Upper Limb Electromyographic Responses to Motor Imagery and Action Observation in Acquired Brain Injury
Abstract
1. Introduction
- To describe the muscle activation in the wrist and finger flexors and extensors that occurs during the use of the CEMIMA as a motor imagery tool via surface electromyographic recordings.
- To describe, using surface electromyographic recordings, the muscle activation in the wrist and finger flexors and extensors that occurs during the application of action observation.
- To analyse whether there are differences between the subjects who evoke better or worse mentally depending on their electromyographic response in MI and AO.
- To analyse the association between the degree of independence in ADLs, upper limb functionality, quality of life, mental evocation, and cognitive impairment with the electromyographic response during MI and AO.
- To analyse, from a gender perspective, the determinants of the study in men and women.
- To study whether there is a relationship between the electromyographic response during MI and AO by age or age groups (over 65 years).
2. Experimental Design
2.1. Type of Study
2.2. Study Setting
2.3. Participants
- Being over 18 years of age with an established diagnosis of stroke.
- Presenting motor response limitations or deficiencies in the upper limbs.
- Maintaining cognitive functions with the capacity to follow the indications of the interventions and assessments in Spanish language (The Montreal Cognitive Assessment, MoCA > 14).
2.4. Power Calculation and Sample Size
3. Materials and Equipment
3.1. Variables Study
3.2. Measurement Instruments
- Degree of disability and dependence: Barthel Index. This is a generic measure that assesses the level of independence of the patient, allowing the degree of performance of the subject in some basic activities of daily living to be determined, assigning different scores and weightings. It is the most widely used scale internationally for the functional assessment of patients with an acute cerebrovascular pathology and its complications, such as vascular dementia. It includes 10 ADLs: eating, transferring between chair and bed, personal grooming, going to the toilet, bathing/showering, transferring (walking on a smooth surface or in a wheelchair), climbing/descending stairs, dressing, stool control, and urine control [16].
- Functionality of the upper limbs: Action Research Arm Test (ARAT). This test determines the functionality of the upper limbs after a cortical injury by assessing the ability to manipulate objects of different sizes, weights, and shapes with both hands. It consists of 19 tests divided into four subscales: gross grasp, grasping, pinching, and gross movement. The ARAT takes between 5 and 15 min to administer. It is a reliable and valid measure to assess the motor function of a person who has suffered a stroke. It also allows an assessment of spontaneous recovery and can detect changes that, after intervention, are in the range of clinically significant values [17].
- Cognitive impairment: Montreal Cognitive Assessment (MoCA). A valid instrument for assessing mild cognitive dysfunction. It consists of the following domains: attention, concentration, executive functions (including the capacity for abstraction), memory, language, visoconstructive abilities, calculation, and orientation. The maximum score is 30. A score of ≥26 is considered normal. Administration time: 10 min [18].
- Loewenstein Occupational Therapy Cognitive Assessment (LOTCA). This is a standardised occupational-therapy-specific test for use with stroke patients. The LOTCA consists of 20 subtests and assesses four areas: orientation, visual and spatial perception, visual motor organisation, and thinking operations. This test suite is consistent, internally valid, and reliable for determining an initial profile of the cognitive and perceptual abilities of the brain-injured patient [19].
- Mental evocation capacity: Mental Evocation of Images, Movements and Activities Questionnaire (CEMIMA). The questionnaire consists of two subscales: one of evocation and the other of sensation. It measures the ability to mentally form visual and kinaesthetic images of the hand/upper limb and can also be used to assess the imagination process of the person being assessed. A study of its reliability and validity determined it as an instrument that allows measurements of the ability to mentally form visual and kinaesthetic images of the hand/upper limb and allows an understanding of the imagination process of the person evaluated [20].
- Perception of quality of life: Stroke Impact Scala 16 (SIS-16). This is a self-report comprising 16 items from four physical domains (strength, hand function, mobility, and ADL/instrumental activities) included in the SIS 3.0. This facilitates its application, as it can be applied in a shorter time, in around 5–10 min [21].
3.3. Electromyographic Equipment
3.4. Videos
4. Detailed Procedure
4.1. Experimental Conditions
4.2. Electromyographic Recording
- The ground electrode will be placed on the clavicle.
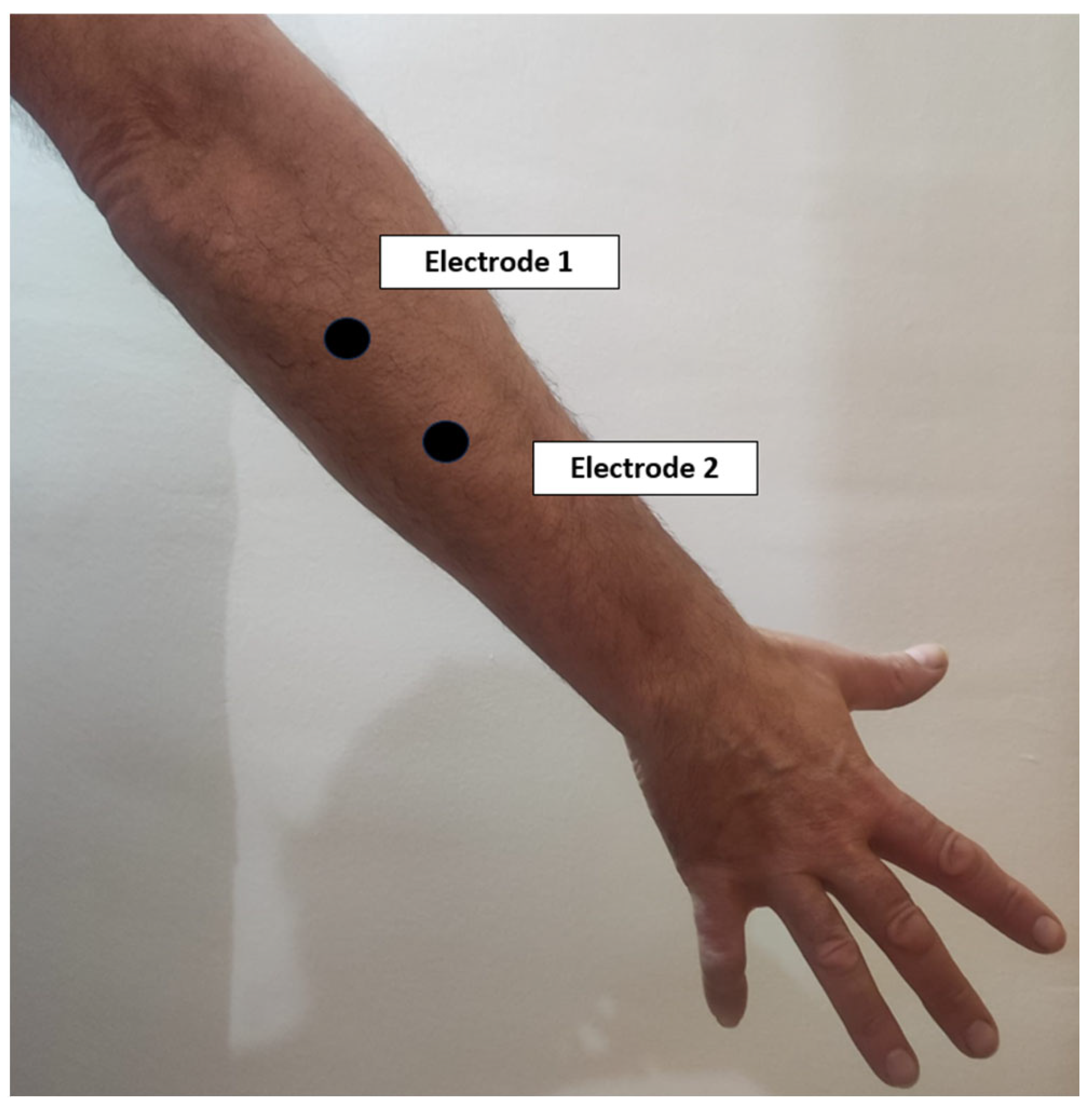
- The electrodes corresponding to the first channel will be placed on the common extensor digitorum on the posterior aspect of the forearm, with a distance between them of approximately 2 cm (Figure 3).
- The electrodes corresponding to the second channel will be placed on the common deep flexor digitorum on the anterior aspect of the forearm, with a distance between them of approximately 2 cm (Figure 4).
- The reference electrode will be placed over the biceps brachii.
4.3. Data Analysis
5. Expected Results
5.1. Strengths and Weaknesses of the Study
5.2. Contribution to Occupational Therapy and Physiotherapy
5.3. Ethics and Dissemination
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tadi, P.; Lui, F. Acute Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535369/ (accessed on 9 January 2024).
- Wafa, H.A.; Wolfe, C.D.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef]
- Truelsen, T.; Piechowski-Jóźwiak, B.; Bonita, R.; Mathers, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in Europe: A review of available data. Eur. J. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef]
- Quezada, M.Y.; Huete, A.; Bascones, L.M. Las Personas con Daño Cerebral Adquirido en España; FEDACE: Madrid, Spain, 2017; Available online: https://fedace.org/files/MSCFEDACE/2021-7/26-14-8-27.admin.Las_personas_con_Dao_Cerebral_en_Espaa.pdf (accessed on 9 January 2024).
- Cook, R.; Bird, G.; Catmur, C.; Press, C.; Heyes, C. Mirror neurons: From origin to function. Behav. Brain Sci. 2014, 37, 177–192. [Google Scholar] [CrossRef]
- Morone, G.; Spitoni, G.F.; De Bartolo, D.; Ghooshchy, S.G.; Di Iulio, F.; Paolucci, S.; Zoccolotti, P.; Iosa, M. Rehabilitative devices for a top-down approach. Expert Rev. Med. Devices 2019, 16, 187–195. [Google Scholar] [CrossRef]
- Silva, S.; Borges, L.R.; Santiago, L.; Lucena, L.; Lindquist, A.R.; Ribeiro, T. Motor imagery for gait rehabilitation after stroke. Cochrane Database Syst. Rev. 2020, 9, CD013019. [Google Scholar]
- Di Rienzo, F.; Debarnot, U.; Daligault, S.; Saruco, E.; Delpuech, C.; Doyon, J.; Collet, C.; Guillot, A. Online and Offline Performance Gains Following Motor Imagery Practice: A Comprehensive Review of Behavioral and Neuroimaging Studies. Front. Hum. Neurosci. 2016, 10, 315. [Google Scholar] [CrossRef]
- Hanakawa, T.; Immisch, I.; Toma, K.; Dimyan, M.A.; Van Gelderen, P.; Hallett, M. Functional Properties of Brain Areas Associated With Motor Execution and Imagery. J. Neurophysiol. 2003, 89, 989–1002. [Google Scholar] [CrossRef]
- Peng, T.-H.; Zhu, J.-D.; Chen, C.-C.; Tai, R.-Y.; Lee, C.-Y.; Hsieh, Y.-W. Action observation therapy for improving arm function, walking ability, and daily activity performance after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosa, J.J.; Natali, F.; Tettamanti, A.; Cursi, M.; Velikova, S.; Comi, G.; Gatti, R.; Leocani, L. Action observation and motor imagery in performance of complex movements: Evidence from EEG and kinematics analysis. Behav. Brain Res. 2015, 281, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Sabari, J.S. Motor learning concepts applied to activity-based intervention with adults with hemiplegia. Am. J. Occup. Ther. 1991, 45, 523–530. [Google Scholar] [CrossRef]
- Johnson, S.H. Imagining the impossible: Intact motor representations in hemiplegics. Neuroreport 2000, 11, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Belmonte, C.; Pamio, A.J.; Gómez-Martínez, M. Cuestionario de evocación mental de imágenes, movimientos y actividades (CEMIMA): Análisis de sus propiedades psicométricas. Cad. Bras. Ter. Ocup. 2022, 30, 3096. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Doussoulin, A.; Rivas, R.; Campos, V. Validation of “Action Research Arm Test” (ARAT) in Chilean patients with a paretic upper limb after a stroke. Rev. Med. Chile 2012, 140, 59–65. [Google Scholar] [PubMed]
- Montreal Cognitive Assessment. RehabMeasures Database. AbilityLab: Chicago, IL, USA, 2020. Available online: https://www.sralab.org/rehabilitation-measures/montreal-cognitive-assessment (accessed on 9 January 2024).
- Katz, N.; Itzkovich, M.; Averbuch, S.; Elazar, B. Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) battery for brain-injured patients: Reliability and validity. Am. J. Occup. Ther. 1989, 43, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zisa, N.S.; Rubio, C.; Gómez, M. Fiabilidad y validez del Cuestionario de evocación mental de imágenes, movimientos y actividades: Estudio piloto. Rehabilitación 2021, 55, 258–265. [Google Scholar] [CrossRef]
- Duncan, P.W.; Lai, S.M.; Bode, R.K.; Perera, S.; DeRosa, J. Stroke Impact Scale-16: A brief assessment of physical function. Neurology 2003, 60, 291–296. [Google Scholar] [CrossRef]
- Criswell, E. Cram’s Introduction to Surface Electromyography, 2nd ed.; Jones and Bartlett Publishers: Sudbury, MA, USA, 2011. [Google Scholar]
- Jarque-Bou, N.J.; Sancho-Bru, J.L.; Vergara, M. A Systematic Review of EMG Applications for the Characterization of Forearm and Hand Muscle Activity during Activities of Daily Living: Results, Challenges, and Open Issues. Sensors 2021, 21, 3035. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.P.; Eugène, F.; Michon, P.E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Gerardin, E.; Sirigu, A.; Lehéricy, S.; Poline, J.B.; Gaymard, B.; Marsault, C.; Agid, Y.; Le Bihan, D. Partially overlapping neural networks for real and imagined hand movements. Cereb. Cortex 2000, 10, 1093–1104. [Google Scholar] [CrossRef]
- Blickenstorfer, A.; Kleiser, R.; Keller, T.; Keisker, B.; Meyer, M.; Riener, R.; Kollias, S. Cortical and subcortical correlates of functional electrical stimulation of wrist extensor and flexor muscles revealed by fMRI. Hum. Brain Mapp. 2009, 30, 963–975. [Google Scholar] [CrossRef]
- Lorey, B.; Pilgramm, S.; Bischoff, M.; Stark, R.; Vaitl, D.; Kindermann, S.; Munzert, J.; Zentgraf, K. Activation of the parieto-premotor network is associated with vivid motor imagery—A parametric FMRI study. PLoS ONE 2011, 6, e20368. [Google Scholar] [CrossRef]
- Schlerf, J.E.; Verstynen, T.D.; Ivry, R.B.; Spencer, R.M.C. Evidence of a Novel Somatopic Map in the Human Neocerebellum During Complex Actions. J. Neurophysiol. 2010, 103, 3330–3336. [Google Scholar] [CrossRef]
- Lehéricy, S.; Benali, H.; Van de Moortele, P.F.; Pélégrini-Issac, M.; Waechter, T.; Ugurbil, K.; Doyon, J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc. Natl. Acad. Sci. USA 2005, 102, 12566–12571. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [CrossRef]
- Gazzola, V.; Keysers, C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cereb. Cortex 2009, 19, 1239–1255. [Google Scholar] [CrossRef]
- Saygin, A.P. Superior temporal and premotor brain areas necessary for biological motion perception. Brain 2007, 130, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Ortigue, S.; Sinigaglia, C.; Rizzolatti, G.; Grafton, S.T. Understanding actions of others: The electrodynamics of the left and right hemispheres. A high-density EEG neuroimaging study. PLoS ONE 2010, 5, e12160. [Google Scholar] [CrossRef] [PubMed]
- Caspers, S.; Zilles, K.; Laird, A.R.; Eickhoff, S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010, 50, 1148–1167. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Merino, B.; Grèzes, J.; Glaser, D.E.; Passingham, R.E.; Haggard, P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 2006, 16, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Rozand, V.; Lebon, F.; Stapley, P.J.; Papaxanthis, C.; Lepers, R. A prolonged motor imagery session alter imagined and actual movement durations: Potential implications for neurorehabilitation. Behav. Brain Res. 2016, 297, 67–75. [Google Scholar] [CrossRef]
- Romano Smith, S.; Wood, G.; Coyles, G.; Roberts, J.W.; Wakefield, C.J. The effect of action observation and motor imagery combinations on upper limb kinematics and EMG during dart-throwing. Scand. J. Med. Sci. Sports 2019, 29, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Bazzini, M.C.; Nuara, A.; Branchini, G.; De Marco, D.; Ferrari, L.; Lanini, M.C.; Paolini, S.; Scalona, E.; Avanzini, P.; Fabbri-Destro, M. The capacity of action observation to drag the trainees’ motor pattern toward the observed model. Sci. Rep. 2023, 13, 9107. [Google Scholar] [CrossRef] [PubMed]



| Baseline Variables | Primary Variables |
|---|---|
| Date of birth Gender Marital Status Level of Education Economic Level Affected Side Dominant Side Type of Injury Time Since Injury Functionally of upper limbs Cognitive impairment | Degree of disability and dependence Cognitive and perceptual impairment Mental evocation capacity Perception of quality of life Surface electromyography |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Martín, S.; Calvo-Vera, A.B.; Bermejo-Gil, B.M.; Martín-Nogueras, A.M. Upper Limb Electromyographic Responses to Motor Imagery and Action Observation in Acquired Brain Injury. Sensors 2024, 24, 1802. https://doi.org/10.3390/s24061802
Santiago-Martín S, Calvo-Vera AB, Bermejo-Gil BM, Martín-Nogueras AM. Upper Limb Electromyographic Responses to Motor Imagery and Action Observation in Acquired Brain Injury. Sensors. 2024; 24(6):1802. https://doi.org/10.3390/s24061802
Chicago/Turabian StyleSantiago-Martín, Sara, Ana Belén Calvo-Vera, Beatriz María Bermejo-Gil, and Ana María Martín-Nogueras. 2024. "Upper Limb Electromyographic Responses to Motor Imagery and Action Observation in Acquired Brain Injury" Sensors 24, no. 6: 1802. https://doi.org/10.3390/s24061802
APA StyleSantiago-Martín, S., Calvo-Vera, A. B., Bermejo-Gil, B. M., & Martín-Nogueras, A. M. (2024). Upper Limb Electromyographic Responses to Motor Imagery and Action Observation in Acquired Brain Injury. Sensors, 24(6), 1802. https://doi.org/10.3390/s24061802







