The Neurological and Hemodynamics Safety of an Airway Clearance Technique in Patients with Acute Brain Injury: An Analysis of Intracranial Pressure Pulse Morphology Using a Non-Invasive Sensor
Abstract
1. Introduction
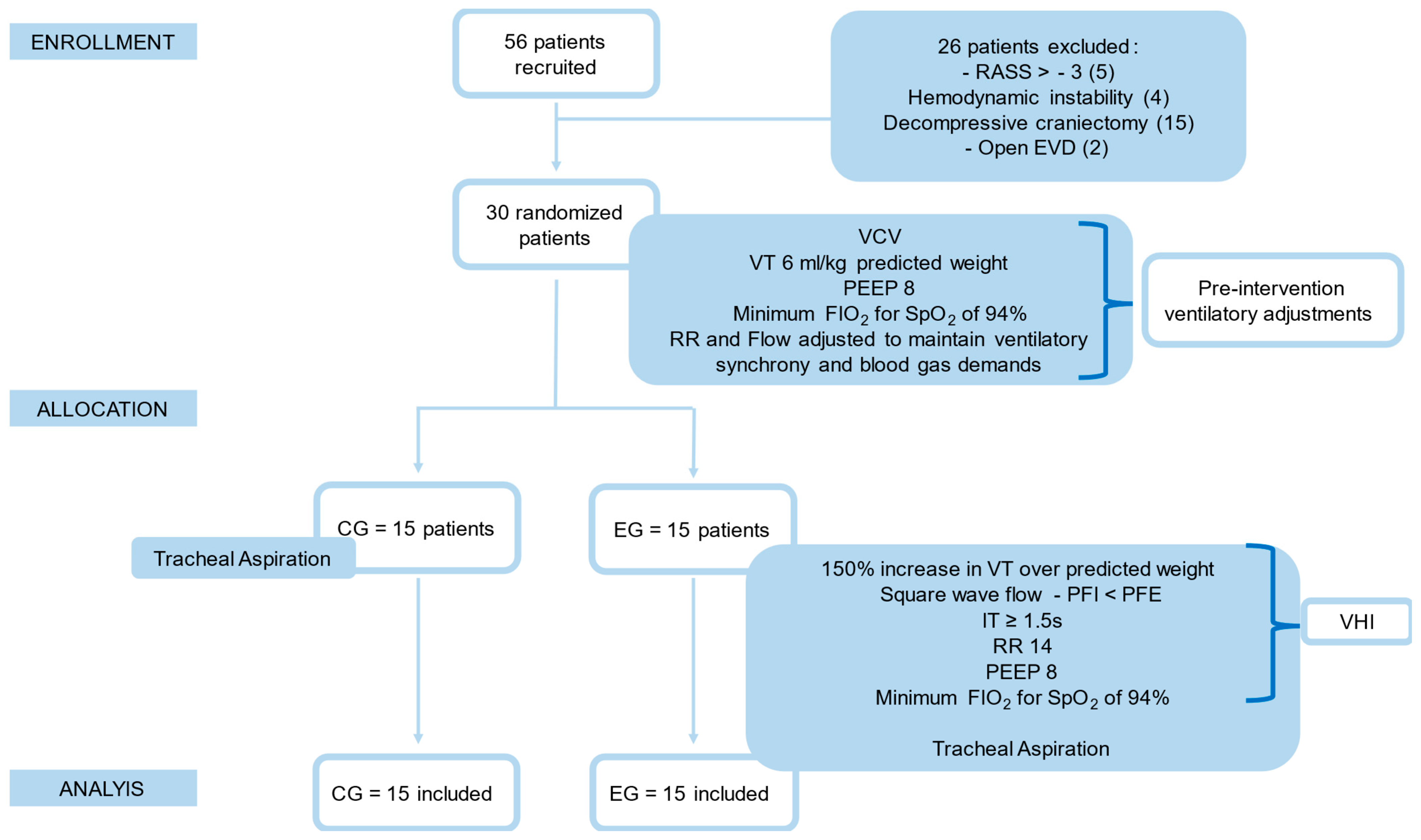
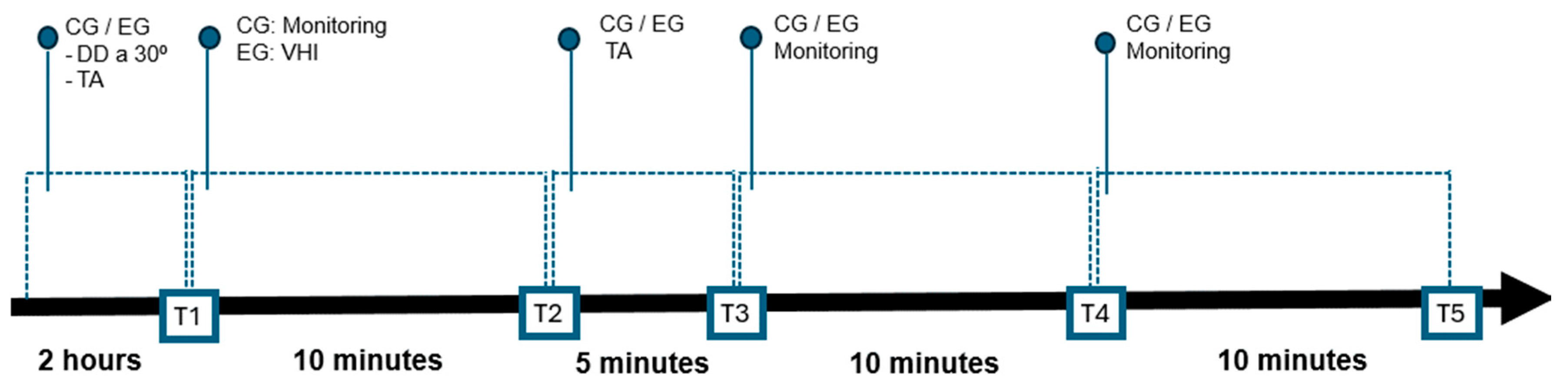
2. Materials and Methods
3. Results
Baseline Characteristics of the Patients
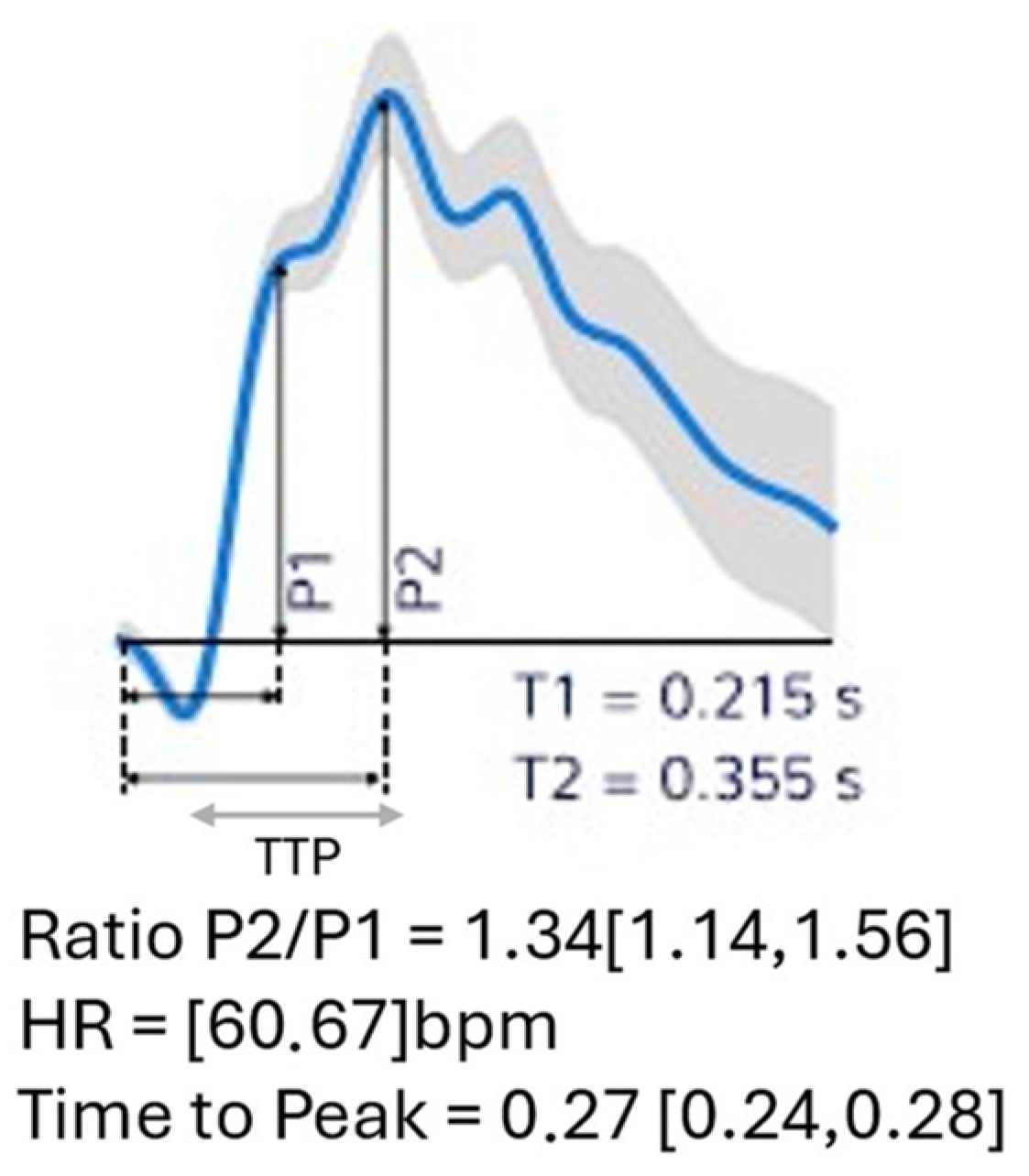
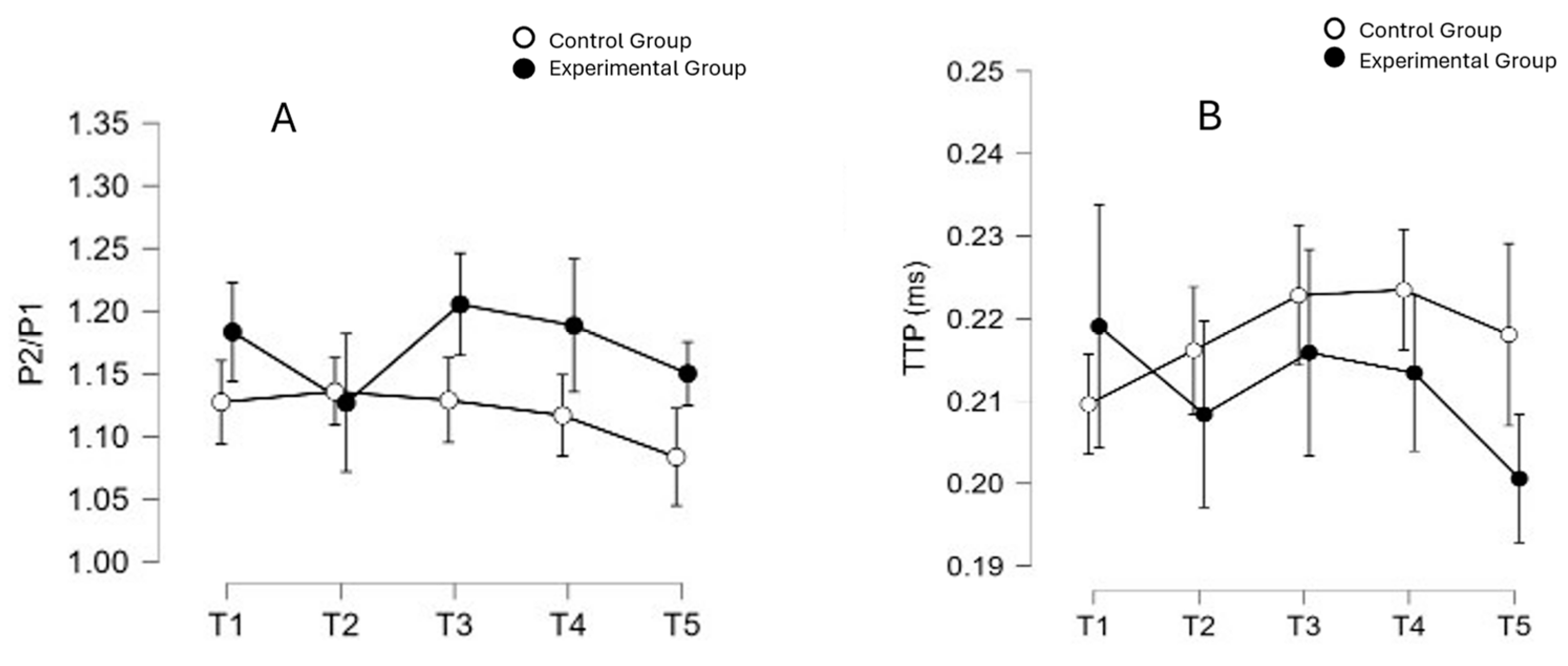
4. Neurological Safety of VHI
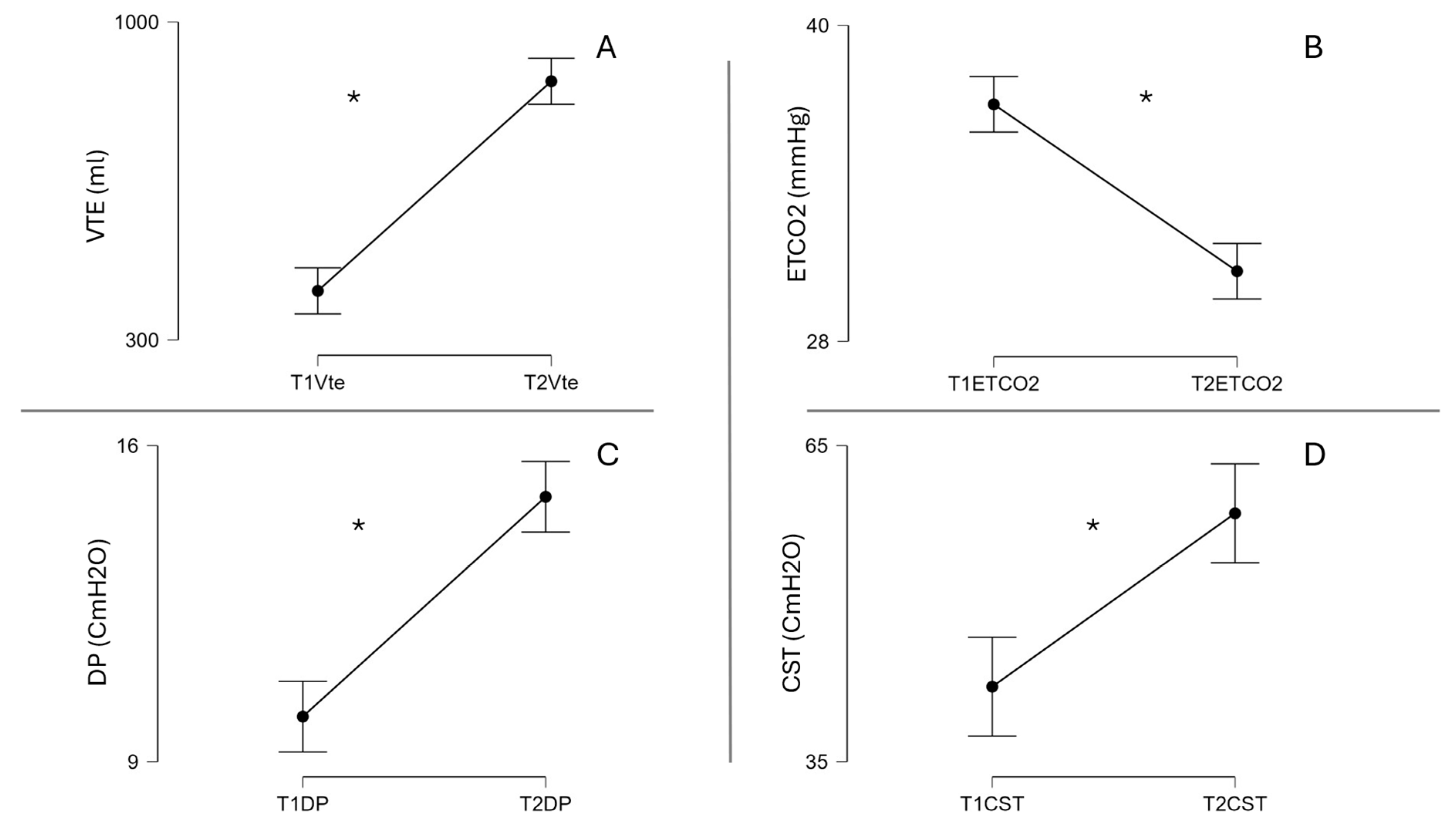
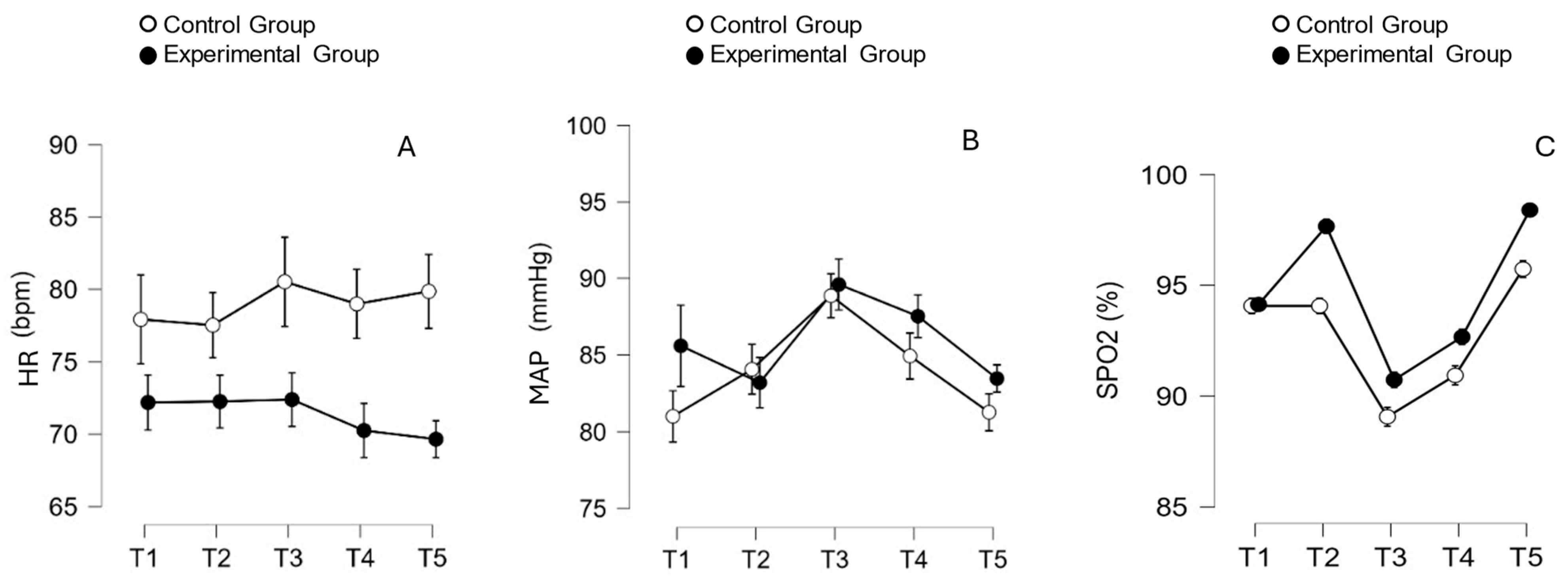
5. Hemodynamics Safety of VHI
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robba, C.; Zanier, E.R.; Soto, C.L.; Park, S.; Sonneville, R.; Helbolk, R.; Sarwal, A.; Newcombe, V.F.J.; van der Jagt, M.; Gunst, J.; et al. Mastering the brain in critical conditions: An update. Intensive Care Med. Exp. 2024, 12, 1. [Google Scholar] [CrossRef]
- Frisvold, S.; Coppola, S.; Ehrmann, S.; Chiumello, D.; Guérin, C. Respiratory challenges and ventilatory management in different types of acute brain-injured patients. Crit. Care 2023, 27, 247. [Google Scholar] [CrossRef] [PubMed]
- Ziaka, M.; Exadaktylos, A. Brain–lung interactions and mechanical ventilation in patients with isolated brain injury. Crit. Care 2021, 25, 358. [Google Scholar] [CrossRef] [PubMed]
- Beqiri, E.; Smielewski, P.; Guérin, C.; Czosnyka, M.; Robba, C.; Bjertnæs, L.; Frisvold, S.K. Neurological and respiratory effects of lung protective ventilation in acute brain injury patients without lung injury: Brain vent, a single centre randomized interventional study. Crit. Care 2023, 27, 115. [Google Scholar] [CrossRef] [PubMed]
- Frisvold, S.K.; Robba, C.; Guérin, C. What respiratory targets should be recommended in patients with brain injury and respiratory failure? Intensive Care Med. 2019, 45, 683–686. [Google Scholar] [CrossRef]
- Paratz, J.; Ntoumenopoulos, G. Detection of Secretion Retention in the Ventilated Patient. Curr. Respir. Med. Rev. 2015, 10, 151–157. [Google Scholar] [CrossRef]
- Asehnoune, K.; Mrozek, S.; Perrigault, P.F.; Seguin, P.; Dahyot-Fizelier, C.; Lasocki, S.S.; Pujol, A.; Martin, M.; Chabanne, R.; Muller, L.; et al. A multi-faceted strategy to reduce ventilation-associated mortality in brain-injured patients. The BI-VILI project: A nationwide quality improvement project. Intensive Care Med. 2017, 43, 957–970. [Google Scholar] [CrossRef]
- Cinotti, R.; Bouras, M.; Roquilly, A.; Asehnoune, K. Management and weaning from mechanical ventilation in neurologic patients. Ann. Transl. Med. 2018, 6, 381. [Google Scholar] [CrossRef]
- Faura, J.; Bustamante, A.; Miró-Mur, F.; Montaner, J. Stroke-induced immunosuppression: Implications for the prevention and prediction of post-stroke infections. J. Neuroinflamm. 2021, 18, 127. [Google Scholar] [CrossRef]
- Rodrigues-Gomes, R.M.; Martí, J.-D.; Rolán, R.M.; Gelabert-González, M. Rapid chest compression effects on intracranial pressure in patients with acute cerebral injury. Trials 2022, 23, 312. [Google Scholar] [CrossRef]
- Jelic, S.; A Cunningham, J.; Factor, P. Clinical review: Airway hygiene in the intensive care unit. Crit. Care 2008, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhou-Suckow, Z.; Duerr, J.; Hagner, M.; Agrawal, R.; Mall, M.A. Airway mucus, inflammation and remodeling: Emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017, 367, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Lai, W.; Alison, J.; Huang, S.; Milross, M. Effect of intrapulmonary percussive ventilation on intensive care unit length of stay, the incidence of pneumonia and gas exchange in critically ill patients: A systematic review. PLoS ONE 2021, 16, e0255005. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.P.; Lobo, L.L.; de Queiroz, R.S.; Saquetto, M.B.; Júnior, L.A.F.; Correia, H.F.; Silva, C.M.d.S.E.; Alves, I.G.N.; Neto, M.G. Effects of ventilator hyperinflation on pulmonary function and secretion clearance in adults receiving mechanical ventilation: A systematic review with meta-analysis. Heart Lung J. Cardiopulm. Acute Care 2022, 56, 8–23. [Google Scholar] [CrossRef]
- Lockstone, J.; Love, A.; Chian, K.; Garnham, K.; Brumby, S.; Parry, S.M. Benefits and risks of ventilator hyperinflation in mechanically ventilated intensive care patients: A systematic review and meta-analysis. Aust. Crit. Care 2023, 36, 1150–1158. [Google Scholar] [CrossRef]
- Robba, C.; Poole, D.; McNett, M.; Asehnoune, K.; Bösel, J.; Bruder, N.; Chieregato, A.; Cinotti, R.; Duranteau, J.; Einav, S.; et al. Mechanical ventilation in patients with acute brain injury: Recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020, 46, 2397–2410. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Citerio, G.; Hutchinson, P.; Kolias, A.; Meyfroidt, G.; Robba, C.; Stocchetti, N.; Chesnut, R. Intracranial pressure: Current perspectives on physiology and monitoring. Intensive Care Med. 2022, 48, 1471–1481. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Sarwal, A. Neuromonitoring in Critically Ill Patients. Crit. Care Med. 2023, 51, 525–542. [Google Scholar] [CrossRef]
- de Moraes, F.M.; Adissy, E.N.B.; Rocha, E.; Barros, F.C.D.; Freitas, F.G.R.; Miranda, M.; Valiente, R.A.; de Andrade, J.B.C.; Chaddad-Neto, E.A.; Silva, G.S. Multimodal monitoring intracranial pressure by invasive and noninvasive means. Sci. Rep. 2023, 13, 18404. [Google Scholar] [CrossRef]
- Nag, D.S.; Sahu, S.; Swain, A.; Kant, S. Intracranial pressure monitoring: Gold standard and recent innovations. World J. Clin. Cases 2019, 7, 1535–1553. [Google Scholar] [CrossRef]
- Brasil, S.; Chesnut, R.; Robba, C. Noninvasive neuromonitoring in acute brain injured patients. Intensive Care Med. 2024, 50, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, S.; Vilela, G.H.F.; Carlotti, C.; Damiano, L.E.G.; Seluque, W.; Colli, B.; Tanaka, K.; Wang, C.C.; Nonaka, K.O. The New ICP Minimally Invasive Method Shows That the Monro–Kellie Doctrine Is Not Valid. In Intracranial Pressure and Brain Monitoring XIV; Schuhmann, M.U., Czosnyka, M., Eds.; Springer: Vienna, Austria, 2012; pp. 117–120. [Google Scholar] [CrossRef]
- Brasil, S.; Solla, D.J.F.; Nogueira, R.D.C.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.S. Intracranial compliance assessed by intracranial pressure pulse waveform. Brain Sci. 2021, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, F.M.; Brasil, S.; Frigieri, G.; Robba, C.; Paiva, W.; Silva, G.S. ICP wave morphology as a screening test to exclude intracranial hypertension in brain-injured patients: A non-invasive perspective. J. Clin. Monit. Comput. 2024, 38, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Czosnyka, Z. Origin of intracranial pressure pulse waveform. Acta Neurochir. 2020, 162, 1815–1817. [Google Scholar] [CrossRef]
- Ocamoto, G.N.; Russo, T.L.; Zambetta, R.M.; Frigieri, G.; Hayashi, C.Y.; Brasil, S.; Rabelo, N.N.; Júnior, D.L.S. Intracranial Compliance Concepts and Assessment: A Scoping Review. Front. Neurol. 2021, 12, 756112. [Google Scholar] [CrossRef]
- de Almeida Souza, D.; Branco, M.W.; Carraro Junior, H.; Zocolotti, A.M.D.; Takeda, S.Y.M.; Valderramas, S. Mechanical hyperinflation maneuver and intracranial compliance of critical neurological patients: Protocol for a randomized controlled equivalence trial. Trials 2023, 24, 348. [Google Scholar] [CrossRef]
- Brasil, S.; Frigieri, G.; Taccone, F.S.; Robba, C.; Solla, D.J.F.; de Carvalho Nogueira, R.; Yoshikawa, M.H.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.S. Noninvasive intracranial pressure waveforms for estimation of intracranial hypertension and outcome prediction in acute brain-injured patients. J. Clin. Monit. Comput. 2022, 37, 753–760. [Google Scholar] [CrossRef]
- Andrade, R.D.A.P.; Oshiro, H.E.; Miyazaki, C.K.; Hayashi, C.Y.; de Morais, M.A.; Brunelli, R.; Carmo, J.P. A Nanometer Resolution Wearable Wireless Medical Device for Non Invasive Intracranial Pressure Monitoring. IEEE Sens. J. 2021, 21, 22270–22284. [Google Scholar] [CrossRef]
- Godoy, D.A.; Brasil, S.; Iaccarino, C.; Paiva, W.; Rubiano, A.M. The intracranial compartmental syndrome: A proposed model for acute brain injury monitoring and management. Crit. Care 2023, 27, 137. [Google Scholar] [CrossRef]
- Karuta, S.C.V.; Folchini, C.M.; Fachi, M.M.; Okumura, L.M.; Manços, G.D.R.; Ricieri, M.C.; Motta, F.A.; Maeda, A.K. Observational study of intracranial compliance analysis in neurologically healthy pediatric patients using a non-invasive device. Sci. Rep. 2024, 14, 19482. [Google Scholar] [CrossRef]
- Ocamoto, G.N.; da Silva, L.N.; Tomaz, C.d.S.R.; Hisatugu, M.T.; Frigieri, G.; Cardim, D.; Gonçalves, R.L.; Russo, T.L.; de Amorim, R.L.O. Characterization of intracranial compliance in healthy subjects using a noninvasive method—Results from a multicenter prospective observational study. J. Clin. Monit. Comput. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, F.M.; Rocha, E.; Barros, F.C.D.; Freitas, F.G.R.; Miranda, M.; Valiente, R.A.; de Andrade, J.B.C.; Neto, F.E.A.C.; Silva, G.S. Waveform Morphology as a Surrogate for ICP Monitoring: A Comparison Between an Invasive and a Noninvasive Method. Neurocrit. Care 2022, 37, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M. Brain Venous Blood Outflow. Neurocrit. Care 2019, 31, 249–250. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Diaz Arrastia, R.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef]
- Godoy, D.A.; Badenes, R.; Robba, C.; Cabezas, F.M. Hyperventilation in Severe Traumatic Brain Injury Has Something Changed in the Last Decade or Uncertainty Continues? A Brief Review. Front. Neurol. 2021, 12, 573237. [Google Scholar] [CrossRef]
- Cardim, D.; Giardina, A.; Ciliberti, P.; Battaglini, D.; Berardino, A.; Uccelli, A.; Czosnyka, M.; Roccatagliata, L.; Matta, B.; Patroniti, N.; et al. Short-term mild hyperventilation on intracranial pressure, cerebral autoregulation, and oxygenation in acute brain injury patients: A prospective observational study. Clin. Monit. Comput. 2024, 38, 753–762. [Google Scholar] [CrossRef]
- Chicayban, L.M. Efeitos agudos da hiperinsuflação com o ventilador com aumento do tempo inspiratório sobre a mecânica respiratória: Ensaio clínico cruzado randomizado. Rev. Bras. Ter. Intensiva 2019, 31, 289–295. [Google Scholar]
- Battaglini, D.; Ciaravolo, E.; Caiffa, S.; Delpiano, L.; Ball, L.; Vena, A.; Giacobbe, D.R.; Bassetti, M.; Matta, B.; Pelosi, P.; et al. Systemic and Cerebral Effects of Physiotherapy in Mechanically Ventilated Subjects. Respir. Care 2023, 68, 452–461. [Google Scholar] [CrossRef]
- Assmann, C.B.; Vieira, P.J.C.; Kutchak, F.; Rieder, M.d.M.; Forgiarini, S.G.I.; Junior, L.A.F. Lung hyperinflation by mechanical ventilation versus isolated tracheal aspiration in the bronchial hygiene of patients undergoing mechanical ventilation. Rev. Bras. Ter. Intensiva 2016, 28, 27–32. [Google Scholar] [CrossRef]
- Segar, J.L.; Merrill, D.C.; Chapleau, M.W.; Robillard, J.E. Hemodynamic Changes during Endotracheal Suctioning Are Mediated by Increased Autonomic Activity. Pediatr. Res. 1993, 33, 649–652. [Google Scholar] [CrossRef][Green Version]
- Valer, B.B.; Bonczynski, G.S.; Scheffer, K.D.; Forgiarini, S.G.I.; Eibel, B.; Lisboa Cordeiro, A.L.; Friedman, G.; Júnior, L.A.F. Ventilator versus manual hyperinflation in adults receiving mechanical ventilation: A systematic review. Physiother. Res. Int. 2022, 27, e1936. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Naue, W.; Herve, B.B.; Vieira, F.N.; Deponti, G.N.; De Fraga Martins, L.; Dias, A.S.; Vieira, S.R.R. Comparison of bronchial hygiene techniques in mechanically ventilated patients: A randomized clinical trial. Rev. Bras. Ter. Intensive 2019, 31, 39–46. [Google Scholar]
- Volpe, M.S.; Guimarães, F.S.; Morais, C.C.A. Airway clearance techniques for mechanically ventilated patients: Insights for optimization. Respir. Care 2020, 65, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Ntoumenopoulos, G. Are Short-Term Changes in Physiological Variables in ICU Patients as a Result of Physiotherapy of Any Clinical Relevance? Respir. Care 2023, 68, 549–552. [Google Scholar] [CrossRef]
| Total Sample (n = 30) | CG (n = 15) | EG (n = 15) | p Value | |
|---|---|---|---|---|
| Age (years) | 64.3 ± 13.9 | 66.4 ± 13.8 | 62.2 ± 14.1 | 0.411 |
| Sex (M/F) | 18/12 | 7/8 | 11/4 | 0.264 |
| BMI (kg/m2) | 21.6 ± 2.1 | 21.9 ± 2.0 | 21.2 ± 2.1 | 0.393 |
| APACHE II | 23.6 ± 3.7 | 22.2 ± 3.4 | 25 ± 3.6 | 0.045 * |
| RASS | −3.2 ± 0.6 | −3.3 ± 0.7 | −3.2 ± 0.5 | 0.577 |
| Intravenous sedation (Y/N) | 6/24 | 4/11 | 2/13 | 0.361 |
| Baseline characteristics for respiratory variables | ||||
| Cst (L/cmH2O) | 45.5 ± 12.7 | 48.9 ± 13.6 | 42.1 ± 11.0 | 0.142 |
| Cdyn (L/cmH2O) | 34.7 ± 8.78 | 37.7 ± 8.9 | 31.6 ± 7.7 | 0.054 |
| R (cmH2O/L/s) | 7 ± 3 | 6.1 ± 2.4 | 7.8 ± 3.3 | 0.123 |
| SD (cmH2O) | 9.43 ± 2.07 | 8.8 ± 2.1 | 10.0 ± 1.9 | 0.138 |
| ETCO2 (mmHg) | 37.5 ± 3.09 | 38.0 ± 3.6 | 37.0 ± 2.5 | 0.385 |
| Severity scores | ||||
| P2/P1 Ratio | 1.16 ± 0.176 | 1.127 ± 0.179 | 1.183 ± 0.176 | 0.395 |
| TTP (ms) | 0.21 ± 0.06 | 0.210 ± 0.054 | 0.219 ± 0.068 | 0.697 |
| GCS < 8 | 30 | 15 | 15 | |
| Type of brain injury | ||||
| HIC/HSA | 23/7 | 11/4 | 12/3 | 1.000 |
| ICH | 23 | 11 | 12 | |
| ICH score 3 | 10 | 3 | 7 | |
| ICH score 4 | 5 | 4 | 1 | |
| ICH score 5 | 8 | 4 | 4 | |
| HSA | 7 | 4 | 3 | |
| FISHER II | 3 | 1 | 2 | |
| FISCHER III | 3 | 2 | 1 | |
| FISCHER IV | 1 | 1 | 0 | |
| Comorbidities | ||||
| 1 Hypertension | 20 | 12 | 8 | |
| 2. Diabetes | 11 | 5 | 6 | |
| 3. Dyslipidaemia | 4 | 2 | 2 | |
| Dead in ICU (A/O) | 20/10 | 11/4 | 9/6 | 0.700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, D.d.A.; Devetak, G.F.; Branco, M.W.; Melo, R.L.; Tonial, J.L.; Delattre, A.M.; Valderramas, S.R. The Neurological and Hemodynamics Safety of an Airway Clearance Technique in Patients with Acute Brain Injury: An Analysis of Intracranial Pressure Pulse Morphology Using a Non-Invasive Sensor. Sensors 2024, 24, 7066. https://doi.org/10.3390/s24217066
Souza DdA, Devetak GF, Branco MW, Melo RL, Tonial JL, Delattre AM, Valderramas SR. The Neurological and Hemodynamics Safety of an Airway Clearance Technique in Patients with Acute Brain Injury: An Analysis of Intracranial Pressure Pulse Morphology Using a Non-Invasive Sensor. Sensors. 2024; 24(21):7066. https://doi.org/10.3390/s24217066
Chicago/Turabian StyleSouza, Daniela de Almeida, Gisele Francini Devetak, Marina Wolff Branco, Reinaldo Luz Melo, Jean Lucas Tonial, Ana Marcia Delattre, and Silvia Regina Valderramas. 2024. "The Neurological and Hemodynamics Safety of an Airway Clearance Technique in Patients with Acute Brain Injury: An Analysis of Intracranial Pressure Pulse Morphology Using a Non-Invasive Sensor" Sensors 24, no. 21: 7066. https://doi.org/10.3390/s24217066
APA StyleSouza, D. d. A., Devetak, G. F., Branco, M. W., Melo, R. L., Tonial, J. L., Delattre, A. M., & Valderramas, S. R. (2024). The Neurological and Hemodynamics Safety of an Airway Clearance Technique in Patients with Acute Brain Injury: An Analysis of Intracranial Pressure Pulse Morphology Using a Non-Invasive Sensor. Sensors, 24(21), 7066. https://doi.org/10.3390/s24217066






