Optimized Synthetic Correlated Diffusion Imaging for Improving Breast Cancer Tumor Delineation
Abstract
1. Introduction
2. Related Works
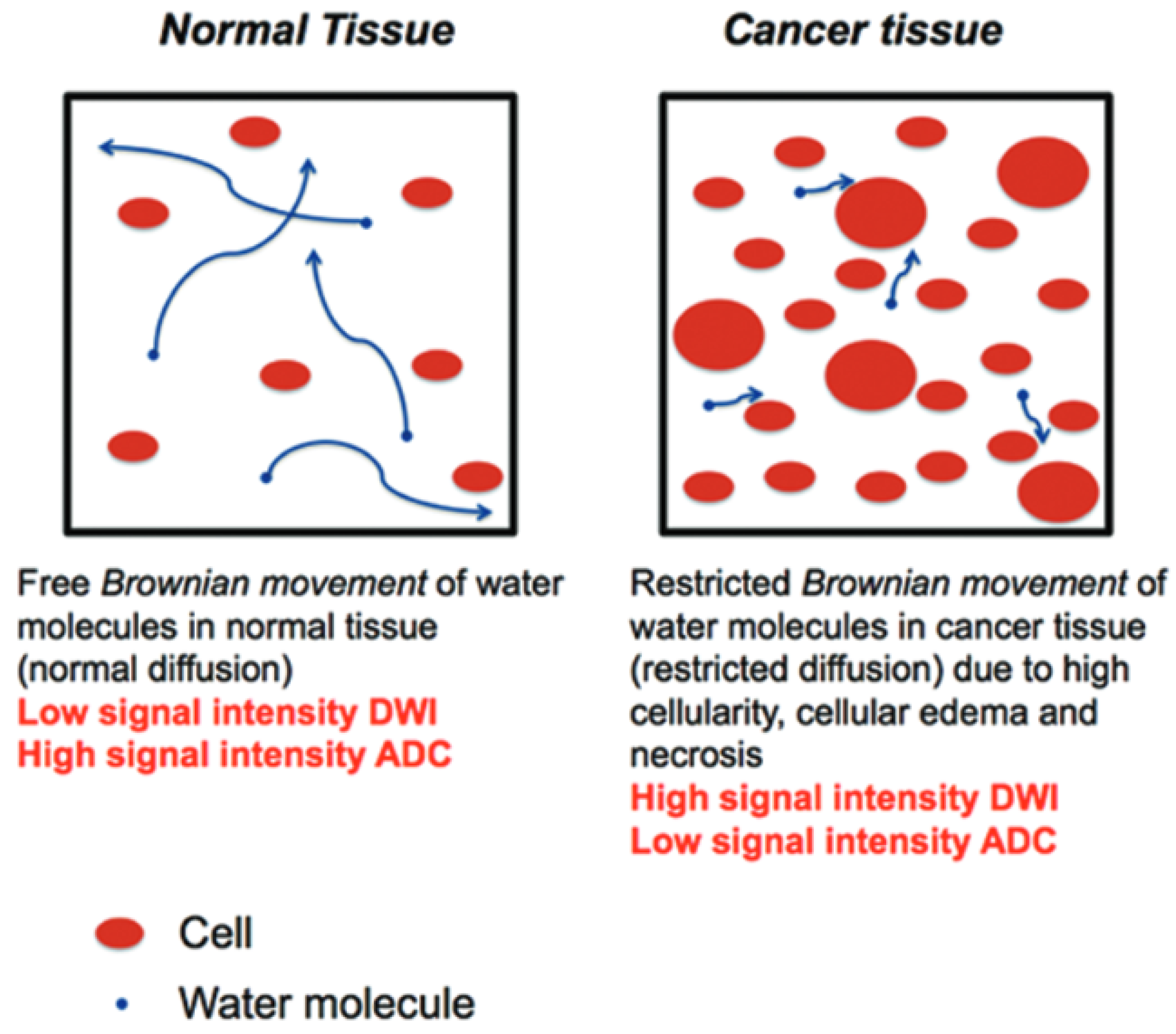
2.1. Diffusion-Weighted Imaging (DWI)
2.2. Apparent Diffusion Coefficient (ADC)
2.3. Synthetic Correlated Diffusion Imaging (CDIs)
2.4. Challenges for Harnessing CDIs for Breast Cancer
2.5. Comparison of CDIs to Positron Emission Tomography (PET) Imaging
3. Materials and Methods
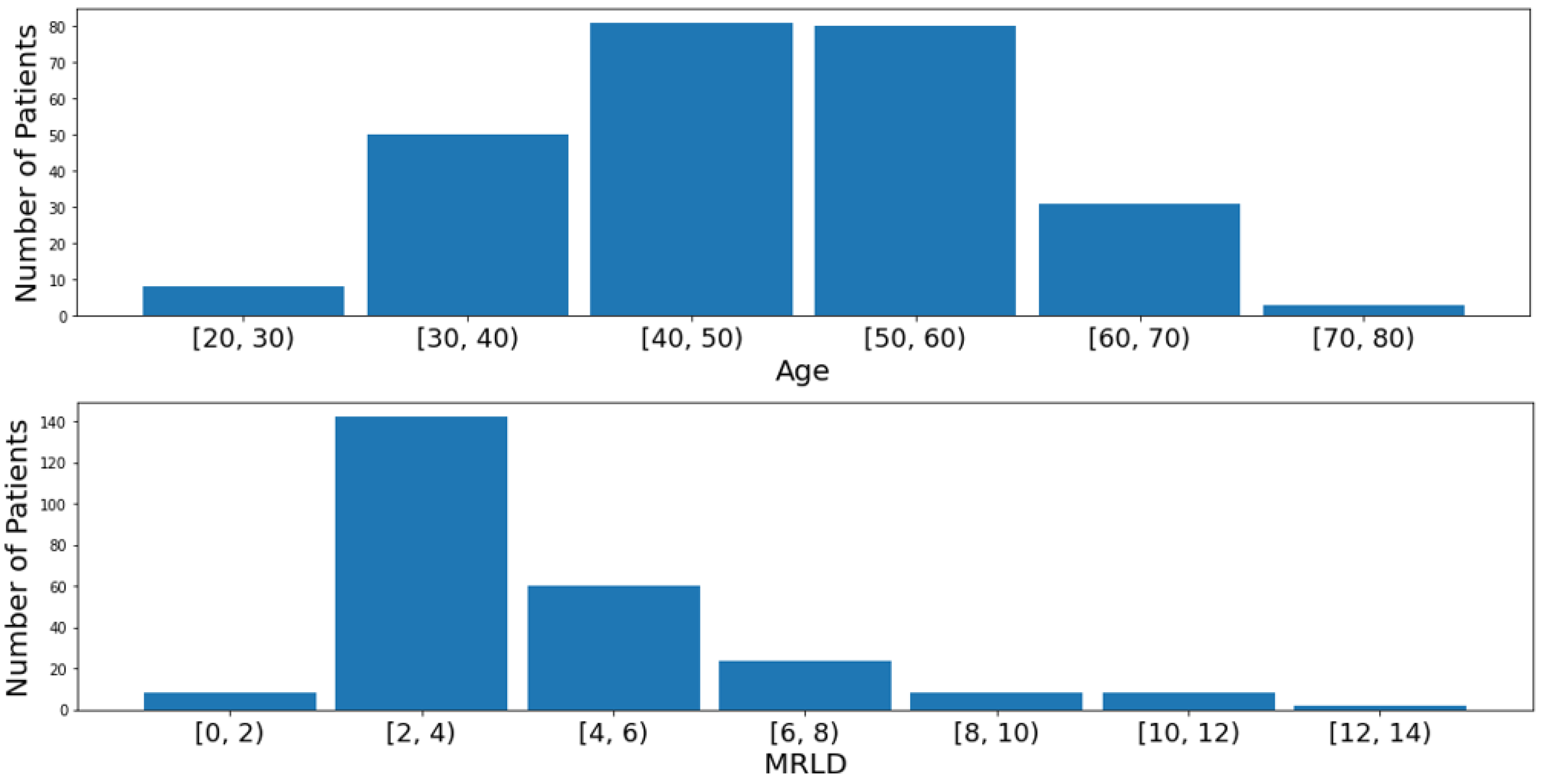
3.1. Dataset
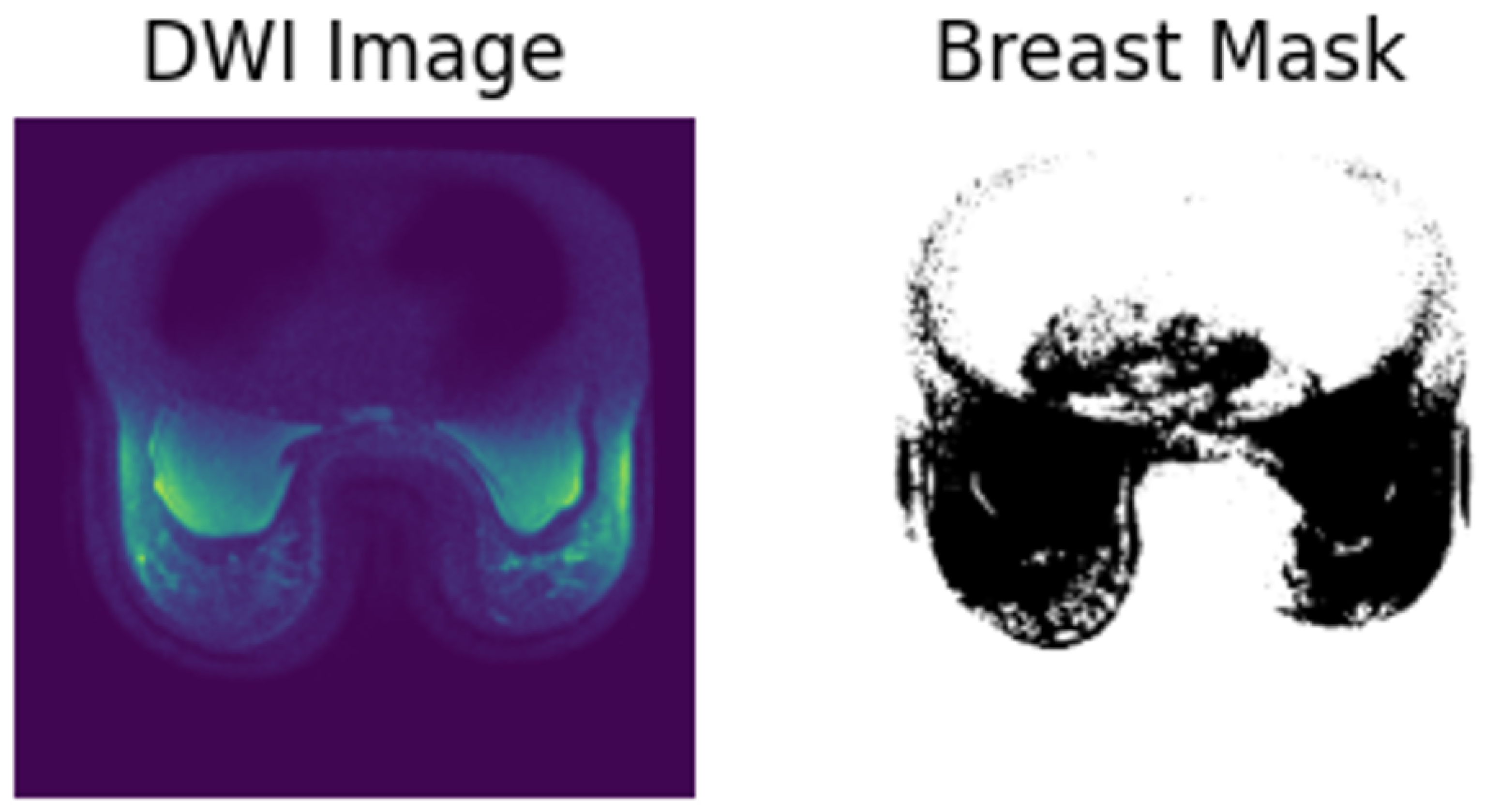
3.2. Data Preprocessing
3.3. Experimental Setup
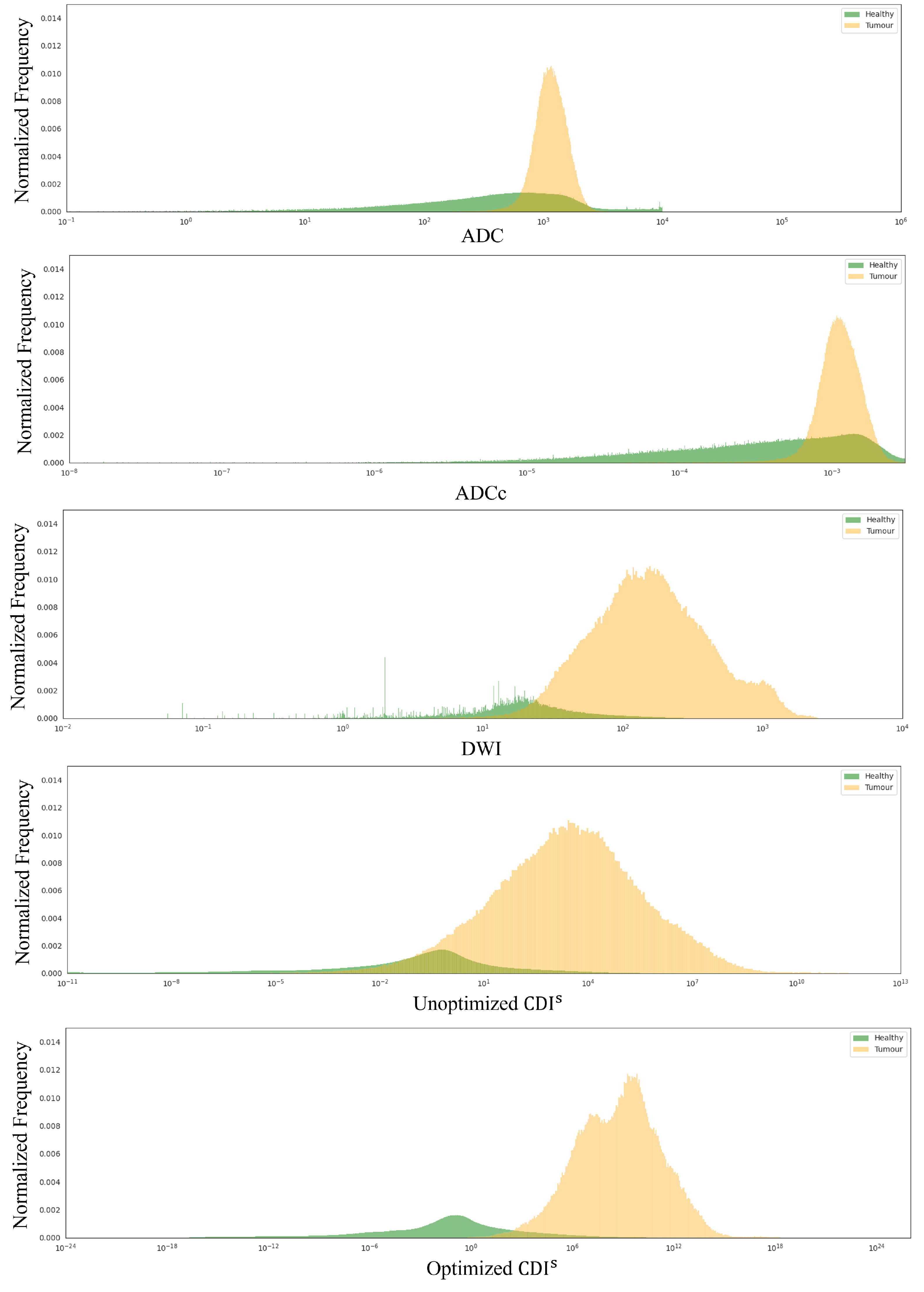
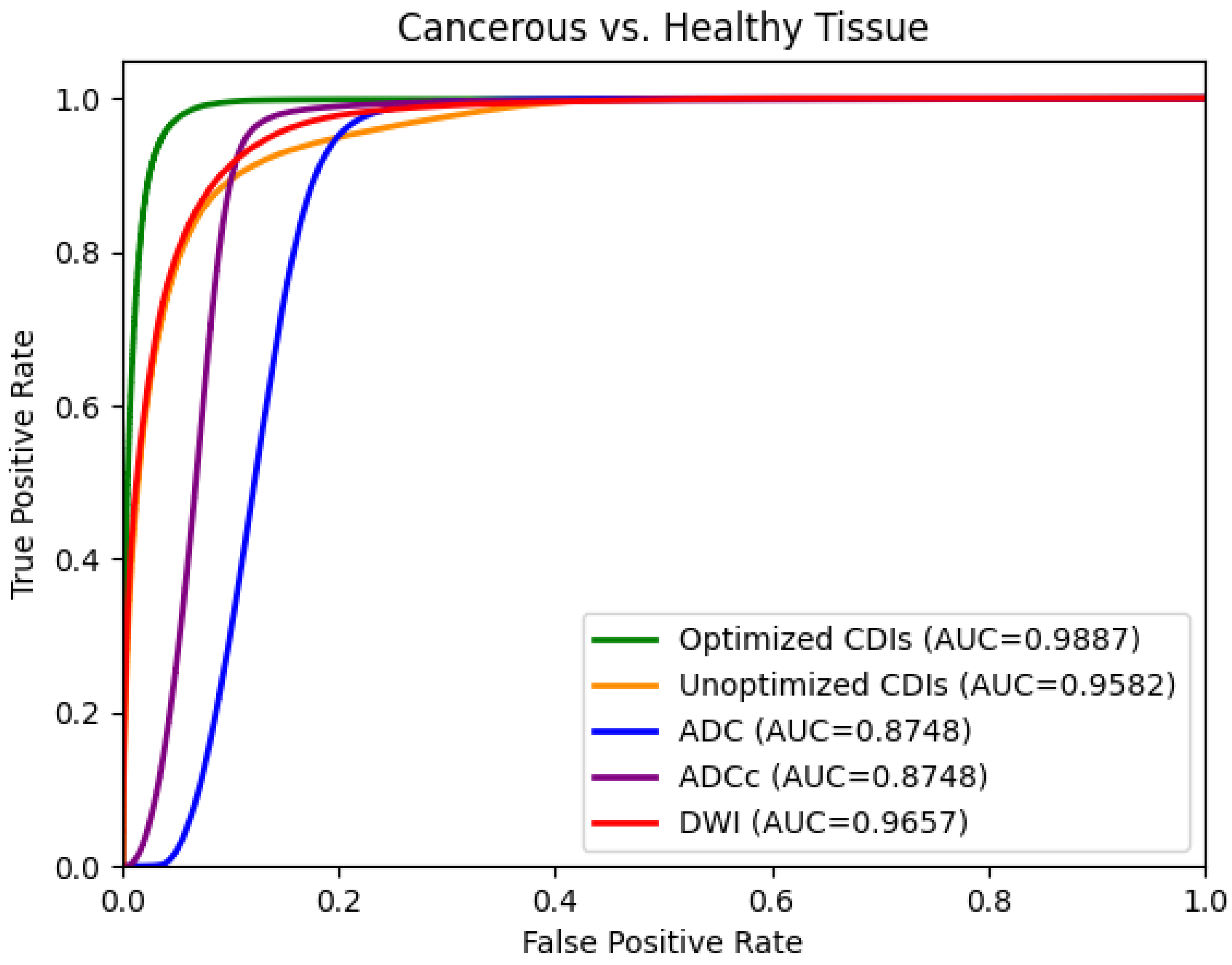
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACRIN | American College of Radiology Imaging Network |
| ADC | apparent diffusion coefficient |
| CDIs | synthetic correlated diffusion imaging |
| CT | computed tomography |
| DCE | dynamic contrast-enhanced imaging |
| DWI | diffusion-weighted imaging |
| MRI | magnetic resonance imaging |
References
- Canadian Cancer Society. Breast Cancer Statistics. 2024. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/statistics (accessed on 6 March 2024).
- Akakuru, O.U.; Zhang, Z.; Iqbal, M.Z.; Zhu, C.; Zhang, Y.; Wu, A. Chemotherapeutic nanomaterials in tumor boundary delineation: Prospects for effective tumor treatment. Acta Pharm. Sin. B 2022, 12, 2640–2657. [Google Scholar] [CrossRef]
- Mahmoud, A.; El-Sharkawy, Y.H. Delineation and detection of breast cancer using novel label-free fluorescence. BMC Med. Imaging 2023, 23, 132. [Google Scholar] [CrossRef]
- Wong, A.; Gunraj, H.; Sivan, V.; Haider, M.A. Synthetic correlated diffusion imaging hyperintensity delineates clinically significant prostate cancer. Sci. Rep. 2022, 12, 3376. [Google Scholar] [CrossRef]
- Westbrook, C.; Talbot, J. MRI in Practice; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Hashemi, R.H.; Bradley, W.G.; Lisanti, C.J. MRI: The Basics: The Basics; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Katti, G.; Ara, S.A.; Shireen, A. Magnetic resonance imaging (MRI)—A review. Int. J. Dent. Clin. 2011, 3, 65–70. [Google Scholar]
- Qayyum, A. Diffusion-weighted imaging in the abdomen and pelvis: Concepts and applications. Radiographics 2009, 29, 1797–1810. [Google Scholar] [CrossRef]
- ScienceDirect Topics. Diffusion Weighted Imaging—An Overview. 2022. Available online: https://www.sciencedirect.com/topics/nursing-and-health-professions/diffusion-weighted-imaging (accessed on 15 July 2024).
- Maas, L.C.; Mukherjee, P. Diffusion MRI: Overview and clinical applications in neuroradiology. Appl. Radiol. 2005, 34, 44–60. [Google Scholar] [CrossRef]
- Le Bihan, D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003, 4, 469–480. [Google Scholar] [CrossRef]
- Bammer, R. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 2003, 45, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Henzler, T.; Goldstraw, P.; Wenz, F.; Pirker, R.; Weder, W.; Apfaltrer, P.; Meyer, M.; Buesing, K.; Crino, L.; Fennell, D.; et al. Perspectives of novel imaging techniques for staging, therapy response assessment, and monitoring of surveillance in lung cancer: Summary of the Dresden 2013 Post WCLC-IASLC State-of-the-Art Imaging Workshop. J. Thorac. Oncol. 2015, 10, 237–249. [Google Scholar]
- Wong, A.; Glaister, J.; Cameron, A.; Haider, M. Correlated diffusion imaging. BMC Med. Imaging 2013, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Unterrainer, M.; Eze, C.; Ilhan, H.; Marschner, S.; Roengvoraphoj, O.; Schmidt-Hegemann, N.S.; Walter, F.; Kunz, W.G.; Rosenschöld, P.M.A.; Jeraj, R.; et al. Recent advances of PET imaging in clinical radiation oncology. Radiat. Oncol. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.J.; Kinahan, P. Positron emission tomography: Current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annu. Rev. Biomed. Eng. 2015, 17, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, F.L.; Dehdashti, F.; Siegel, B.A. PET in breast cancer. In Proceedings of the Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 1998; Volume 28, pp. 290–302. [Google Scholar]
- Hadebe, B.; Harry, L.; Ebrahim, T.; Pillay, V.; Vorster, M. The role of PET/CT in breast cancer. Diagnostics 2023, 13, 597. [Google Scholar] [CrossRef]
- Baptist Health. PET Scan vs. MRI: What’s the Difference. 2021. Available online: https://www.baptisthealth.com/blog/health-and-wellness/pet-scan-vs-mri-what-s-the-difference (accessed on 8 December 2024).
- Partridge, S.C.; Zhang, Z.; Newitt, D.C.; Gibbs, J.E.; Chenevert, T.L.; Rosen, M.A.; Bolan, P.J.; Marques, H.S.; Romanoff, J.; Cimino, L.; et al. Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial. Radiology 2018, 289, 618–627. [Google Scholar] [CrossRef]
- Newitt, D.C.; Zhang, Z.; Gibbs, J.E.; Partridge, S.C.; Chenevert, T.L.; Rosen, M.A.; Bolan, P.J.; Marques, H.S.; Aliu, S.; Li, W.; et al. Test-retest repeatability and reproducibility of ADC measures by breast DWI: Results from the ACRIN 6698 trial. J. Magn. Reson. Imaging 2018, 49, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Newitt, D.C.; Partridge, S.C.; Zhang, Z.; Gibbs, J.; Chenevert, T.; Rosen, M.; Bolan, P.; Marques, H.; Romanoff, J.; Cimino, L.; et al. ACRIN 6698/I-SPY2 Breast DWI [Data set]. Cancer Imaging Arch. 2021. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.e.A.; Hodzic, N.; Flanagan, N.; Gunraj, H.; Wong, A. Cancer-net bca: Breast cancer pathologic complete response prediction using volumetric deep radiomic features from synthetic correlated diffusion imaging. arXiv 2022, arXiv:2211.05308. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Nachar, N. The Mann-Whitney U: A test for assessing whether two independent samples come from the same distribution. Tutorials Quant. Methods Psychol. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Cruz-Roa, A.; Gilmore, H.; Basavanhally, A.; Feldman, M.; Ganesan, S.; Shih, N.N.; Tomaszewski, J.; González, F.A.; Madabhushi, A. Accurate and reproducible invasive breast cancer detection in whole-slide images: A Deep Learning approach for quantifying tumor extent. Sci. Rep. 2017, 7, 46450. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]



| Parameter | T2w | DWI |
|---|---|---|
| Sequence type | FSE or STIR | DW SE-EPI |
| 2D or 3D sequence | 2D | 2D |
| Slice orientation | Axial or sagittal | Axial |
| Laterality | Bilateral | Bilateral |
| Freqency direction | A/P | A/P |
| Phase direction | R/L (axial) | R/L |
| S/I (sagittal) | ||
| FOV–frequency | 260–360 mm (axial) | 260–360 |
| 180–220 mm (sagittal) | ||
| FOV–phase | 300–360 mm (axial) | 300–360 |
| 180–220 mm (sagittal) | ||
| Matrix–frequency (acquired) | 256–512 | 128–192 |
| Matrix–phase (acquired) | ≥256 | 128–192 |
| Reconstruction Matrix | 512 × 512 | 256 × 256 |
| In-plane resolution | ≤1.4 mm | 1.7–2.8 mm |
| Fat-suppression | Active fat-sat | Active fat-sat |
| recommended | ||
| TR | 2000–10,000 ms | ≥4000 ms |
| TE | 70–140 ms | Minimum |
| (STIR 70 ms) | (50–100 ms) | |
| Echo Train Length | ≤16 | N/A |
| TI (STIR sequence) | 170 ms (1.5T) | N/A |
| 230 ms (3.0T) | ||
| Flip Angle | 90 degrees | 90 degrees |
| Readout Bandwidth (per pixel) | N/A | N/A |
| b values | N/A | 0, 100, 600, 800 s/ |
| Slice thickness (acquired) | ≤4 mm | 4–5 mm |
| # of slices | Variable; complete | Variable; bilateral |
| bilateral coverage | coverage; adjust to keep | |
| w/in single acquisition | ||
| Slice Gap | ≤1.0 mm | No gap |
| Parallel imaging factor | ≤2 | ≥2 |
| # of excitations/averages | ≤2 | ≥2 |
| k-space ordering | N/A | N/A |
| Sequence | ≤7 min | 4–6 min |
| acquisition time | (multi-b seq ~5 min) | |
| Total post-contrast | N/A | N/A |
| imaging duration |
| Race | Percentage |
|---|---|
| White | 70.8% |
| Black | 10.7% |
| Asian | 6.3% |
| Unknown | 11.1% |
| Multiple Races | 0.4% |
| Native Hawaiian or other Pacific Islander | 0.4% |
| American Indian or Alaska Native | 0.4% |
| Config. | Optimized Values | |||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 1000 | 2000 | 3000 | 4000 | 5000 | 6000 | 7000 | |
| Initial | 1.616 | 1.521 | 1.201 | 0.836 | 1.163 | 0.867 | 1.142 | −0.464 |
| Optimized | 3.517 | 3.122 | 1.740 | 1.00 | 0.607 | 0.804 | −1.124 | −0.655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, C.-e.A.; Wong, A. Optimized Synthetic Correlated Diffusion Imaging for Improving Breast Cancer Tumor Delineation. Sensors 2024, 24, 8173. https://doi.org/10.3390/s24248173
Tai C-eA, Wong A. Optimized Synthetic Correlated Diffusion Imaging for Improving Breast Cancer Tumor Delineation. Sensors. 2024; 24(24):8173. https://doi.org/10.3390/s24248173
Chicago/Turabian StyleTai, Chi-en Amy, and Alexander Wong. 2024. "Optimized Synthetic Correlated Diffusion Imaging for Improving Breast Cancer Tumor Delineation" Sensors 24, no. 24: 8173. https://doi.org/10.3390/s24248173
APA StyleTai, C.-e. A., & Wong, A. (2024). Optimized Synthetic Correlated Diffusion Imaging for Improving Breast Cancer Tumor Delineation. Sensors, 24(24), 8173. https://doi.org/10.3390/s24248173






