In Medio Stat Virtus: Moderate Cognitive Flexibility as a Key to Affective Flexibility Responses in Long-Term HRV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion Criteria
2.3. Procedure
- -
- Cognitive Flexibility Inventory—CFI [20,47]: This consists of 19 items that form two different subclasses, which are Alternatives (12 items) and Control (7 items), which are rated on a 7-point Likert scale (1 = strongly disagree, 7 = strongly agree). The “Alternatives” sub-dimension assesses the capacity to produce several answers to issues and to consider circumstances from different viewpoints. On the other hand, the “Control” sub-dimension evaluates the extent to which an individual believes in their capability to successfully implement these alternative methods in various situations. In general, higher scores on the overall measure represent high cognitive flexibility.
- -
- Cognitive Control and Flexibility Questionnaire—CCFQ [22]: The 18-item questionnaire measures an individual’s perceived ability to exercise control over intrusive, unwanted (negative) thoughts and emotions (sub-component named as “Cognitive Control over Emotion”) and their ability to cope flexibly with a stressful situation (sub-component named as “Appraisal and Coping Flexibility”). The rating for each item is measured using a 7-point Likert scale, where 1 represents severe disagreement and 7 represents strong agreement.
- -
- Cognitive Flexibility Scale—CFS [21]: This consists of 12 items and was developed to measure the components of cognitive flexibility related to interpersonal communication competence. Each item is scored using a 6-point Likert scale (1 = strongly disagree, 6 = strongly agree). The CFS does not consist of separate sub-dimensions but rather assesses the overall quality of adaptability in communication. Interpersonal communication flexibility refers to an individual’s capacity to adapt their communication style based on the social situation and the requirements of the encounter, indicating a wide range of flexibility in interpersonal interactions.
2.4. Recording of Psychophysiological Signals
2.5. Psychophysiological Signal Processing
2.6. Statistical Analyses
3. Results
3.1. Temporal Emotional Dynamics
3.2. Spectral Emotional Dynamics
3.3. Non-Linear Emotional Dynamics
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanai, R.; Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Kappeler, P.; Kraus, C. Levels and mechanisms of behavioural variability. In Animal Behaviour: Evolution and Mechanisms; Springer: Berlin/Heidelberg, Germany, 2010; pp. 655–684. Available online: https://link.springer.com/chapter/10.1007/978-3-642-02624-9_21 (accessed on 20 November 2024).
- Borghesi, F.; Chirico, A.; Cipresso, P. Outlining a novel psychometric model of mental flexibility and affect dynamics. Front. Psychol. 2023, 14, 1183316. [Google Scholar] [CrossRef] [PubMed]
- Baudier, L.; Gros, H.; Clément, E. Cognitive Flexibility or Flexibilities? New Insights From A Classroom Study. 2023. Available online: https://hal-cyu.archives-ouvertes.fr/hal-04070133 (accessed on 20 November 2024).
- Kuppens, P. Exploring the nature of cognitive flexibility. New Ideas Psychol. 2012, 30, 190–200. [Google Scholar]
- Southwick, S.M.; Charney, D.S. Cognitive and emotional flexibility. In Resilience: The Science of Mastering Life’s Greatest Challenges; Cambridge University Press: Cambridge, UK, 2012; pp. 165–183. [Google Scholar] [CrossRef]
- Berlin, G.S.; Hollander, E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr. 2014, 19, 62–68. Available online: https://www.cambridge.org/core/journals/cns-spectrums/article/abs/compulsivity-impulsivity-and-the-dsm5-process/3AF93AFEE4A085161FD78D34CD10F00A (accessed on 26 October 2024). [CrossRef] [PubMed]
- D’Cruz, A.M.; Ragozzino, M.E.; Mosconi, M.W.; Shrestha, S.; Cook, E.H.; Sweeney, J.A. Reduced Behavioral Flexibility in Autism Spectrum Disorders. Neuropsychology 2013, 27, 152. [Google Scholar] [CrossRef] [PubMed]
- Mora-Maltas, B.; Lucas, I.; Granero, R.; Vintró-Alcaraz, C.; Miranda-Olivos, R.; Baenas, I.; Sánchez, I.; Jiménez-del Toro, J.; Sánchez-González, J.; Krug, I.; et al. Cognitive flexibility and DSM-5 severity criteria for eating disorders: Assessing drive for thinness and duration of illness as alternative severity variables. J. Eat. Disord. 2023, 11, 155. Available online: https://link.springer.com/articles/10.1186/s40337-023-00875-z (accessed on 26 October 2024). [CrossRef]
- Tchanturia, K.; Harrison, A.; Davies, H.; Roberts, M.; Oldershaw, A.; Nakazato, M.; Stahl, D.; Morris, R.; Schmidt, U.; Treasure, J. Cognitive Flexibility and Clinical Severity in Eating Disorders. PLoS ONE 2011, 6, e20462. [Google Scholar] [CrossRef]
- Friederich, H.C.; Herzog, W. Cognitive-Behavioral Flexibility in Anorexia Nervosa. Curr. Top Behav. Neurosci. 2010, 6, 111–123. [Google Scholar]
- Kashdan, T.B.; Rottenberg, J. Psychological flexibility as a fundamental aspect of health. Clin. Psychol. Rev. 2010, 30, 865–878. [Google Scholar] [CrossRef]
- Grol, M.; De Raedt, R. The link between resting heart rate variability and affective flexibility. Cogn. Affect. Behav. Neurosci. 2020, 20, 746–756. [Google Scholar] [CrossRef]
- Lee, J.K.; Orsillo, S.M. Investigating cognitive flexibility as a potential mechanism of mindfulness in Generalized Anxiety Disorder. J. Behav. Ther. Exp. Psychiatry 2014, 45, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Christensen, R.E.; Ruggieri, A.; Schettini, E.; Freeman, J.B.; Garcia, A.M.; Flessner, C.; Stewart, E.; Conelea, C.; Dickstein, D.P. Cognitive performance of youth with primary generalized anxiety disorder versus primary obsessive–compulsive disorder. Depress. Anxiety 2019, 36, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Ansari, T.L.; Derakshan, N.; Richards, A. Effects of anxiety on task switching: Evidence from the mixed antisaccade task. Cogn. Affect. Behav. Neurosci. 2008, 8, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Chelune, G.J.; Baer, R.A. Developmental norms for the wisconsin card sorting test. J. Clin. Exp. Neuropsychol. 1986, 8, 219–228. [Google Scholar] [CrossRef]
- Classics in the History of Psychology—Stroop (1935) [Internet]. Available online: https://psychclassics.yorku.ca/Stroop/?c=002 (accessed on 26 October 2024).
- Dennis, J.P.; Vander Wal, J.S. The cognitive flexibility inventory: Instrument development and estimates of reliability and validity. Cognit. Ther. Res. 2010, 34, 241–253. [Google Scholar] [CrossRef]
- Martin, M.M.; Rubin, R.B. A New Measure of Cognitive Flexibility. Psychol. Rep. 1995, 76, 623–626. [Google Scholar] [CrossRef]
- Gabrys, R.L.; Tabri, N.; Anisman, H.; Matheson, K. Cognitive control and flexibility in the context of stress and depressive symptoms: The cognitive control and flexibility questionnaire. Front. Psychol. 2018, 9, 376606. [Google Scholar] [CrossRef]
- Hollenstein, T. This Time, Its Real: Affective Flexibility, Time Scales, Feedback Loops, and the Regulation of Emotion. Emot. Rev. 2015, 7, 308–315. [Google Scholar] [CrossRef]
- Kraft, D.; Rademacher, L.; Eckart, C.; Fiebach, C.J. Cognitive, Affective, and Feedback-Based Flexibility—Disentangling Shared and Different Aspects of Three Facets of Psychological Flexibility. J. Cogn. 2020, 3, 21. [Google Scholar] [CrossRef]
- Koval, P.; Brose, A.; Pe, M.L.; Houben, M.; Erbas, Y.; Champagne, D.; Kuppens, P. Emotional inertia and external events: The roles of exposure, reactivity, and recovery. Emotion 2015, 15, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Technical Manual and Affective Ratings; NIMH Center for the Study of Emotion and Attention; University of Florida: Gainesville, FL, USA, 1997; pp. 39–58. Available online: https://acordo.net/acordo/wp-content/uploads/2020/08/instructions.pdf (accessed on 8 July 2024).
- Hektner, J.M.; Schmidt, J.A.; Csikszentmihalyi, M. Experience sampling methods. In Measuring the Quality of Everydaylife; Sage Publications: New York, NY, USA, 2007; p. 369. Available online: https://books.google.com/books/about/Experience_Sampling_Method.html?hl=it&id=05e5d_KBYY0C (accessed on 8 July 2024).
- Bernstein, E.E.; Curtiss, J.E.; Wu, G.W.Y.; Barreira, P.J.; McNally, R.J. Exercise and emotion dynamics: An experience sampling study. Emotion 2019, 19, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Malooly, A.M.; Genet, J.J.; Siemer, M. Individual differences in reappraisal effectiveness: The role of affective flexibility. Emotion 2013, 13, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Twivy, E.; Grol, M.; Fox, E. Individual differences in affective flexibility predict future anxiety and worry. Cogn. Emot. 2021, 35, 425–434. [Google Scholar] [CrossRef]
- Genet, J.J.; Siemer, M. Flexible control in processing affective and non-affective material predicts individual differences in trait resilience. Cogn. Emot. 2011, 25, 380–388. [Google Scholar] [CrossRef]
- Genet, J.J.; Malooly, A.M.; Siemer, M. Flexibility is not always adaptive: Affective flexibility and inflexibility predict rumination use in everyday life. Cogn. Emot. 2013, 27, 685–695. Available online: https://www.tandfonline.com/doi/abs/10.1080/02699931.2012.733351 (accessed on 21 November 2024). [CrossRef]
- Grant, A.; Cassidy, S. Exploring the relationship between psychological flexibility and self-report and task-based measures of cognitive flexibility. J. Contextual. Behav. Sci. 2022, 23, 144–150. [Google Scholar] [CrossRef]
- Grol, M.; De Raedt, R. The relationship between affective flexibility, spontaneous emotion regulation and the response to induced stress. Behav. Res. Ther. 2021, 143, 103891. [Google Scholar] [CrossRef]
- Pumprla, J.; Howorka, K.; Groves, D.; Chester, M.; Nolan, J. Functional assessment of heart rate variability: Physiological basis and practical applications. Int. J. Cardiol. 2002, 84, 1–14. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Alloy, L.B. Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clin. Psychol. Rev. 2016, 50, 67–79. [Google Scholar] [CrossRef]
- Weber, C.S.; Thayer, J.F.; Rudat, M.; Wirtz, P.H.; Zimmermann-Viehoff, F.; Thomas, A.; Perschel, F.H.; Arck, P.C.; Deter, H.C. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur. J. Appl. Physiol. 2010, 109, 201–211. Available online: https://link.springer.com/article/10.1007/s00421-009-1341-x (accessed on 26 October 2024). [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Casagrande, M. Heart rate variability and cognitive function: A systematic review. Front. Neurosci. 2019, 13, 204–436. [Google Scholar] [CrossRef] [PubMed]
- Hovland, A.; Pallesen, S.; Hammar, Å.; Hansen, A.L.; Thayer, J.F.; Tarvainen, M.P.; Nordhus, I.H. The relationships among heart rate variability, executive functions, and clinical variables in patients with panic disorder. Int. J. Psychophysiol. 2012, 86, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D.; Adams, J.; Krohn, J.; Wenzel, M.; Mann, C.G.; Gomille, L.K.; Jacobi-Scherbening, V.; Kubiak, T. Heart rate variability and self-control—A meta-analysis. Biol. Psychol. 2016, 115, 9–26. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Rademacher, L.; Kraft, D.; Eckart, C.; Fiebach, C.J. Individual differences in resilience to stress are associated with affective flexibility. Psychol. Res. 2023, 87, 1862–1879. Available online: https://link.springer.com/article/10.1007/s00426-022-01779-4 (accessed on 9 September 2024). [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. Available online: https://link-springer-com.bibliopass.unito.it/article/10.3758/bf03193146 (accessed on 21 November 2024). [CrossRef]
- Borghesi, F.; Chirico, A.; Pedroli, E.; Cipriani, G.E.; Canessa, N.; Amanzio, M.; Cipresso, P. Exploring Biomarkers of Mental Flexibility in Healthy Aging: A Computational Psychometric Study. Sensors 2023, 23, 6983. [Google Scholar] [CrossRef]
- Cipresso, P.; Borghesi, F.; Chirico, A. Affects affect affects: A Markov Chain. Front. Psychol. 2023, 14, 1162655. [Google Scholar] [CrossRef]
- Flaig, N.K.; Large, E.W. Dynamic musical communication of core affect. Front. Psychol. 2014, 5, 72. [Google Scholar] [CrossRef]
- Portoghese, I.; Lasio, M.; Conti, R.; Mascia, M.L.; Hitchcott, P.; Agus, M.; Gemignani, A.; Penna, M.P. Cognitive Flexibility Inventory: Factor structure, invariance, reliability, convergent, and discriminant validity among Italian university students. PsyCh J. 2020, 9, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.; Russell, J.A.; Peterson, B.S. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005, 17, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. A circumplex model of affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Russell, J.A. Core Affect and the Psychological Construction of Emotion. Psychol. Rev. 2003, 110, 145–172. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Borghesi, F.; Mancuso, V.; Bruni, F.; Cremascoli, R.; Bianchi, L.; Mendolicchio, L.; Cattaldo, S.; Chirico, A.; Mauro, A.; Pedroli, E. Mental flexibility assessment: A research protocol for patients with Parkinson’s Disease and Anorexia Nervosa. PLoS ONE 2023, 18, e0293921. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0293921 (accessed on 18 March 2024). [CrossRef]
- Simoncini, G.; Borghesi, F.; Cipresso, P. Linking Affect Dynamics and Well-Being: A Novel Methodological Approach for Mental Health. Healthcare 2024, 12, 1690. [Google Scholar] [CrossRef]
- MathWorks—Creatori di MATLAB e Simulink—MATLAB e Simulink—MATLAB & Simulink [Internet]. Available online: https://it.mathworks.com/?s_tid=gn_logo (accessed on 19 July 2024).
- Von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Gullett, N.; Zajkowska, Z.; Walsh, A.; Harper, R.; Mondelli, V. Heart rate variability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. Int. J. Psychophysiol. 2023, 192, 35–42. [Google Scholar] [CrossRef]
- Perna, G.; Riva, A.; Defillo, A.; Sangiorgio, E.; Nobile, M.; Caldirola, D. Heart rate variability: Can it serve as a marker of mental health resilience?: Special Section on “Translational and Neuroscience Studies in Affective Disorders” Section Editor, Maria Nobile MD, PhD. J. Affect. Disord. 2020, 263, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 45733. [Google Scholar] [CrossRef] [PubMed]
- von Rosenberg, W.; Chanwimalueang, T.; Adjei, T.; Jaffer, U.; Goverdovsky, V.; Mandic, D.P. Resolving ambiguities in the LF/HF ratio: LF-HF scatter plots for the categorization of mental and physical stress from HRV. Front. Physiol. 2017, 8, 360. [Google Scholar] [CrossRef]
- Brennan, M.; Palaniswami, M.; Kamen, P. Poincaré plot interpretation using a physiological model of HRV based on a network of oscillators. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H1873–H1886. [Google Scholar] [CrossRef]
- Dimitriev, D.A.; Saperova, E.V.; Dimitriev, A.D. State Anxiety and Nonlinear Dynamics of Heart Rate Variability in Students. PLoS ONE 2016, 11, e0146131. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0146131 (accessed on 18 March 2024). [CrossRef]
- Balocchi, R.; Cantini, F.; Varanini, M.; Raimondi, G.; Legramante, J.M.; Macerata, A. Revisiting the potential of time-domain indexes in short-term HRV analysis. Biomed. Tech. 2006, 51, 190–193. Available online: https://www.degruyter.com/document/doi/10.1515/BMT.2006.034/html (accessed on 21 November 2024). [CrossRef]
- Bodenes, L.; N’Guyen, Q.T.; Le Mao, R.; Ferrière, N.; Pateau, V.; Lellouche, F.; L’Her, E. Early heart rate variability evaluation enables to predict ICU patients’ outcome. Sci. Rep. 2022, 12, 2498. Available online: https://www.nature.com/articles/s41598-022-06301-9 (accessed on 21 November 2024). [CrossRef]
- Nickerson, R.S. Null hypothesis significance testing: A review of an old and continuing controversy. Psychol. Methods 2000, 5, 241–301. [Google Scholar] [CrossRef]
- Kemp, A.H.; Quintana, D.S. The relationship between mental and physical health: Insights from the study of heart rate variability. Int. J. Psychophysiol. 2013, 89, 288–296. [Google Scholar] [CrossRef]
- Pham, T.; Lau, Z.J.; Chen, S.H.A.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 2021, 21, 3998. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.G.L.; Mendonça-de-Souza, A.C.F.; Barros, E.M.; Coutinho, E.F.S.; Oliveira, L.; Mendlowicz, M.V.; Figueira, I.; Volchan, E. Resilience and vagal tone predict cardiac recovery from acute social stress. Stress 2007, 10, 368–374. Available online: https://www.tandfonline.com/doi/abs/10.1080/10253890701419886 (accessed on 22 November 2024). [CrossRef] [PubMed]
- Sokhadze, E.M. Effects of music on the recovery of autonomic and electrocortical activity after stress induced by aversive visual stimuli. Appl. Psychophysiol. Biofeedback 2007, 32, 31–50. Available online: https://link.springer.com/article/10.1007/s10484-007-9033-y (accessed on 22 November 2024). [CrossRef] [PubMed]
- Watson, D.; Wiese, D.; Vaidya, J.; Tellegen, A. The two general activation systems of affect: Structural evolutionary considerations, and psychobiological evidence. J. Pers. Soc. Psychol. 1999, 76, 820–838. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, R.; Yang, Q.; Luo, Y.J. How Do Amusement, Anger and Fear Influence Heart Rate and Heart Rate Variability? Front. Neurosci. 2019, 13, 490153. [Google Scholar] [CrossRef]
- Cattaneo, L.A.; Franquillo, A.C.; Grecucci, A.; Beccia, L.; Caretti, V.; Dadomo, H. Is Low Heart Rate Variability Associated with Emotional Dysregulation, Psychopathological Dimensions, and Prefrontal Dysfunctions? An Integrative View. J. Pers. Med. 2021, 11, 872. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, J.; Kwon, O.S.; Kim, M.J.; Ryu, Y.H.; Park, J.E. Is heart rate variability (HRV) an adequate tool for evaluating human emotions?—A focus on the use of the International Affective Picture System (IAPS). Psychiatry Res. 2017, 251, 192–196. [Google Scholar] [CrossRef]
- Cipresso, P.; Colombo, D.; Riva, G. Computational Psychometrics Using Psychophysiological Measures for the Assessment of Acute Mental Stress. Sensors 2019, 19, 781. [Google Scholar] [CrossRef]
- Maner, J.K.; Kenrick, D.T. When Adaptations Go Awry: Functional and Dysfunctional Aspects of Social Anxiety. Soc. Issues Policy Rev. 2010, 4, 111–142. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1751-2409.2010.01019.x (accessed on 21 November 2024). [CrossRef]
- Schmitt, D.P.; Pilcher, J.J. Evaluating evidence of psychological adaptation: How do we know one when we see one? Psychol. Sci. 2004, 15, 643–649. Available online: https://journals.sagepub.com/doi/full/10.1111/j.0956-7976.2004.00734 (accessed on 21 November 2024). [CrossRef]
- Cserjési, R.; Molnár, D.; Luminet, O.; Lénárd, L. Is there any relationship between obesity and mental flexibility in children? Appetite 2007, 49, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.F.; Reynolds, C.F., III; Butters, M.A.; Dew, M.A.; Mazumdar, S.; Begley, A.E.; Lenze, E.; Weiner, D.K. The Relationship Between Pain and Mental Flexibility in Older Adult Pain Clinic Patients. Pain Med. 2006, 7, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Wecker, N.S.; Kramer, J.H.; Hallam, B.J.; Delis, D.C. Mental flexibility: Age effects on switching. Neuropsychology 2005, 19, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, F.; Murtas, V.; Chirico, A.; Pizzolante, M.; Cipresso, P. Affect Dynamics through Virtual Reality. Annu. Rev. Cybertherapy Telemed. 2023, 21, 11–13. Available online: http://hdl.handle.net/10807/260504 (accessed on 25 February 2024).
- Giles, S.; Hughes, E.K.; Castle, D.; Jenkins, Z.; Phillipou, A.; Rossell, S.; Urbini, G.; Fuller-Tyszkiewicz, M.; Krug, I. A new network analysis model in anorexia nervosa patients based on self-reported eating disorder symptoms, psychological distress, and cognitive flexibility. Br. J. Clin. Psychol. 2023, 63, 118–134. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/bjc.12451 (accessed on 25 November 2024). [CrossRef]
- Tyndall, I.; Vagos, P.; Cano, S.; Pinto, H.; Trindade, I.A. Assessing psychological flexibility in chronic illness. Psychol. Rec. 2023, 73, 565–574. [Google Scholar] [CrossRef]
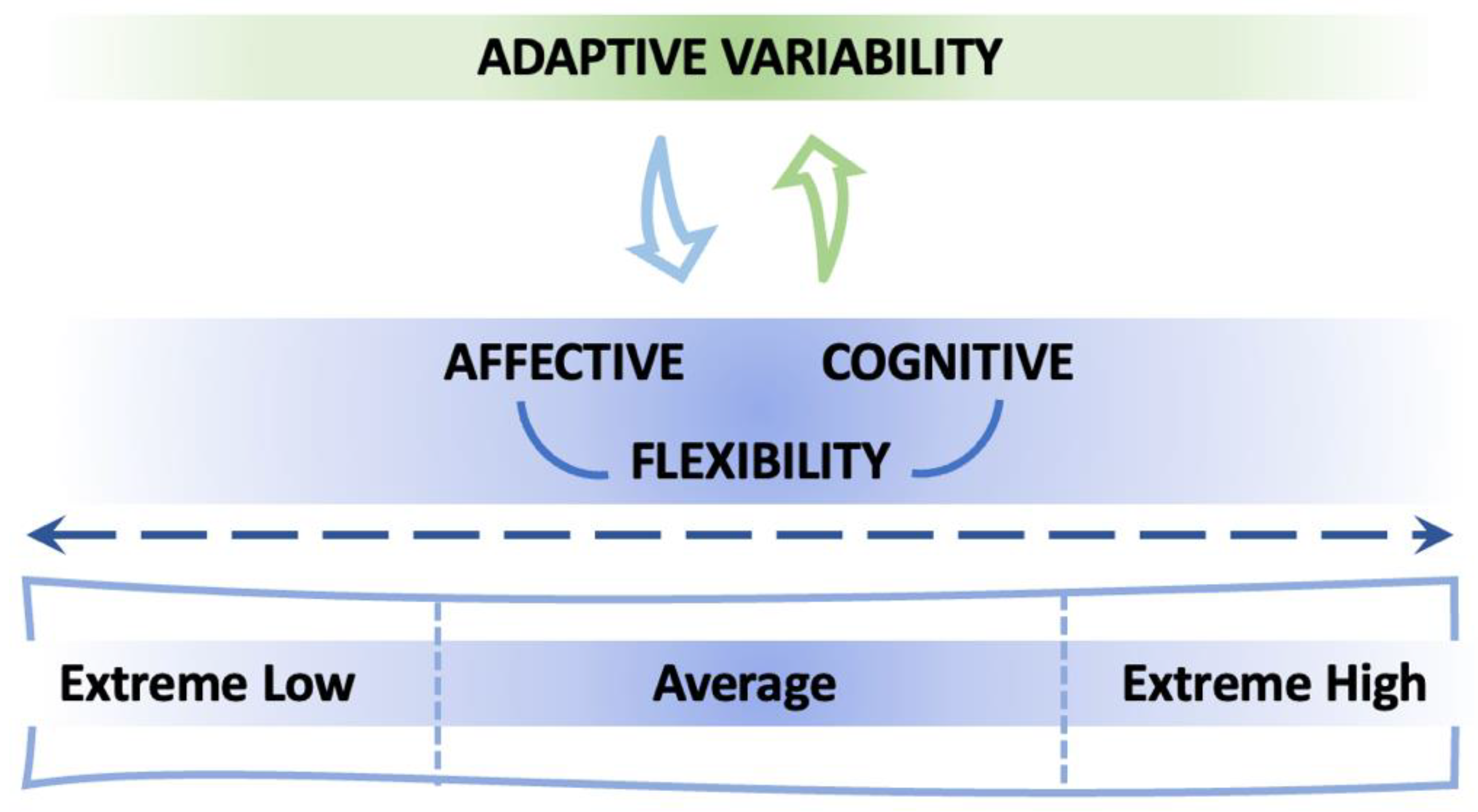
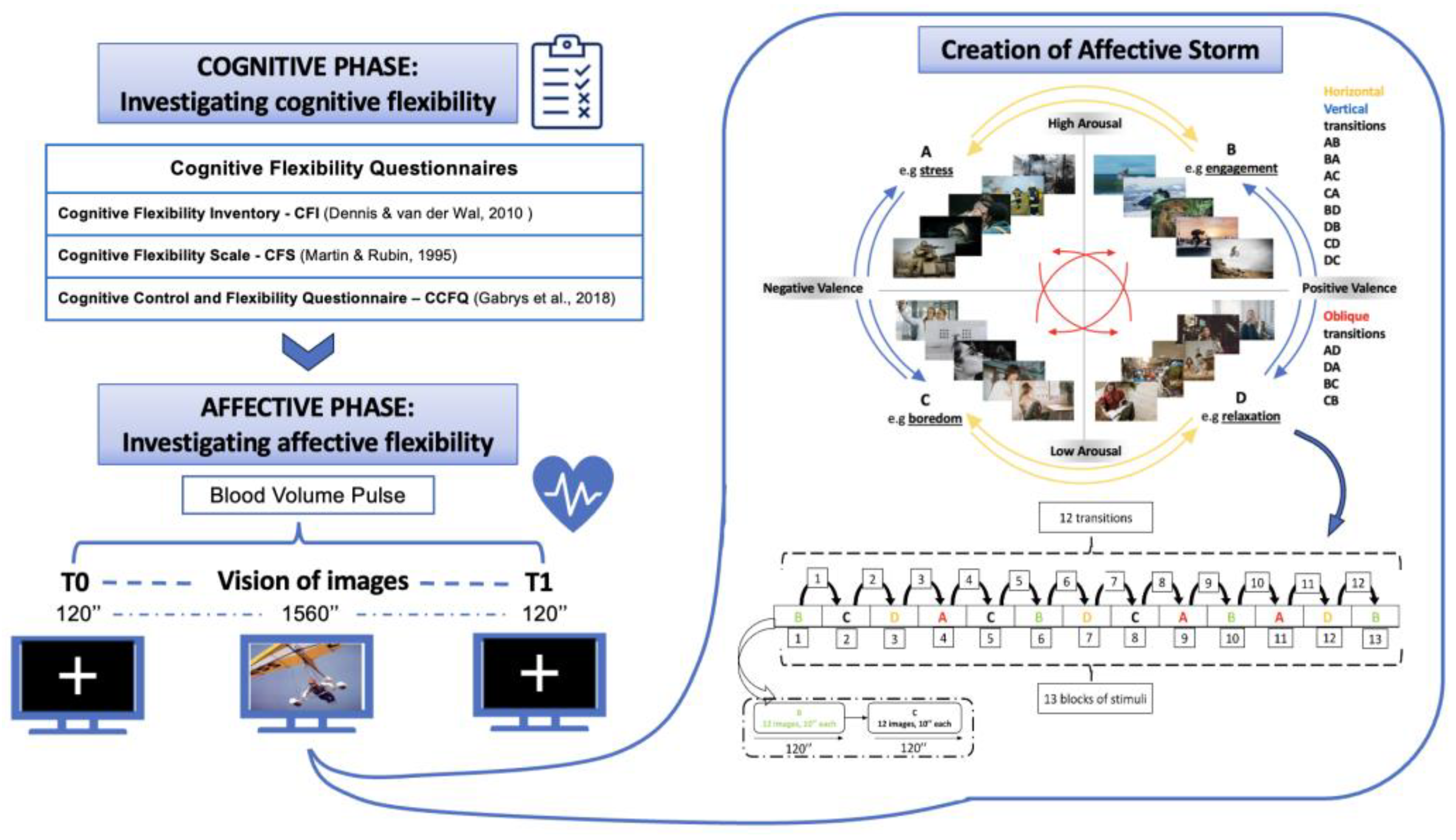


| Questionnaire | Aim | Examples of Items |
|---|---|---|
| Cognitive Flexibility Inventory (CFI) [20] | To assess an individual’s cognitive flexibility, specifically their ability to consider alternative perspectives and manage challenges flexibly. |
|
| Cognitive Flexibility Scale (CFS) [21] | To measure an individual’s perceived control over intrusive negative thoughts and emotions, as well as their ability to respond flexibly to stress. |
|
| Cognitive Control and Flexibility Questionnaire (CCFQ) [22] | To measure cognitive flexibility in interpersonal communication. This scale assesses the adaptability of an individual’s communication style to fit different social situations and the needs of the interaction. |
|
| Constructs | Type of Measurements | Measures | Group | N | Mean | Std. Dev. | Std. Error |
|---|---|---|---|---|---|---|---|
| Cognitive Flexibility | Self-report | CFI Alternative | Average | 24 | 5.40 | 0.1132 | 0.555 |
| Extreme | 20 | 5.38 | 0.1919 | 0.858 | |||
| CFI Control | Average | 24 | 4.93 | 0.1111 | 0.544 | ||
| Extreme | 20 | 4.74 | 0.2943 | 1.316 | |||
| CFS | Average | 24 | 4.47 | 0.0903 | 0.442 | ||
| Extreme | 20 | 4.50 | 0.1605 | 0.718 | |||
| CCFQ Cognitive Control over Emotion | Average | 24 | 35.63 | 1.0033 | 4.915 | ||
| Extreme | 20 | 35.90 | 2.5214 | 11.276 | |||
| CCFQ Appraisal and Coping Flexibility | Average | 24 | 45.46 | 1.1500 | 5.634 | ||
| Extreme | 20 | 45.35 | 2.4401 | 10.912 |
| T0 | T1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physio | Type | Indexes | Group | N | Mean | SE | SD | N | Mean | SE | SD |
| Heart Rate Variability | Temporal Domain | RMSSD (ms) | Average | 23 | 55.11 | 7.31 | 35.07 | 23 | 56.13 | 7.41 | 35.55 |
| Extreme | 20 | 44.23 | 4.20 | 18.79 | 20 | 61.34 | 5.71 | 25.52 | |||
| SDNN (ms) | Average | 23 | 54.09 | 5.76 | 27.64 | 23 | 63.64 | 5.89 | 28.26 | ||
| Extreme | 20 | 50.95 | 4.43 | 19.81 | 20 | 66.73 | 4.99 | 22.30 | |||
| SD HR (bpm) | Average | 23 | 4.77 | 0.43 | 2.04 | 23 | 5.58 | 0.52 | 2.48 | ||
| Extreme | 20 | 4.45 | 0.38 | 1.72 | 20 | 6.94 | 1.16 | 5.19 | |||
| LF power AR (n.u.) | Average | 23 | 47.2 | 3.61 | 17.3 | 23 | 65.1 | 4.13 | 19.8 | ||
| Frequency Domain | Extreme | 20 | 58.8 | 4.52 | 20.2 | 20 | 52.5 | 4.53 | 20.3 | ||
| HF power AR (n.u.) | Average | 23 | 52.8 | 3.61 | 17.3 | 23 | 34.9 | 4.12 | 19.8 | ||
| Extreme | 20 | 41.2 | 4.52 | 20.2 | 20 | 47.4 | 4.53 | 20.2 | |||
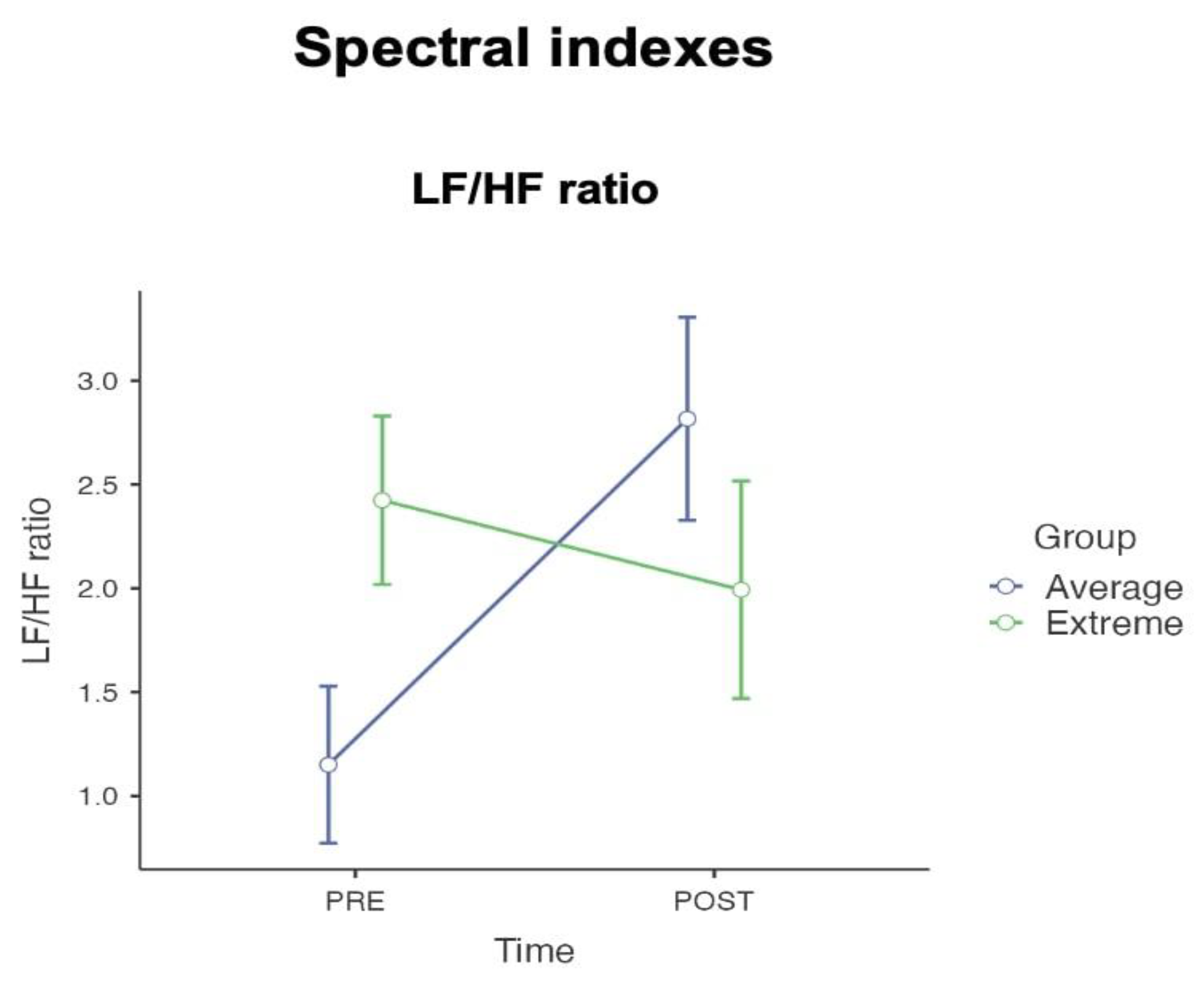
| LF/HF ratio | Average | 23 | 1.15 | 0.18 | 0.88 | 23 | 2.82 | 0.40 | 1.91 | ||
| Extreme | 20 | 2.42 | 0.56 | 2.49 | 20 | 1.99 | 0.62 | 2.76 | |||
| Non-linear | SD1 (ms) | Average | 23 | 39.1 | 5.20 | 24.9 | 23 | 39.9 | 5.29 | 25.4 | |
| Extreme | 20 | 31.4 | 2.99 | 13.4 | 20 | 43.6 | 4.05 | 18.1 | |||
| SD2 (ms) | Average | 23 | 65.3 | 6.56 | 31.5 | 23 | 80.0 | 6.94 | 33.3 | ||
| Extreme | 20 | 64.2 | 5.96 | 26.6 | 20 | 83.2 | 6.22 | 27.8 | |||
| SD2/SD1 ratio | Average | 23 | 1.87 | 0.12 | 0.57 | 23 | 2.37 | 0.17 | 0.81 | ||
| Extreme | 20 | 2.18 | 0.17 | 0.75 | 20 | 2.03 | 0.14 | 0.63 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghesi, F.; Simoncini, G.; Chirico, A.; Cipresso, P. In Medio Stat Virtus: Moderate Cognitive Flexibility as a Key to Affective Flexibility Responses in Long-Term HRV. Sensors 2024, 24, 8047. https://doi.org/10.3390/s24248047
Borghesi F, Simoncini G, Chirico A, Cipresso P. In Medio Stat Virtus: Moderate Cognitive Flexibility as a Key to Affective Flexibility Responses in Long-Term HRV. Sensors. 2024; 24(24):8047. https://doi.org/10.3390/s24248047
Chicago/Turabian StyleBorghesi, Francesca, Gloria Simoncini, Alice Chirico, and Pietro Cipresso. 2024. "In Medio Stat Virtus: Moderate Cognitive Flexibility as a Key to Affective Flexibility Responses in Long-Term HRV" Sensors 24, no. 24: 8047. https://doi.org/10.3390/s24248047
APA StyleBorghesi, F., Simoncini, G., Chirico, A., & Cipresso, P. (2024). In Medio Stat Virtus: Moderate Cognitive Flexibility as a Key to Affective Flexibility Responses in Long-Term HRV. Sensors, 24(24), 8047. https://doi.org/10.3390/s24248047







