Research Progress on the Measurement Methods and Clinical Significance of Capillary Refill Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Literature Search Strategy
2.3. Literature Screening
3. Results
3.1. Results of Literature Search
3.2. Normal Range of CRT
3.3. Measurement Method of CRT
3.3.1. Manual Measurement
3.3.2. Semi-Automatic Measurement Technology
3.3.3. Fully Automatic Measurement Technology
3.4. Clinical Significance of CRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klibus, M.; Smirnova, D.; Marcinkevics, Z.; Rubins, U.; Grabovskis, A.; Vanags, I.; Sabelnikovs, O. Quantitative Evaluation of Microcirculatory Alterations in Patients with COVID-19 and Bacterial Septic Shock through Remote Photoplethysmography and Automated Capillary Refill Time Analysis. Medicina 2024, 60, 1680. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.; Gotlib, A.; King, J. Capillary Refill Time. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ding, X.; Zhou, Y.; Zhang, X.; Sun, T.; Cui, N.; Wang, S.; Su, D.; Yu, Z. Application of microcirculatory indicators in predicting the prognosis of patients with septic shock. Heliyon 2024, 10, e38035. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Hernandez, G. Can Peripheral Skin Perfusion Be Used to Assess Organ Perfusion and Guide Resuscitation Interventions? Front. Med. 2020, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, Y.; Ke, L.; Hu, C.; Chen, P.; Hu, B. Perspectives for capillary refill time in clinical practice for sepsis. Intensive Crit. Care Nurs. 2024, 84, 103743. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Ujihara, Y.; Nakamura, M.; Sugita, S. Relationships Among Capillary Refill Time, Peripheral Blood Flow Rate, and Fingertip Temperature: Advances in Peripheral Artery Contractility Diagnosis. J. Biomech. Eng. 2025, 147, 021005. [Google Scholar] [CrossRef]
- Falotico, J.M.; Shinozaki, K.; Saeki, K.; Becker, L.B. Advances in the Approaches Using Peripheral Perfusion for Monitoring Hemodynamic Status. Front. Med. 2020, 7, 614326. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Xie, Z.Y. Capillary refill and its application progress in peripheral circulation monitoring in critically ill patients. Chin. J. Emerg. Med. 2018, 27, 1062–1066. [Google Scholar]
- Jacquet-Lagreze, M.; Wiart, C.; Schweizer, R.; Didier, L.; Ruste, M.; Coutrot, M.; Legrand, M.; Baudin, F.; Javouhey, E.; Dépret, F.; et al. Capillary refill time for the management of acute circulatory failure: A survey among pediatric and adult intensivists. BMC Emerg. Med. 2022, 22, 131. [Google Scholar] [CrossRef]
- Champion, H.R.; Sacco, W.J.; Carnazzo, A.J.; Copes, W.; Fouty, W.J. Trauma score. Crit. Care Med. 1981, 9, 672–676. [Google Scholar] [CrossRef]
- Schriger, D.L.; Baraff, L. Defining normal capillary refill: Variation with age, sex, and temperature. Ann. Emerg. Med. 1988, 17, 932–935. [Google Scholar] [CrossRef]
- Shinozaki, K.; Jacobson, L.S.; Saeki, K.; Kobayashi, N.; Weisner, S.; Falotico, J.M.; Li, T.; Kim, J.; Lampe, J.W.; Becker, L.B. The standardized method and clinical experience may improve the reliability of visually assessed capillary refill time. Am. J. Emerg. Med. 2021, 44, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Gill, P.J.; Van den Bruel, A.; Thompson, M. Capillary refill time in sick children: A clinical guide for general practice. Br. J. Gen. Pract. 2016, 66, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.H.; Prasad, N.H.; Whitley, T.W. Adverse lighting condition effects on the assessment of capillary refill. Am. J. Emerg. Med. 1994, 12, 46–47. [Google Scholar] [CrossRef]
- Mongkolpun, W.; Orbegozo, D.; Cordeiro, C.P.; Franco, C.J.; Vincent, J.L.; Creteur, J. Alterations in Skin Blood Flow at the Fingertip Are Related to Mortality in Patients With Circulatory Shock. Crit. Care Med. 2020, 48, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Valenzuela, E.D.; Kattan, E.; Castro, R.; Guzmán, C.; Kraemer, A.E.; Sarzosa, N.; Alegría, L.; Contreras, R.; Oviedo, V.; et al. Capillary refill time response to a fluid challenge or a vasopressor test: An observational, proof-of-concept study. Ann. Intensive Care 2024, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Fage, N.; Moretto, F.; Rosalba, D.; Shi, R.; Lai, C.; Teboul, J.L.; Monnet, X. Effect on capillary refill time of volume expansion and increase of the norepinephrine dose in patients with septic shock. Crit. Care 2023, 27, 429. [Google Scholar] [CrossRef]
- Raia, L.; Gabarre, P.; Bonny, V.; Urbina, T.; Missri, L.; Boelle, P.Y.; Baudel, J.-L.; Guidet, B.; Maury, E.; Joffre, J.; et al. Kinetics of capillary refill time after fluid challenge. Ann. Intensive Care 2022, 12, 74. [Google Scholar] [CrossRef]
- Cruz, G.; Pedroza Gómez, S.; Arango, A.; Guevara, P.A.; González, C.; Aguirre, J.; Valencia-Orozco, A.; Suguimoto, A.J. Capillary Refill Time and Serum Lactate as Predictors of Mortality and Postoperative Extracorporeal Membrane Oxygenation Requirement in Congenital Heart Surgery. Children 2023, 10, 875. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Basu, S.; Bhatia, B. Determinants of Capillary Refill Time in Healthy Neonates. J. Clin. Diagn. Res. 2015, 9, SC01–SC03. [Google Scholar] [CrossRef]
- LeFlore, J.L.; Engle, W.D. Capillary refill time is an unreliable indicator of cardiovascular status in term neonates. Adv. Neonatal Care 2005, 5, 147–154. [Google Scholar] [CrossRef]
- Strozik, K.S.; Pieper, C.H.; Roller, J. Capillary refilling time in newborn babies: Normal values. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, F193–F196. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.V.; Maisels, M.J.; Kring, E.; Schwarz-Warner, L. Capillary refill time in the hands and feet of normal newborn infants. Clin. Pediatr. 1999, 38, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Taylor, R.M. The agreement of fingertip and sternum capillary refill time in children. Arch. Dis. Child. 2013, 98, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Karpitskaya, Y.; Miller, J.; Otsuka, N.Y. The influence of arterial flow on capillary refill in pediatric lower extremity. J. Surg. Orthop. Adv. 2008, 17, 74–76. [Google Scholar]
- Anderson, B.; Kelly, A.M.; Kerr, D.; Clooney, M.; Jolley, D. Impact of patient and environmental factors on capillary refill time in adults. Am. J. Emerg. Med. 2008, 26, 62–65. [Google Scholar] [CrossRef]
- La Via, L.; Sanfilippo, F.; Continella, C.; Triolo, T.; Messina, A.; Robba, C.; Astuto, M.; Hernandez, G.; Noto, A. Agreement between Capillary Refill Time measured at Finger and Earlobe sites in different positions: A pilot prospective study on healthy volunteers. BMC Anesthesiol. 2023, 23, 30. [Google Scholar] [CrossRef]
- Misango, D.; Pattnaik, R.; Baker, T.; Dünser, M.W.; Dondorp, A.M.; Schultz, M.J. Hemodynamic Assessment and Support in Sepsis and Septic Shock in Resource-Limited Settings. In Sepsis Management in Resource-Limited Settings; Dondorp, A.M., Dünser, M.W., Schultz, M.J., Eds.; Springer: Cham, Switzerland, 9 February 2019; pp. 151–162. [Google Scholar]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Bakker, J. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs. Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Bige, N.; Boelle, P.Y.; Pichereau, C.; Alves, M.; Bertinchamp, R.; Baudel, J.L.; Galbois, A.; Maury, E.; Guidet, B. Capillary refill time exploration during septic shock. Intensive Care Med. 2014, 40, 958–964. [Google Scholar] [CrossRef]
- Saito, D.; Nakada, T.A.; Imaeda, T.; Takahashi, N.; Shinozaki, M.; Shimizu, R.; Nakaguchi, T. Impact of posture on capillary refilling time. Am. J. Emerg. Med. 2022, 56, 378–379. [Google Scholar] [CrossRef]
- Lima, A.; Bakker, J. Clinical assessment of peripheral circulation. Curr. Opin. Crit. Care 2015, 21, 226–231. [Google Scholar] [CrossRef]
- Jacquet-Lagrèze, M.; Bouhamri, N.; Portran, P.; Schweizer, R.; Baudin, F.; Lilot, M.; Fornier, W.; Fellahi, J.-L. Capillary refill time variation induced by passive leg raising predicts capillary refill time response to volume expansion. Crit. Care 2019, 23, 281. [Google Scholar] [CrossRef] [PubMed]
- Maurin, C.; Portran, P.; Schweizer, R.; Allaouchiche, B.; Junot, S.; Jacquet-Lagrèze, M.; Fellahi, J.L. Effects of methylene blue on microcirculatory alterations following cardiac surgery: A prospective cohort study. Eur. J. Anaesthesiol. 2021, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Lamprea, S.; Fernández-Sarmiento, J.; Barrera, S.; Mora, A.; Fernández-Sarta, J.P.; Acevedo, L. Capillary refill time in sepsis: A useful and easily accessible tool for evaluating perfusion in children. Front. Pediatr. 2022, 10, 1035567. [Google Scholar] [CrossRef] [PubMed]
- Shavit, I.; Brant, R.; Nijssen-Jordan, C.; Galbraith, R.; Johnson, D.W. A novel imaging technique to measure capillary-refill time: Improving diagnostic accuracy for dehydration in young children with gastroenteritis. Pediatrics 2006, 118, 2402–2408. [Google Scholar] [CrossRef]
- Alsma, J.; van Saase, J.L.; Nanayakkara, P.W.; Schouten, W.I.; Baten, A.; Bauer, M.P.; de Bie, S. The power of flash mob research: Conducting a nationwide observational clinical study on capillary refill time in a single day. Chest 2017, 151, 1106–1113. [Google Scholar] [CrossRef]
- Shinozaki, K.; Jacobson, L.S.; Saeki, K.; Kobayashi, N.; Weisner, S.; Falotico, J.M.; Li, T.; Kim, J.; Lampe, J.W.; Becker, L.B. Does training level affect the accuracy of visual assessment of capillary refill time? Crit. Care 2019, 23, 157. [Google Scholar] [CrossRef]
- Shinozaki, M.; Saito, D.; Nakada, T.A.; Nomura, Y.; Nakaguchi, T. Feasibility study of wearable capillary refill time measurement device. Artif. Life Robotics 2024, 29, 334–339. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Nakada, T.A.; Oshima, T.; Shinozaki, M.; Nakaguchi, T.; Haneishi, H.; Oda, S. Optimal pressing strength and time for capillary refilling time. Crit. Care 2019, 23, 4. [Google Scholar] [CrossRef]
- Zaman, T.; Kyriacou, P.A.; Pal, S.K. Free flap pulse oximetry utilizing reflectance photoplethysmography. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4046–4049. [Google Scholar]
- John, R.T.; Henricson, J.; Nilsson, G.E.; Wilhelms, D.; Anderson, C.D. Reflectance spectroscopy: To shed new light on the capillary refill test. J. Biophotonics 2018, 11, e201700043. [Google Scholar] [CrossRef]
- John, R.T.; Henricson, J.; Anderson, C.D.; Wilhelms, D.B. Man versus machine: Comparison of naked-eye estimation and quantified capillary refill. Emerg. Med. J. 2019, 36, 465–471. [Google Scholar] [CrossRef]
- Liu, C.; Correia, R.; Ballaji, H.; Korposh, S.; Hayes-Gill, B.; Morgan, S. Optical Fibre Sensor for Simultaneous Measurement of Capillary Refill Time and Contact Pressure. Sensors 2020, 20, 1388. [Google Scholar] [CrossRef]
- Shinozaki, K.; Capilupi, M.J.; Saeki, K.; Hirahara, H.; Horie, K.; Kobayashi, N.; Becker, L.B. Low temperature increases capillary blood refill time following mechanical fingertip compression of healthy volunteers: Prospective cohort study. J. Clin. Monit. Comput. 2019, 33, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ballaji, H.K.; Correia, R.; Liu, C.; Korposh, S.; Hayes-Gill, B.R.; Musgrove, A.; Morgan, S.P. Optical Fibre Sensor for Capillary Refill Time and Contact Pressure Measurements under the Foot. Sensors 2021, 21, 6072. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, L.L.; Morris, D.E.; Crowe, J.A.; Henry, C.; Hill, S.; Sharkey, D.; Hayes-Gill, B.R. An automated quasi-continuous capillary refill timing device. Physiol. Meas. 2016, 37, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Saeki, K.; Jacobson, L.S.; Falotico, J.M.; Li, T.; Hirahara, H.; Horie, K.; Kobayashi, N.; Weisner, S.; Lampe, J.W.; et al. Evaluation of accuracy of capillary refill index with pneumatic fingertip compression. J. Clin. Monit. Comput. 2021, 35, 135–145. [Google Scholar] [CrossRef]
- Wang, Z. Measuring Mechanism and Method of Capillary Refill Time. 201811142255.6[P].2019-01-11. Available online: https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=changgui (accessed on 6 November 2024).
- Kerr, E.; Coleman, S.; McGinnity, T.M.; Shepherd, A. Measurement of capillary refill time (CRT) in healthy subjects using a robotic hand. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition Workshops, Salt Lake City, UT, USA, 18–22 June 2018; pp. 1372–1379. [Google Scholar] [CrossRef]
- Ruste, M.; Cazenave, L.; Tardif, M.; Saint-Jean, C.; Fellahi, J.L.; Lagrèze, M.J. Measurement of capillary refill time with a handheld prototype device: A comparative validation study in healthy volunteers. J. Clin. Monit. Comput. 2022, 36, 1271–1278. [Google Scholar] [CrossRef]
- Morimura, N.; Takahashi, K.; Doi, T.; Ohnuki, T.; Sakamoto, T.; Uchida, Y.; Takahashi, H.; Fujita, T.; Ikeda, H. A pilot study of quantitative capillary refill time to identify high blood lactate levels in critically ill patients. Emerg. Med. J. 2015, 32, 444–448. [Google Scholar] [CrossRef]
- Takazawa, K.; Tanaka, N.; Fujita, M.; Matsuoka, O.; Saiki, T.; Aikawa, M.; Tamura, S.; Ibukiyama, C. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension 1998, 32, 365–370. [Google Scholar] [CrossRef]
- Shen, Y.; Voisin, M.; Aliamiri, A.; Avati, A.; Hannun, A.; Ng, A. Ambulatory atrial fibrillation monitoring using wearable photoplethysmography with deep learning. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019; pp. 1909–1916. [Google Scholar]
- Takayesu, J.K.; Lozner, A.W. Pediatrics, dehydration. Pediatrics 1910, 8, 18. [Google Scholar]
- Guedel, A.E. Cyclopropane anesthesia. Anesthesiology 1940, 1, 13–25. [Google Scholar] [CrossRef]
- Crismon, J.M.; Fuhrman, F.A. Studies on gangrene following cold injury: VI. capillary blood flow after cold injury, the effects of rapid warming, and sympathetic block. J. Clin. Investig. 1947, 26, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Zanner, R.; Schneider, G.; Schmid, R.; Lahmer, T. Assessment of Regional Perfusion and Organ Function: Less and Non-invasive Techniques. Front. Med. 2019, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.A.; Samuy, N. Clinical guideline highlights for the hospitalist: International consensus criteria for pediatric sepsis and septic shock. J. Hosp. Med. 2024, 19, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Greif, R.; Bray, J.E.; Djärv, T.; Drennan, I.R.; Liley, H.G.; Ng, K.C.; Cheng, A.; Douma, M.J.; Scholefield, B.R.; Smyth, M.; et al. 2024 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation 2024, 87, e15. [Google Scholar] [CrossRef]
- Weiss, S.L.M.; Peters, M.J.M.; Alhazzani, W.M.; Agus, M.S.D.M.; Flori, H.R.M.; Inwald, D.P.M.; Nadel, S.M.; Schlapbach, L.J.F.; Tasker, R.C.M.B.; Argent, A.C.M.B.; et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr. Crit. Care Med. 2020, 21, e52–e106. [Google Scholar] [CrossRef]
- Paul, S.P.; Kini, P.K.; Tibrewal, S.R.; Heaton, P.A. NICE guideline review: Fever in under 5s: Assessment and initial management (NG143). Arch. Dis. Child. Educ. Pract. Ed. 2022, 107, 212–216. [Google Scholar] [CrossRef]
- Davis, A.L.; Carcillo, J.A.; Aneja, R.K.; Deymann, A.J.; Lin, J.C.; Nguyen, T.C.; Okhuysen-Cawley, R.S.; Relvas, M.S.; Rozenfeld, R.A.; Skippen, P.W.; et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit. Care Med. 2017, 45, e993. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Meningitis (Bacterial) and Meningococcal Disease: Recognition, Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2024. [Google Scholar]
- Fleming, S.; Gill, P.; Jones, C.; Taylor, J.A.; Van den Bruel, A.; Heneghan, C.; Roberts, N.; Thompson, M. The diagnostic value of capillary refill time for detecting serious illness in children: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138155. [Google Scholar] [CrossRef]
- Hariri, G.; Joffre, J.; Leblanc, G.; Bonsey, M.; Lavillegrand, J.R.; Urbina, T.; Guidet, B.; Maury, E.; Bakker, J.; Ait-Oufella, H. Narrative review: Clinical assessment of peripheral tissue perfusion in septic shock. Ann. Intensive Care 2019, 9, 37. [Google Scholar] [CrossRef]
- Sebat, C.; Vandegrift, M.A.; Oldroyd, S.; Kramer, A.; Sebat, F. Capillary refill time as part of an early warning score for rapid response team activation is an independent predictor of outcomes. Resuscitation 2020, 153, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ruste, M.; Sghaier, R.; Chesnel, D.; Didier, L.; Fellahi, J.L.; Jacquet-Lagrèze, M. Perfusion-based deresuscitation during continuous renal replacement therapy: A before-after pilot study. J. Crit. Care 2022, 72, 154169. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Henricson, J.; Anderson, C.D.; Wilhelms, D.B. The Effect of Repeated Capillary Refill Tests on the Cutaneous Microcirculation. J. Biophotonics 2024, 17, e202400098. [Google Scholar] [CrossRef] [PubMed]
- Amson, H.; Vacheron, C.H.; Thiolliere, F.; Piriou, V.; Magnin, M.; Allaouchiche, B. Core-to-skin temperature gradient measured by thermography predicts day-8 mortality in septic shock: A prospective observational study. J. Crit. Care 2020, 60, 294–299. [Google Scholar] [CrossRef]
- Van der Mullen, J.; Wise, R.; Vermeulen, G.; Moonen, P.J.; Malbrain, M.L.N.G. Assessment of hypovolaemia in the critically ill. Anaesthesiol. Intensive Ther. 2018, 50, 141–149. [Google Scholar] [CrossRef]
- Vera, M.; Kattan, E.; Castro, R.; Hernández, G. The seven Ts of capillary refill time: More than a clinical sign for septic shock patients. Eur. J. Emerg. Med. 2020, 27, 169–171. [Google Scholar] [CrossRef]
- Brunauer, A.; Koköfer, A.; Bataar, O.; Gradwohl-Matis, I.; Dankl, D.; Bakker, J.; Dünser, M.W. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: A pilot study. J. Crit. Care 2016, 35, 105–109. [Google Scholar] [CrossRef]
- van Genderen, M.E.; Paauwe, J.; de Jonge, J.; van der Valk, R.J.; Lima, A.; Bakker, J.; van Bommel, J. Clinical assessment of peripheral perfusion to predict postoperative complications after major abdominal surgery early: A prospective observational study in adults. Crit. Care 2014, 18, R114. [Google Scholar] [CrossRef]
- Hernandez, G.; Bruhn, A.; Castro, R.; Regueira, T. The holistic view on perfusion monitoring in septic shock. Curr. Opin. Crit. Care 2012, 18, 280–286. [Google Scholar] [CrossRef]
- van Genderen, M.E.; Lima, A.; Akkerhuis, M.; Bakker, J.; van Bommel, J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit. Care Med. 2012, 40, 2287–2294. [Google Scholar] [CrossRef]
- Yamamoto, M.; Doi, K.; Hayase, N.; Asada, T.; Akamatsu, N.; Kaneko, J.; Hasegawa, K.; Morimura, N. Pulse oximetry-based capillary refilling evaluation predicts postoperative outcomes in liver transplantation: A prospective observational cohort study. BMC Anesthesiol. 2020, 20, 251. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, D.C.; Cloutier, R.L.; Samatham, R.; Hansen, M.L. Point-Of-Care Capillary Refill Technology Improves Accuracy of Peripheral Perfusion Assessment. Front. Med. 2021, 8, 694241. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tong, M.; Tian, Z. Prolonged capillary refill time and short-term mortality of critically ill patients: A meta-analysis. Am. J. Emerg. Med. 2024, 79, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Zarate, D.; Rosas-Sanchez, K.; Zaragoza, J.J. Clinical evaluation of peripheral tissue perfusion as a predictor of mortality in sepsis and septic shock inthe intensive care unit: Systematic review and meta-analysis. Med. Intensiva 2023, 47, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Kattan, E.; Hernández, G.; Ospina-Tascón, G.; Valenzuela, E.D.; Bakker, J.; Castro, R. ANDROMEDA-SHOCK Study Investigators and the Latin America Intensive Care Network (LIVEN) A lactate-targeted resuscitation strategy may be associated with higher mortality in patients with septic shock and normal capillary refill time: A post hoc analysis of the ANDROMEDA-SHOCK study. Ann. Intensive Care 2020, 10, 114. [Google Scholar]
- Zampieri, F.G.; Damiani, L.P.; Bakker, J.; Ospina-Tascón, G.A.; Castro, R.; Cavalcanti, A.B.; Hernandez, G. Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock. A bayesian reanalysis of the ANDROMEDA-SHOCK trial. Am. J. Respir. Crit. Care Med. 2020, 201, 423–429. [Google Scholar] [CrossRef]
- Guven, G.; Hilty, M. Can Ince Microcirculation: Physiology, Pathopysiology and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef]
- Valenzuela Espinoza, E.D.; Pozo, M.O.; Kanoore Edul, V.S.; Furche, M.; Motta, M.F.; Risso Vazquez, A.; Rubatto Birri, P.N.; Dubin, A. Effects of short-term hyperoxia on sytemic hemodynamics, oxygen transport, and microcirculation: An observational study in patients with septic shock and healthy volunteers. J. Crit. Care 2019, 53, 62–68. [Google Scholar] [CrossRef]
- Yasufumi, O.; Morimura, N.; Shirasawa, A.; Honzawa, H.; Oyama, Y.; Niida, S.; Abe, T.; Imaki, S.; Takeuchi, I. Quantitative capillary refill time predicts sepsis in patients with suspected infection in the emergency department: An observational study. J. Intensive Care 2019, 7, 29. [Google Scholar] [CrossRef]
- Huang, W.; Xiang, H.; Hu, C.; Wu, T.; Zhang, D.; Ma, S.; Hu, B.; Li, J. Association of Sublingual Microcirculation Parameters and Capillary Refill Time in the Early Phase of ICU Admission. Crit. Care Med. 2023, 51, 913–923. [Google Scholar] [CrossRef]
- Kattan, E.; Bakker, J.; Estenssoro, E.; Ospina-Tascón, G.A.; Cavalcanti, A.B.; Backer, D.; Vieillard-Baron, A.; Teboul, J.L.; Castro, R.; Hernández, G. Hemodynamic phenotype-based, capillary refill time-targeted resuscitation in early septic shock: The ANDROMEDA-SHOCK-2 randomized clinical trial study protocol. Rev. Bras. Ter. Intensiva 2022, 34, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ramasco, F.; Aguilar, G.; Aldecoa, C.; Bakker, J.; Carmona, P.; Dominguez, D.; Galiana, M.; Hernández, G.; Kattan, E.; Olea, C.; et al. Towards the personalization of septic shock resuscitation: The fundamentals of ANDROMEDA-SHOCK-2 trial. Rev. Esp. Anestesiol. Reanim. 2024, 71, 112–124. [Google Scholar] [CrossRef]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19 (Suppl. 3), S8. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; McCoy, J.V.; Phillip Dellinger, R.; Arnold, R.C.; Rizzuto, M.; Abate, N.L.; Shapiro, N.I.; Parrillo, J.E.; Hollenberg, S.M. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008, 34, 2210–2217. [Google Scholar] [CrossRef]
- Lima, A.; Jansen, T.C.; van Bommel, J.; Ince, C.; Bakker, J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit. Care Med. 2009, 37, 934–938. [Google Scholar] [CrossRef]
- Contreras, R.; Hernández, G.; Valenzuela, E.D.; González, C.; Ulloa, R.; Soto, D.; Castro, R.; Guzmán, C.; Oviedo, V.; Alegría, L.; et al. Exploring the relationship between capillary refill time, skin blood flow and microcirculatory reactivity during early resuscitation of patients with septic shock: A pilot study. J. Clin. Monit. Comput. 2023, 37, 839–845. [Google Scholar] [CrossRef]
- Hernandez, G.; Bruhn, A.; Castro, R.; Pedreros, C.; Rovegno, M.; Kattan, E.; Veas, E.; Fuentealba, A.; Regueira, T.; Ruiz, C.; et al. Persistent Sepsis-Induced Hypotension without Hyperlactatemia: A Distinct Clinical and Physiological Profile within the Spectrum of Septic Shock. Crit. Care Res. Pract. 2012, 2012, 536852. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Lamprea, S.; Barrera, S.; Acevedo, L.; Duque, C.; Trujillo, M.; Aguirre, V.; Jimenez, C. The association between prolonged capillary refill time and microcirculation changes in children with sepsis. BMC Pediatr. 2024, 24, 68. [Google Scholar] [CrossRef]
- Merdji, H.; Curtiaud, A.; Aheto, A.; Studer, A.; Harjola, V.P.; Monnier, A.; Duarte, K.; Girerd, N.; Kibler, M.; Ait-Oufella, H.; et al. Performance of Early Capillary Refill Time Measurement on Outcomes in Cardiogenic Shock: An Observational, Prospective Multicentric Study. Am. J. Respir. Crit. Care Med. 2022, 206, 1230–1238. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef]
- Yamada, N.K.; Szyld, E.; Strand, M.L.; Finan, E.; Illuzzi, J.L.; Kamath-Rayne, B.D.; Kapadia, V.S.; Niermeyer, S.; Schmölzer, G.M.; Williams, A.; et al. 2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2024, 149, e157–e166. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Duff, J.P.; Topjian, A.A.; Berg, M.D.; Chan, M.; Haskell, S.E.; Joyner, B.L., Jr.; Lasa, J.J.; Ley, S.J.; Raymond, T.T.; Sutton, R.M.; et al. 2019 American Heart Association Focused Update on Pediatric Advanced Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2019, 140, e904–e914. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef]
- Slovinski, A.P.; Hajjar, L.A.; Ince, C. Microcirculation in cardiovascular diseases. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3458–3468. [Google Scholar] [CrossRef]
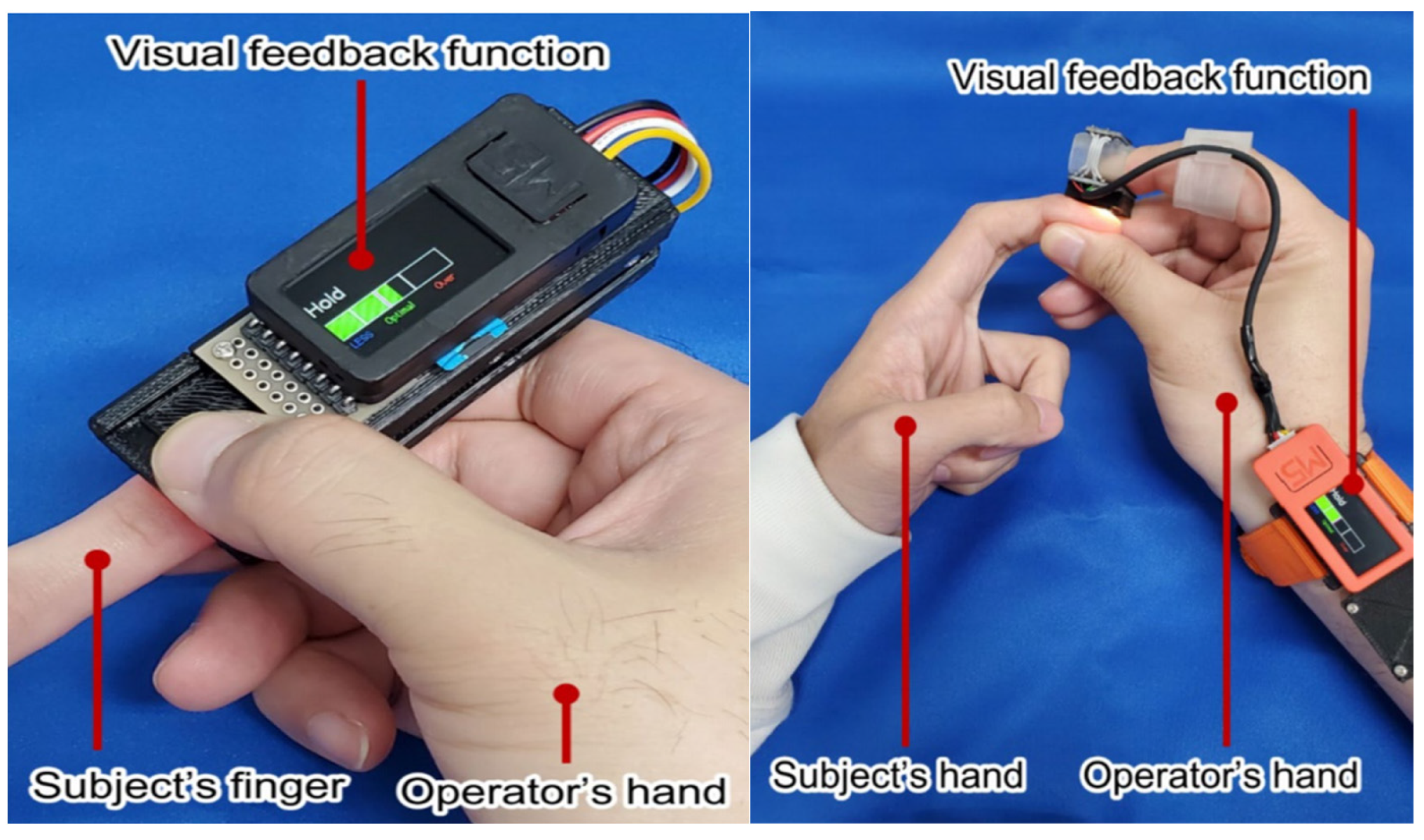
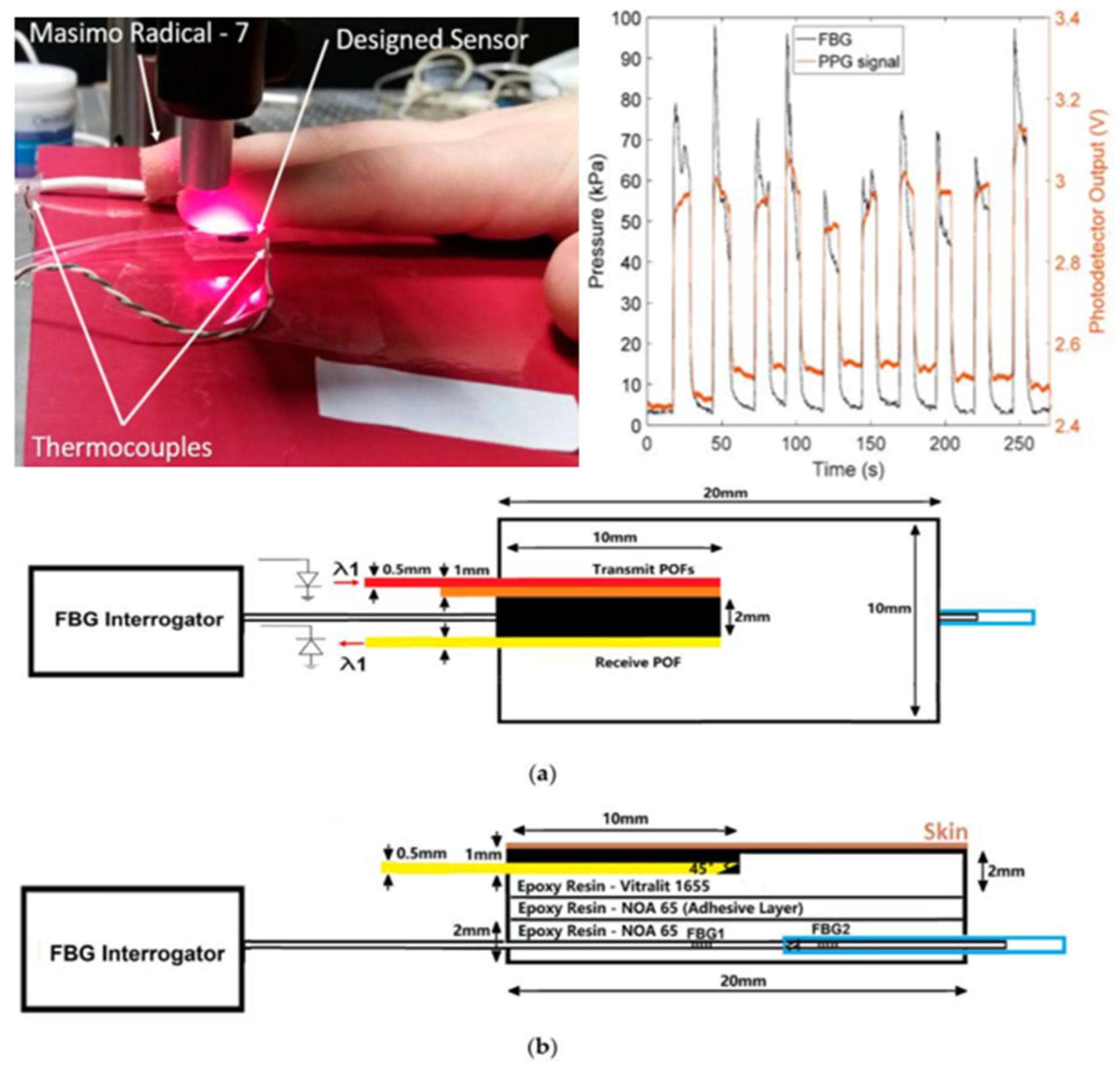
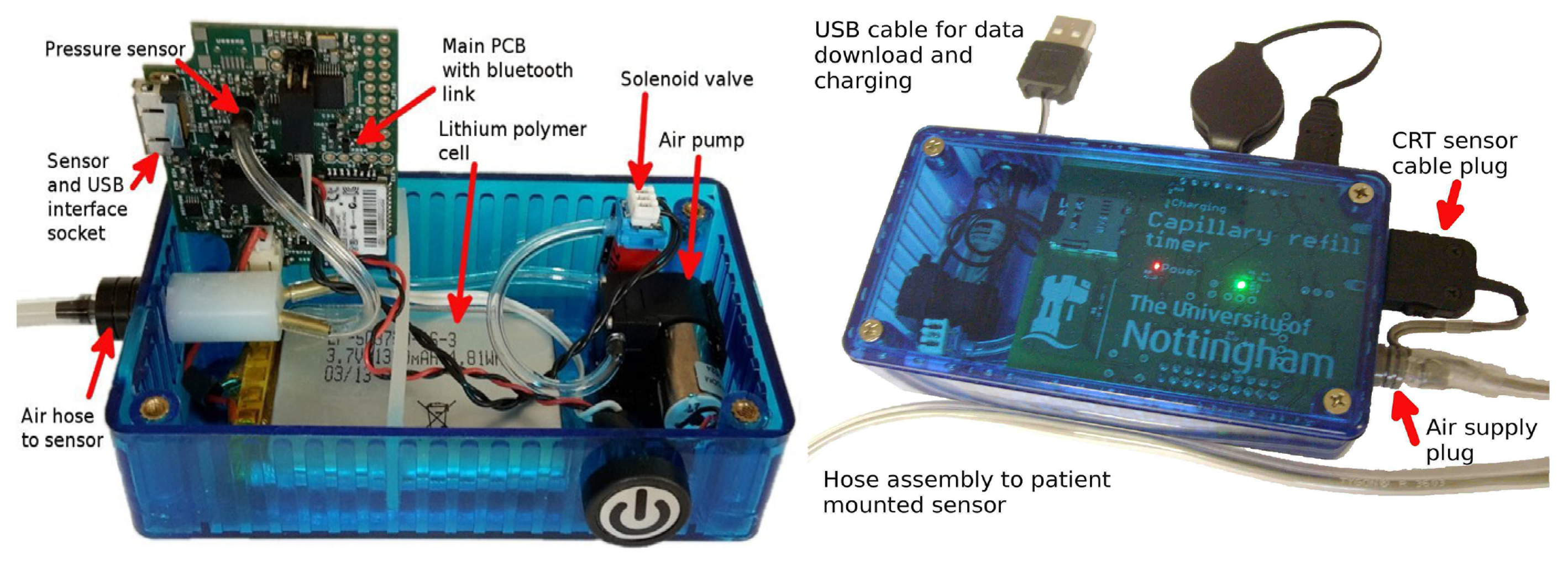
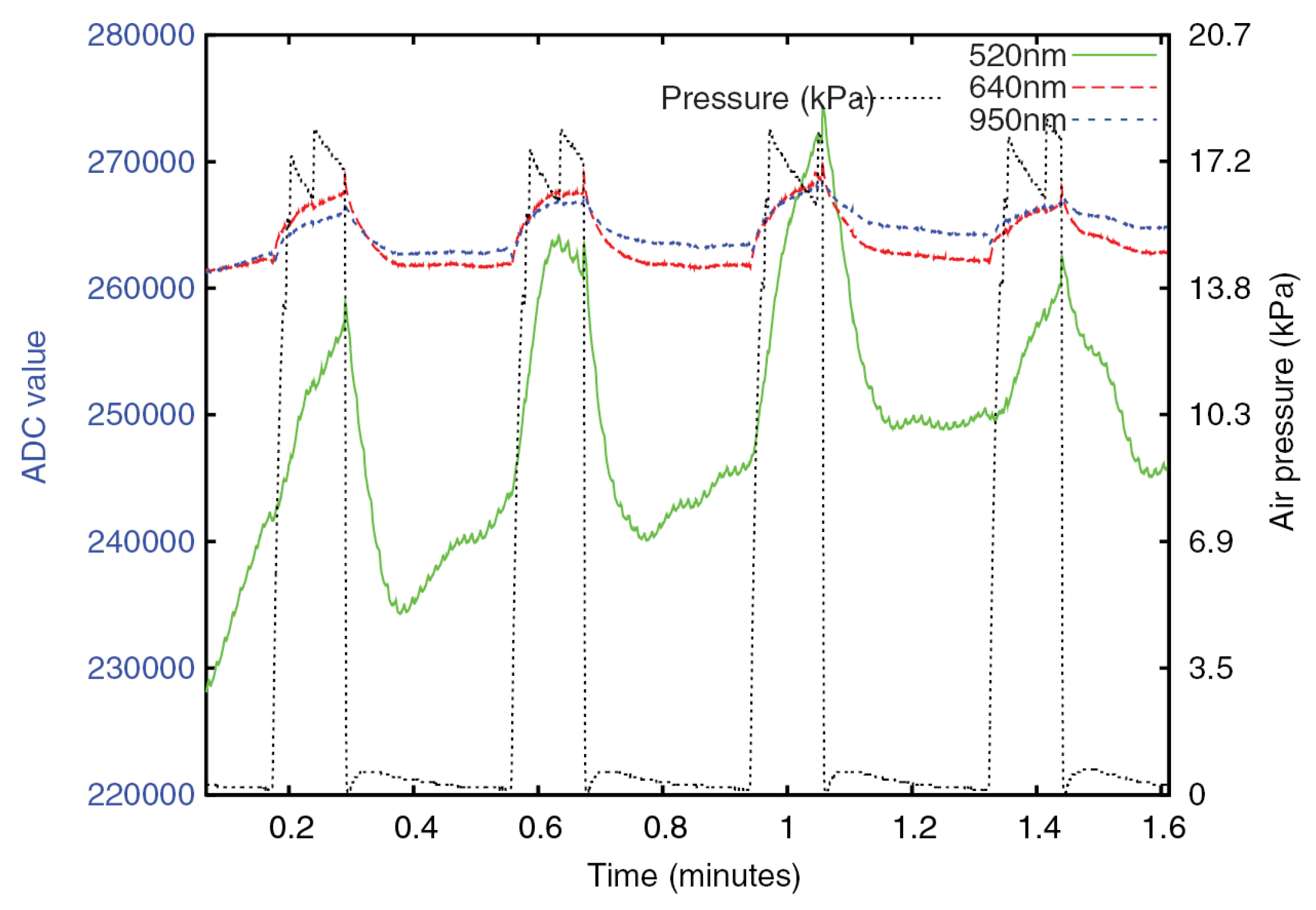
| Age Range | Sample Size | Type | Measurement Methods | Upper Limit of CRT | Refs. |
|---|---|---|---|---|---|
| Early neonatal period | 42 | Observational, cross-sectional | Use the fingers, soles of the heels, and sternum to press for 3–4 s, using a digital stopwatch. | Fingers: 2 s, chest, feet: 4 s | [20] |
| Newborn | 627 | Observational | Press the middle of the sternum for 5 s (supine position), take a measurement three times, and calculate the average. | 3 s | [21] |
| 469 | Cross-sectional | Press the chest, head, palms, and heels for 5 s, manually taking the time. | 3 s | [22] | |
| 137 | Cross-sectional | Apply pressure to the right hand and the instep of the right foot for 5 s. | Hand: 4.23 ± 1.47 s, foot: 4.64 ± 1.41 s | [23] | |
| Infants and children | 92 | Prospective, method comparison study | Apply enough pressure to the fingertips and sternum for 5 s to blanch the skin. | 2–3 s | [24] |
| Teenager | 20 | Observational | Using a digital camera, the average of the first and fifth toes is used as the CRT. | 3.5 s | [25] |
| Adult | 1000 | Prospective observational study | Use medium pressure to press the index finger of your right hand to the white point for 5 s and use a stopwatch to measure the time. | 3.5 s | [26] |
| Elderly | 1000 | Prospective observational study | Same as above. | 4.5 s | [26] |
| Methods | Advantages | Disadvantages | Refs | |
|---|---|---|---|---|
| 1. Manual measurement | Hand pressing method. | Fast, convenient, non-invasive, and inexpensive. | Observer bias and poor reproducibility. | [28] |
| Slide press method. | The color changes significantly, and the accuracy is higher than that of the bare hand pressing method. | Observer bias and poor reproducibility. | [29] | |
| 2. Semi-automatic measurement technology | Based on digital camera technology. | Accurate judgment of blood emptying and filling time points. | Data collected in a cumbersome, cluttered clinical environment are unstable and difficult to focus on; not suitable for people with darker skin or in poorly illuminated testing environments. | [36] |
| Wearable devices based on visual feedback technology. | Quantification of compression intensity and time to avoid their influence on measurement results. | It requires manual pressing by the operator and is easily affected by external light. | [39] | |
| Based on pressure-sensing technology and photoelectric capacitance wave technology. | It ensures blood drainage by measuring the contact pressure. | CRT measurement for fingers only. | [40,41,42,43,44,45,46] | |
| 3. Fully automatic measurement technology | Based on diffuse reflection and pneumatic pressure application system. | It eliminates observer bias through the standardization of pressure application and release and through the electronic measurement of diffuse reflectance. | Unable to release all the pressure instantly, resulting in larger measurement results. | [47,48] |
| Based on mechanical press actuators. | Accurate and efficient. | Single function, high cost, large instrument size, and inconvenient to carry. | [49,50,51] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Guo, Z.; Wang, X.; Wang, Z.; Wang, X.; Wang, Z. Research Progress on the Measurement Methods and Clinical Significance of Capillary Refill Time. Sensors 2024, 24, 7941. https://doi.org/10.3390/s24247941
Xia Y, Guo Z, Wang X, Wang Z, Wang X, Wang Z. Research Progress on the Measurement Methods and Clinical Significance of Capillary Refill Time. Sensors. 2024; 24(24):7941. https://doi.org/10.3390/s24247941
Chicago/Turabian StyleXia, Yuxiang, Zhe Guo, Xinrui Wang, Ziyi Wang, Xuesong Wang, and Zhong Wang. 2024. "Research Progress on the Measurement Methods and Clinical Significance of Capillary Refill Time" Sensors 24, no. 24: 7941. https://doi.org/10.3390/s24247941
APA StyleXia, Y., Guo, Z., Wang, X., Wang, Z., Wang, X., & Wang, Z. (2024). Research Progress on the Measurement Methods and Clinical Significance of Capillary Refill Time. Sensors, 24(24), 7941. https://doi.org/10.3390/s24247941






