Z.; Yang, J.; Duan, L.; Li, F.; Zhang, Q. Pore size dependent acetic acid gas sensing performance of mesoporous CuO. Sens. Actuators B 2021, 334, 129639. [Google Scholar] [CrossRef]
- Lee, J.-H.; Nguyen, T.-B.; Nguyen, D.-K.; Kim, J.-H.; Kim, J.-Y.; Phan, B.T.; Kim, S.S. Gas Sensing Properties of Mg-Incorporated Metal–Organic Frameworks. Sensors 2019, 19, 3323. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Jung, G.; Shin, W.; Park, J.; Lee, C.; Kim, D.; Shin, H.; Choi, W.Y.; Lee, J.-H. NO2 gas response improvement method by adopting oxygen vacancy controlled In2O3 double sensing layers. Solid·State Electron. 2024, 216, 108926. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B 2019, 301, 126845. [Google Scholar] [CrossRef]
- Patil, L.A.; Bari, A.R.; Shinde, M.D.; Deo, V.; Kaushik, M.P. Detection of dimethyl methyl phosphonate—A simulant of sarin: The highly toxic chemical warfare–using platinum activated nanocrystalline ZnO thick films. Sens. Actuators B 2012, 161, 372–380. [Google Scholar] [CrossRef]
- Patil, L.A.; Deo, V.V.; Shinde, M.D.; Bari, A.R.; Patil, D.M.; Kaushik, M.P. Ultrasonically Sprayed Nanostructured Perovskite-Type CdSnO3 Thin Films for Sensing of CWA Simulants. IEEE Sens. J. 2014, 14, 3014–3020. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Cao, S.; Yang, J.; Yan, C.; Zhang, L.; Huang, Q.; Zhao, J. High-performance metal-oxide gas sensors based on hierarchical core-shell ZnFe(2)O(4) microspheres for detecting 2-chloroethyl ethyl sulfide. Anal Methods 2023, 15, 3084–3091. [Google Scholar] [CrossRef]
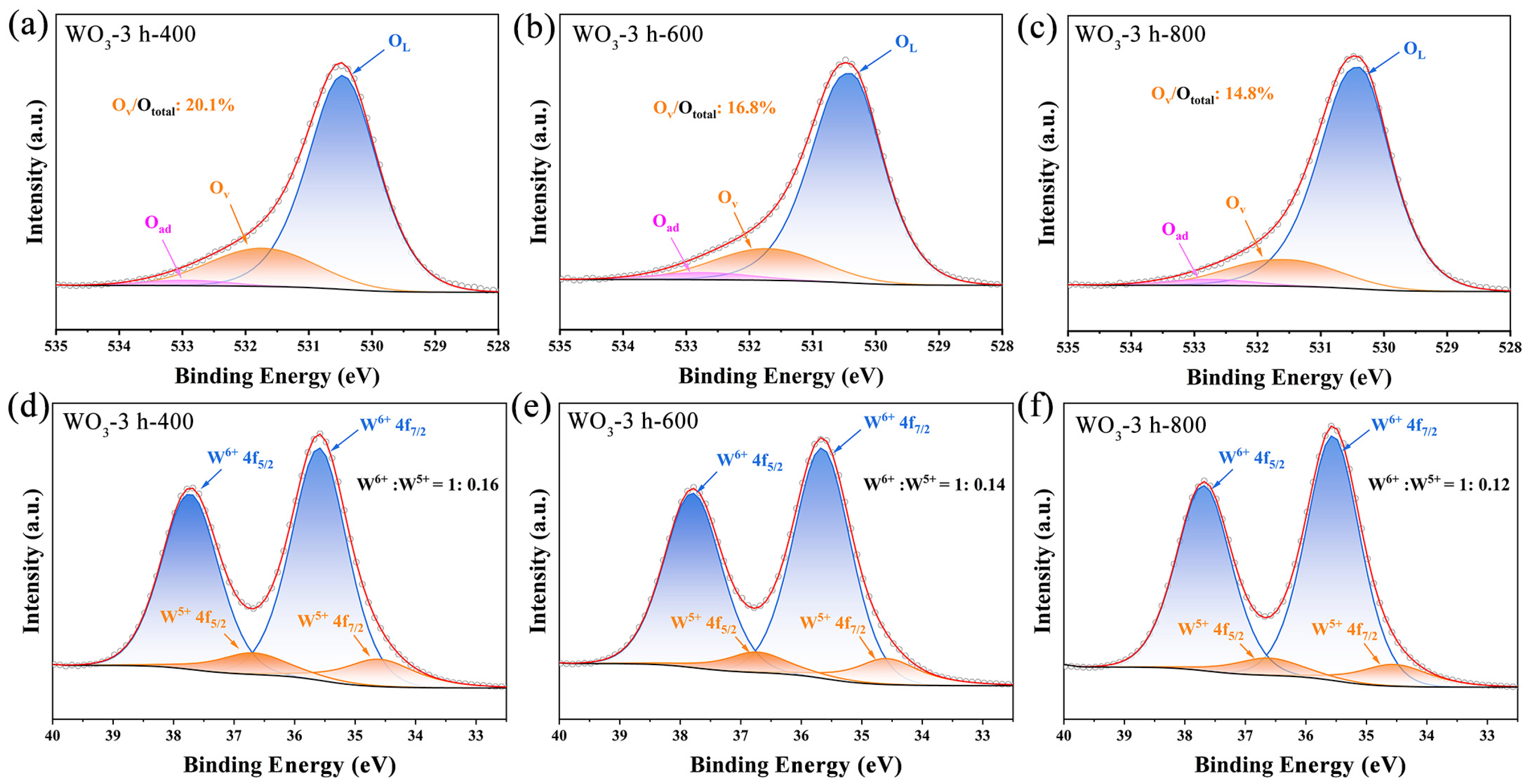
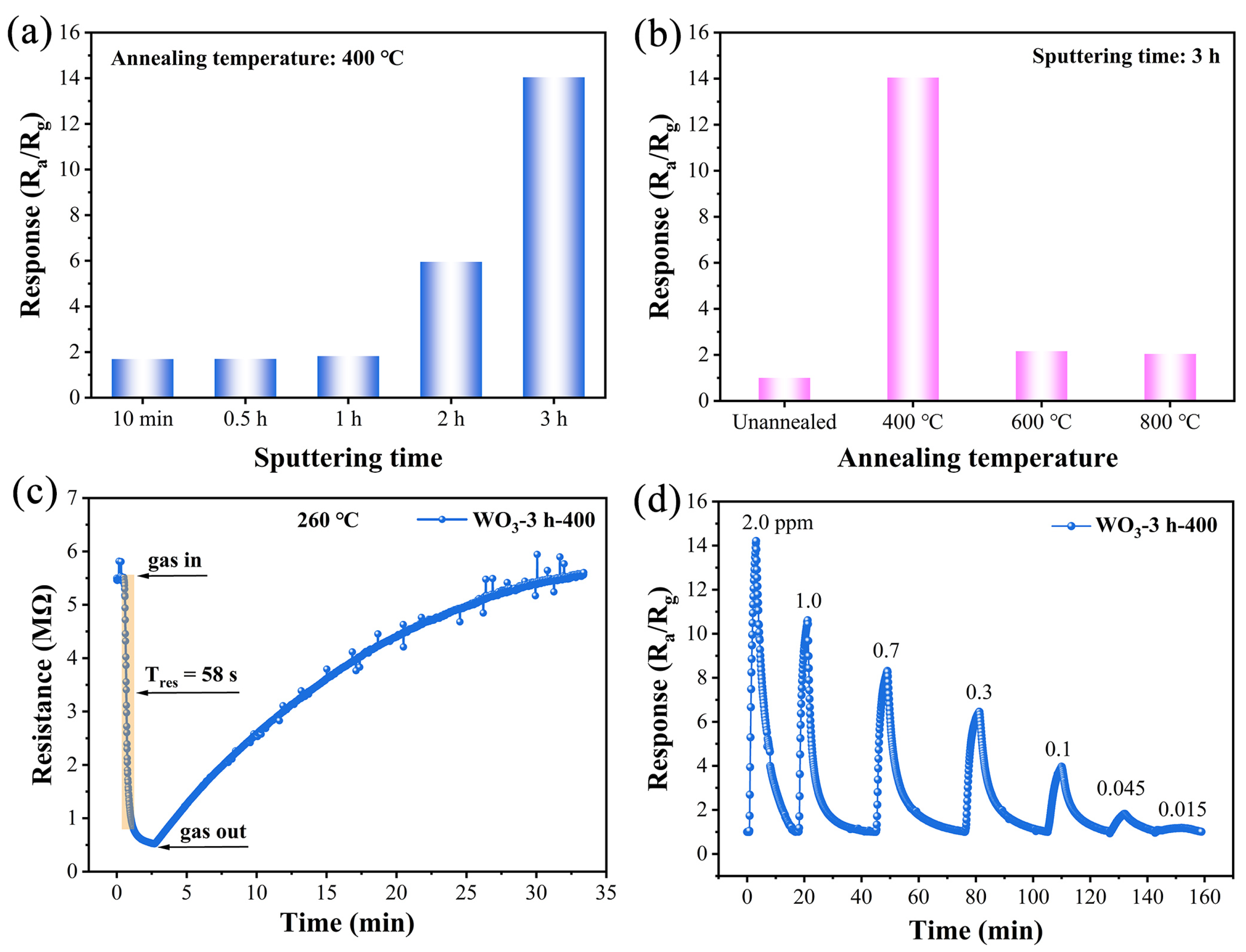
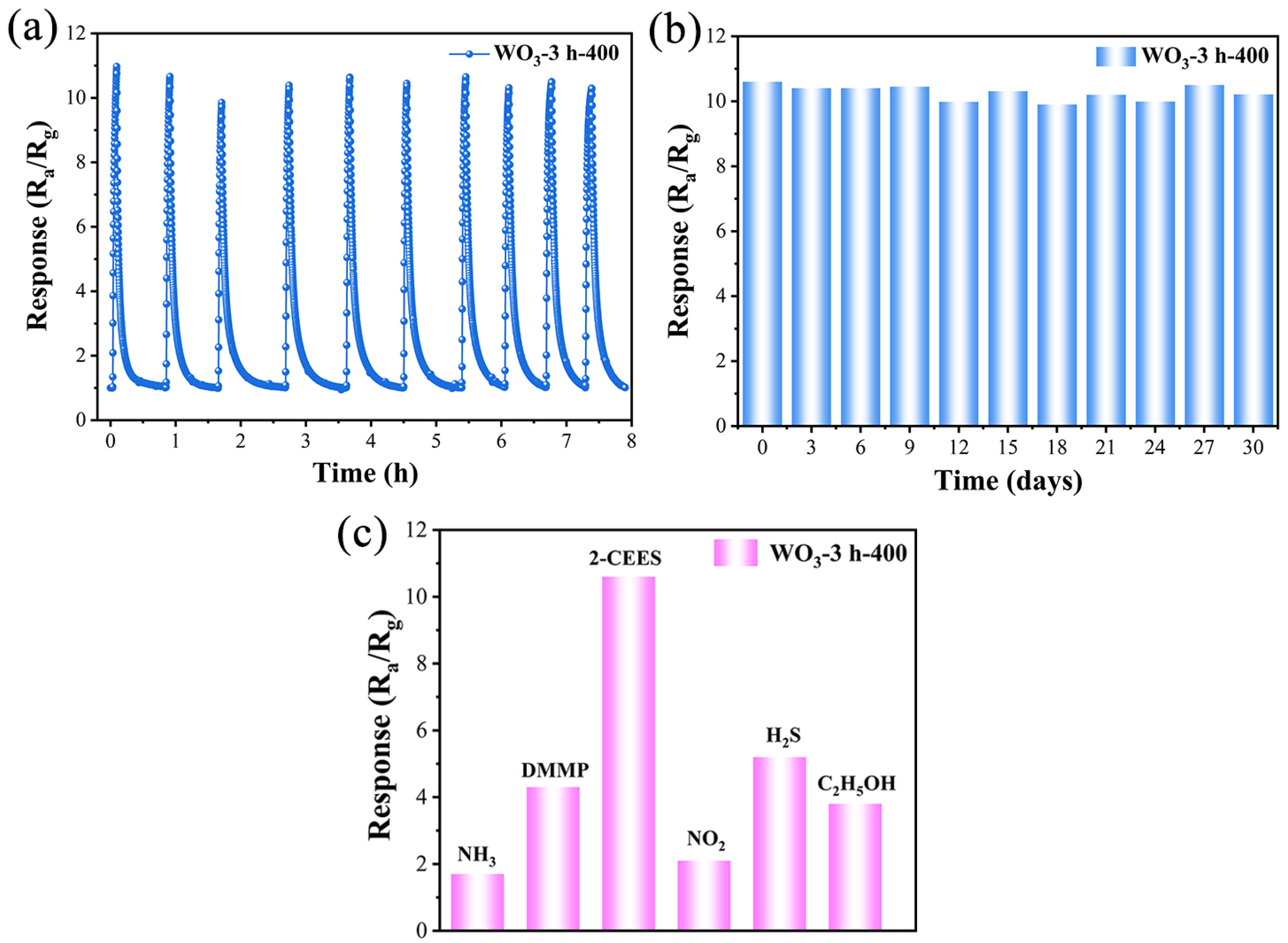
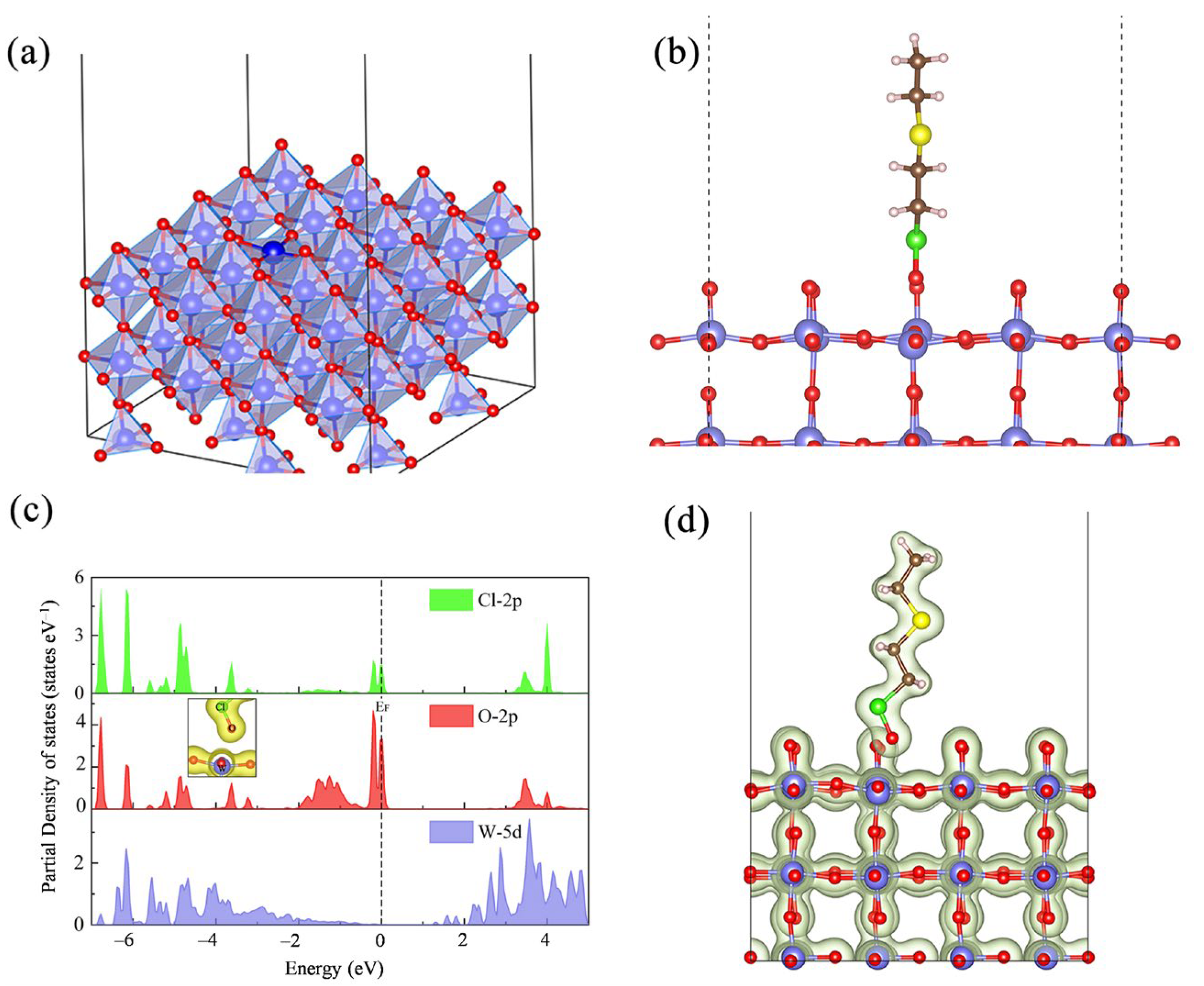
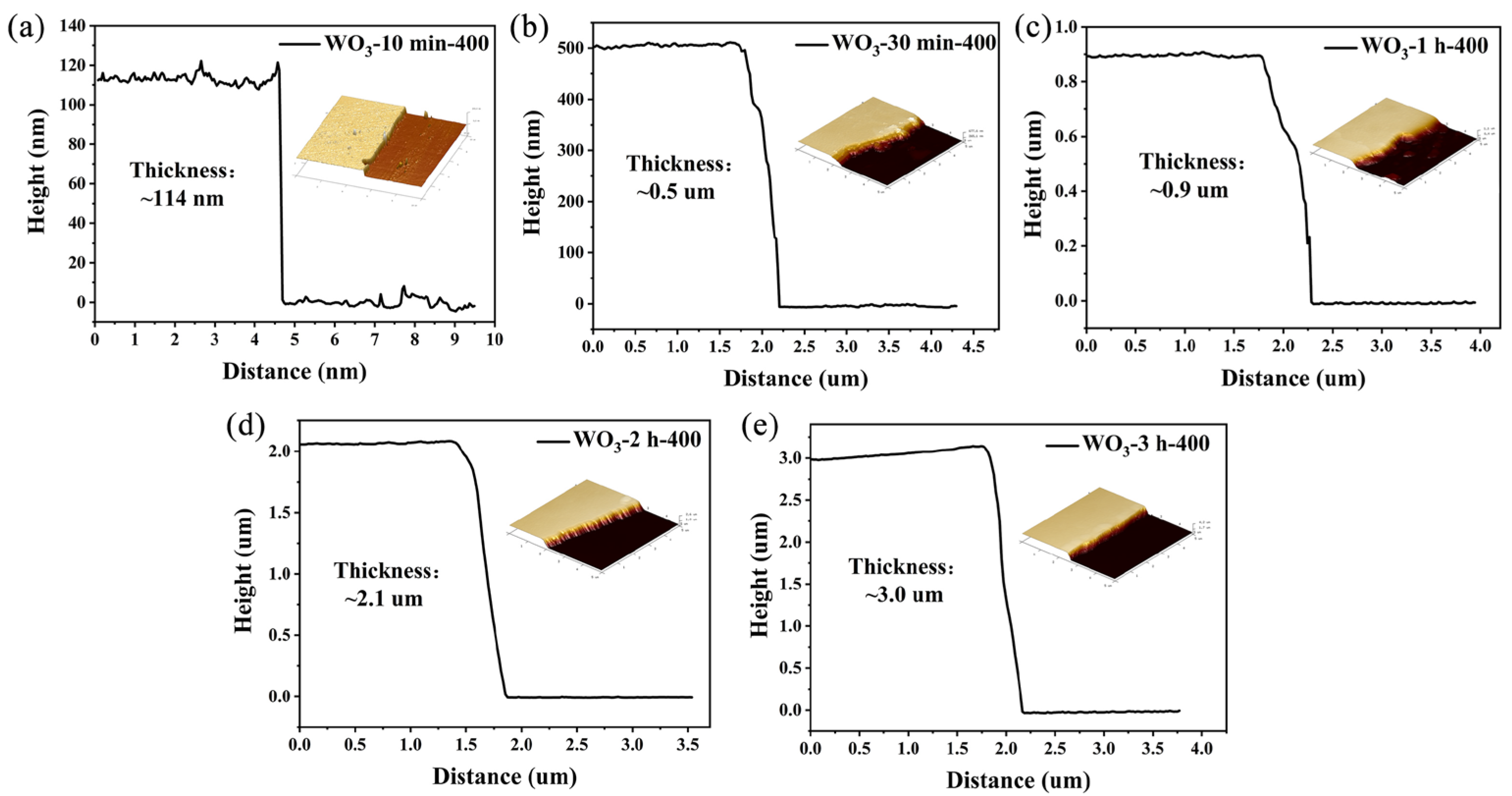
| OL | OV | Oad | OV/Ototal | |
|---|---|---|---|---|
| WO3-3 h-400 | 530.39 | 531.55 | 532.77 | 20.1% |
| WO3-3 h-600 | 530.47 | 531.73 | 532.90 | 16.8% |
| WO3-3 h-800 | 530.40 | 531.72 | 532.72 | 14.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yan, C.; Shen, J.; Liu, S.; Ma, Q.; Zheng, Y. Fabrication of Oxygen Vacancy-Rich WO3 Porous Thin Film by Sputter Deposition for Ultrasensitive Mustard-Gas Simulants Sensor. Sensors 2025, 25, 3049. https://doi.org/10.3390/s25103049
Li H, Yan C, Shen J, Liu S, Ma Q, Zheng Y. Fabrication of Oxygen Vacancy-Rich WO3 Porous Thin Film by Sputter Deposition for Ultrasensitive Mustard-Gas Simulants Sensor. Sensors. 2025; 25(10):3049. https://doi.org/10.3390/s25103049
Chicago/Turabian StyleLi, Haizhen, Cancan Yan, Jun Shen, Shuai Liu, Qingyu Ma, and Yongchao Zheng. 2025. "Fabrication of Oxygen Vacancy-Rich WO3 Porous Thin Film by Sputter Deposition for Ultrasensitive Mustard-Gas Simulants Sensor" Sensors 25, no. 10: 3049. https://doi.org/10.3390/s25103049
APA StyleLi, H., Yan, C., Shen, J., Liu, S., Ma, Q., & Zheng, Y. (2025). Fabrication of Oxygen Vacancy-Rich WO3 Porous Thin Film by Sputter Deposition for Ultrasensitive Mustard-Gas Simulants Sensor. Sensors, 25(10), 3049. https://doi.org/10.3390/s25103049






