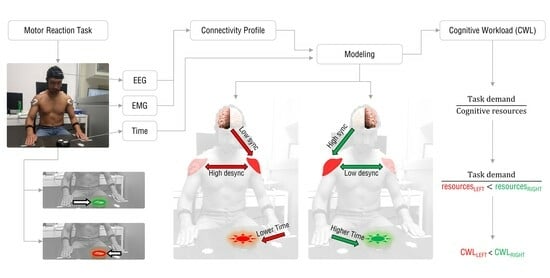
Assessing Cognitive Workload in Motor Decision-Making through Functional Connectivity Analysis: Towards Early Detection and Monitoring of Neurodegenerative Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Instrumentation
2.4. Signal Pre-Processing
2.5. Motor Planning Phase Identification
2.6. Functional Connectivity Computation
2.7. Statistical Procedure for Modeling and Testing
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | connectivity change |
| CMC | corticomuscular coherence |
| CWL | cognitive workload |
| EEG | electroencephalography |
| EMG | electromyography |
| ICA | Independent Component Analysis |
| IMC | intermuscular coherence |
| MDM | motor decision-making |
| ND | neurodegenerative disease |
| PLAN | motor planning phase |
| PMA | premotor area |
| RT | reaction time |
| SMA | supplementary motor area |
References
- McMackin, R.; Muthuraman, M.; Groppa, S.; Babiloni, C.; Taylor, J.P.; Kiernan, M.C.; Nasseroleslami, B.; Hardiman, O. Measuring network disruption in neurodegenerative diseases: New approaches using signal analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Teruya, P.Y.; Farfán, F.D.; Pizá, Á.G.; Soletta, J.H.; Lucianna, F.A.; Albarracín, A.L. Quantifying muscle alterations in a Parkinson’s disease animal model using electromyographic biomarkers. Med. Biol. Eng. Comput. 2021, 59, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Gratwicke, J.; Jahanshahi, M.; Foltynie, T. Parkinson’s disease dementia: A neural networks perspective. Brain 2015, 138, 1454–1476. [Google Scholar] [CrossRef] [PubMed]
- Nasseroleslami, B.; Dukic, S.; Broderick, M.; Mohr, K.; Schuster, C.; Gavin, B.; McLaughlin, R.; Heverin, M.; Vajda, A.; Iyer, P.M.; et al. Characteristic Increases in EEG Connectivity Correlate With Changes of Structural MRI in Amyotrophic Lateral Sclerosis. Cereb. Cortex 2017, 29, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Omer, T.; Finegan, E.; Chipika, R.H.; Iyer, P.M.; Doherty, M.A.; Vajda, A.; Pender, N.; McLaughlin, R.L.; Hutchinson, S.; et al. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: A multimodal neuroimaging study. Brain Imaging Behav. 2018, 12, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Wooten, G.F. Diagnosis and Initial Management of Parkinson’s Disease. N. Engl. J. Med. 2005, 353, 1021–1027. [Google Scholar] [CrossRef]
- Benbrika, S.; Desgranges, B.; Eustache, F.; Viader, F. Cognitive, Emotional and Psychological Manifestations in Amyotrophic Lateral Sclerosis at Baseline and Overtime: A Review. Front. Neurosci. 2019, 13, 951. [Google Scholar] [CrossRef]
- Herzog–Krzywoszanska, R.; Krzywoszanski, L. Sleep Disorders in Huntington’s Disease. Front. Psychiatry 2019, 10, 221. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Meigal, A.Y.; Rissanen, S.M.; Zaripova, Y.R.; Miroshnichenko, G.G.; Karjalainen, P. Nonlinear parameters of surface electromyogram for diagnostics of neuromuscular disorders and normal conditions of the human motor system. Hum. Physiol. 2015, 41, 672–679. [Google Scholar] [CrossRef]
- Hancock, P.A.; Meshkati, N. Human Mental Workload; North-Holland: Oxford, UK, 1988; Volume 382, p. Xvi. [Google Scholar]
- Kantowitz, B.H. Attention and Mental Workload. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2000, 44, 3-456–3-459. [Google Scholar] [CrossRef]
- Brouwer, A.M.; Hogervorst, M.A.; Van Erp, J.B.F.; Heffelaar, T.; Zimmerman, P.H.; Oostenveld, R. Estimating workload using EEG spectral power and ERPs in the n-back task. J. Neural Eng. 2012, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, J. Measurement of Cognitive Load in HCI Systems Using EEG Power Spectrum: An Experimental Study. Procedia Comput. Sci. 2016, 84, 70–78. [Google Scholar] [CrossRef]
- Plechawska-Wójcik, M.; Tokovarov, M.; Kaczorowska, M.; Zapała, D. A Three-Class Classification of Cognitive Workload Based on EEG Spectral Data. Appl. Sci. 2019, 9, 5340. [Google Scholar] [CrossRef]
- Tremmel, C.; Herff, C.; Sato, T.; Rechowicz, K.; Yamani, Y.; Krusienski, D.J. Estimating Cognitive Workload in an Interactive Virtual Reality Environment Using EEG. Front. Hum. Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, G.N.; Kakkos, I.; Dai, Z.; Lim, J.; de Souza, J.J.; Bezerianos, A.; Sun, Y. Task-Independent Mental Workload Classification Based Upon Common Multiband EEG Cortical Connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Taori, T.J.; Ladekar, M.Y.; Manthalkar, R.R.; Gajre, S.S.; Joshi, Y.V. Classification of cross task cognitive workload using deep recurrent network with modelling of temporal dynamics. Biomed. Signal Process. Control 2021, 70, 103070. [Google Scholar] [CrossRef]
- Taori, T.J.; Gupta, S.S.; Gajre, S.S.; Manthalkar, R.R. Cognitive workload classification: Towards generalization through innovative pipeline interface using HMM. Biomed. Signal Process. Control 2022, 78, 104010. [Google Scholar] [CrossRef]
- Chikhi, S.; Matton, N.; Blanchet, S. EEG power spectral measures of cognitive workload: A meta-analysis. Psychophysiology 2022, 59, e14009. [Google Scholar] [CrossRef]
- Wong, A.L.; Haith, A.M.; Krakauer, J.W. Motor Planning. Neuroscientist 2015, 21, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P.; Kalaska, J.F. Neural Mechanisms for Interacting with a World Full of Action Choices. Annu. Rev. Neurosci. 2010, 33, 269–298. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.P.; Vaz, J.R.; Domingos, C.; Freitas, S.R. From thinking fast to moving fast: Motor control of fast limb movements in healthy individuals. Rev. Neurosci. 2022, 33, 919–950. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.N.; Chen, C.Y.; Muggleton, N.G. Sport, time pressure, and cognitive performance. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 234, pp. 85–99. [Google Scholar] [CrossRef]
- Nuri, L.; Shadmehr, A.; Ghotbi, N.; Attarbashi Moghadam, B. Reaction time and anticipatory skill of athletes in open and closed skill-dominated sport. Eur. J. Sport Sci. 2013, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Vaeyens, R.; Lenoir, M.; Williams, A.M.; Philippaerts, R.M. Mechanisms Underpinning Successful Decision Making in Skilled Youth Soccer Players: An Analysis of Visual Search Behaviors. J. Mot. Behav. 2007, 39, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Cano, L.; Pizá, A.; Fernández, E.; Farfán, F. Motor planning efficiency based on reaction time. A measure for cognitive demand. Rev. Argent. Bioingeniería 2022, in press. [Google Scholar]
- Boonstra, T.W. The potential of corticomuscular and intermuscular coherence for research on human motor control. Front. Hum. Neurosci. 2013, 7, 855. [Google Scholar] [CrossRef]
- Bigot, J.; Longcamp, M.; Dal Maso, F.; Amarantini, D. A new statistical test based on the wavelet cross-spectrum to detect time–frequency dependence between non-stationary signals: Application to the analysis of cortico-muscular interactions. NeuroImage 2011, 55, 1504–1518. [Google Scholar] [CrossRef]
- Dal Maso, F.; Longcamp, M.; Cremoux, S.; Amarantini, D. Effect of training status on beta-range corticomuscular coherence in agonist vs. antagonist muscles during isometric knee contractions. Exp. Brain Res. 2017, 235, 3023–3031. [Google Scholar] [CrossRef]
- Desmyttere, G.; Mathieu, E.; Begon, M.; Simoneau-Buessinger, E.; Cremoux, S. Effect of the phase of force production on corticomuscular coherence with agonist and antagonist muscles. Eur. J. Neurosci. 2018, 48, 3288–3298. [Google Scholar] [CrossRef]
- Elie, D.; Barbier, F.; Ido, G.; Cremoux, S. Corticomuscular Coherence and Motor Control Adaptations after Isometric Maximal Strength Training. Brain Sci. 2021, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Fauvet, M.; Gasq, D.; Chalard, A.; Tisseyre, J.; Amarantini, D. Temporal Dynamics of Corticomuscular Coherence Reflects Alteration of the Central Mechanisms of Neural Motor Control in Post-Stroke Patients. Front. Hum. Neurosci. 2021, 15, 682080. [Google Scholar] [CrossRef] [PubMed]
- Glories, D.; Soulhol, M.; Amarantini, D.; Duclay, J. Specific modulation of corticomuscular coherence during submaximal voluntary isometric, shortening and lengthening contractions. Sci. Rep. 2021, 11, 6322. [Google Scholar] [CrossRef] [PubMed]
- Kenville, R.; Maudrich, T.; Vidaurre, C.; Maudrich, D.; Villringer, A.; Nikulin, V.V.; Ragert, P. Corticomuscular interactions during different movement periods in a multi-joint compound movement. Sci. Rep. 2020, 10, 5021. [Google Scholar] [CrossRef] [PubMed]
- Tisseyre, J.; Cremoux, S.; Amarantini, D.; Tallet, J. Increased intensity of unintended mirror muscle contractions after cervical spinal cord injury is associated with changes in interhemispheric and corticomuscular coherences. Behav. Brain Res. 2022, 417, 113563. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, Y.; Liu, H. Corticomuscular Coherence and Its Applications: A Review. Front. Hum. Neurosci. 2019, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Sanuki, F.; Iramina, K. Electroencephalogram-Electromyogram Functional Coupling and Delay Time Change Based on Motor Task Performance. Sensors 2021, 21, 4380. [Google Scholar] [CrossRef]
- Dum, R.; Strick, P. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 1991, 11, 667–689. [Google Scholar] [CrossRef]
- Graziano, M.S.; Aflalo, T.N. Mapping Behavioral Repertoire onto the Cortex. Neuron 2007, 56, 239–251. [Google Scholar] [CrossRef]
- He, S.; Dum, R.; Strick, P. Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the medial surface of the hemisphere. J. Neurosci. 1995, 15, 3284–3306. [Google Scholar] [CrossRef]
- Lemon, R.N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Xu, D.; Dong, M.; Chen, Y.; Delgado, A.M.; Hughes, N.C.; Zhang, L.; O’Connor, D.H. Cortical processing of flexible and context-dependent sensorimotor sequences. Nature 2022, 603, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Aikio, R.; Laaksonen, K.; Sairanen, V.; Parkkonen, E.; Abou Elseoud, A.; Kujala, J.; Forss, N. CMC is more than a measure of corticospinal tract integrity in acute stroke patients. NeuroImage Clin. 2021, 32, 102818. [Google Scholar] [CrossRef] [PubMed]
- Von Carlowitz-Ghori, K.; Bayraktaroglu, Z.; Hohlefeld, F.U.; Losch, F.; Curio, G.; Nikulin, V.V. Corticomuscular coherence in acute and chronic stroke. Clin. Neurophysiol. 2014, 125, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Cremoux, S.; Tallet, J.; Dal Maso, F.; Berton, E.; Amarantini, D. Impaired corticomuscular coherence during isometric elbow flexion contractions in humans with cervical spinal cord injury. Eur. J. Neurosci. 2017, 46, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Klug, M.; Gramann, K. Identifying key factors for improving ICA-based decomposition of EEG data in mobile and stationary experiments. Eur. J. Neurosci. 2021, 54, 8406–8420. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Cohen, P.; West, S.; Aiken, L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed.; Routledge: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Johnson-Frey, S.H.; Newman-Norlund, R.; Grafton, S.T. A Distributed Left Hemisphere Network Active During Planning of Everyday Tool Use Skills. Cereb. Cortex 2005, 15, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Mancini, C.; Mirabella, G. Handedness Does Not Impact Inhibitory Control, but Movement Execution and Reactive Inhibition Are More under a Left-Hemisphere Control. Symmetry 2021, 13, 1602. [Google Scholar] [CrossRef]
- Vingerhoets, G.; Acke, F.; Alderweireldt, A.S.; Nys, J.; Vandemaele, P.; Achten, E. Cerebral lateralization of praxis in right- and left-handedness: Same pattern, different strength. Hum. Brain Mapp. 2012, 33, 763–777. [Google Scholar] [CrossRef]






| Right Hand | Left Hand | |||||
|---|---|---|---|---|---|---|
| Subject | (s) | (-) | (-) | (s) | (-) | (-) |
| P1 | 0.298 | −0.186 | 0.451 | 0.244 | −0.424 | −0.402 |
| P2 | 0.225 | 1.057 | 0.739 | 0.198 | 1.215 | 1.266 |
| P3 | 0.271 | −0.005 | 0.145 | 0.188 | −0.132 | 0.023 |
| P5 | 0.257 | −0.317 | 0.969 | 0.154 | 0.057 | 0.315 |
| P6 | 0.348 | −0.668 | −0.184 | 0.229 | −0.508 | −0.171 |
| P7 | 0.148 | 0.019 | −0.318 | 0.213 | −0.132 | 0.022 |
| P8 | 0.304 | −0.387 | −0.225 | 0.189 | 0.623 | 0.919 |
| P9 | 0.245 | 0.167 | −0.079 | 0.260 | −0.570 | 0.155 |
| P10 | 0.236 | 0.106 | 0.285 | 0.251 | −0.052 | 0.054 |
| P11 | 0.240 | −0.628 | 0.330 | 0.165 | −0.249 | 0.134 |
| P13 | 0.319 | 0.168 | 0.040 | 0.302 | −0.585 | −0.061 |
| P14 | 0.231 | −0.070 | 0.213 | 0.347 | 0.653 | 0.957 |
| P15 | 0.372 | 0.523 | −0.078 | 0.278 | −0.660 | −0.061 |
| P16 | 0.316 | −0.174 | −0.252 | 0.308 | −0.662 | −0.147 |
| P17 | 0.224 | −0.030 | 1.069 | 0.199 | 0.370 | −0.047 |
| P18 | 0.273 | 1.255 | 0.192 | 0.192 | −0.154 | −0.001 |
| P19 | 0.266 | 1.919 | −0.032 | 0.183 | 1.174 | 0.201 |
| Mean | 0.269 | - | - | 0.229 | - | - |
| Median | - | −0.005 | 0.145 | - | −0.132 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, L.A.; Albarracín, A.L.; Pizá, A.G.; García-Cena, C.E.; Fernández-Jover, E.; Farfán, F.D. Assessing Cognitive Workload in Motor Decision-Making through Functional Connectivity Analysis: Towards Early Detection and Monitoring of Neurodegenerative Diseases. Sensors 2024, 24, 1089. https://doi.org/10.3390/s24041089
Cano LA, Albarracín AL, Pizá AG, García-Cena CE, Fernández-Jover E, Farfán FD. Assessing Cognitive Workload in Motor Decision-Making through Functional Connectivity Analysis: Towards Early Detection and Monitoring of Neurodegenerative Diseases. Sensors. 2024; 24(4):1089. https://doi.org/10.3390/s24041089
Chicago/Turabian StyleCano, Leonardo Ariel, Ana Lía Albarracín, Alvaro Gabriel Pizá, Cecilia Elisabet García-Cena, Eduardo Fernández-Jover, and Fernando Daniel Farfán. 2024. "Assessing Cognitive Workload in Motor Decision-Making through Functional Connectivity Analysis: Towards Early Detection and Monitoring of Neurodegenerative Diseases" Sensors 24, no. 4: 1089. https://doi.org/10.3390/s24041089
APA StyleCano, L. A., Albarracín, A. L., Pizá, A. G., García-Cena, C. E., Fernández-Jover, E., & Farfán, F. D. (2024). Assessing Cognitive Workload in Motor Decision-Making through Functional Connectivity Analysis: Towards Early Detection and Monitoring of Neurodegenerative Diseases. Sensors, 24(4), 1089. https://doi.org/10.3390/s24041089