A Sensorized 3D-Printed Knee Test Rig for Preliminary Experimental Validation of Patellar Tracking and Contact Simulation
Abstract
1. Introduction
2. Material and Methods
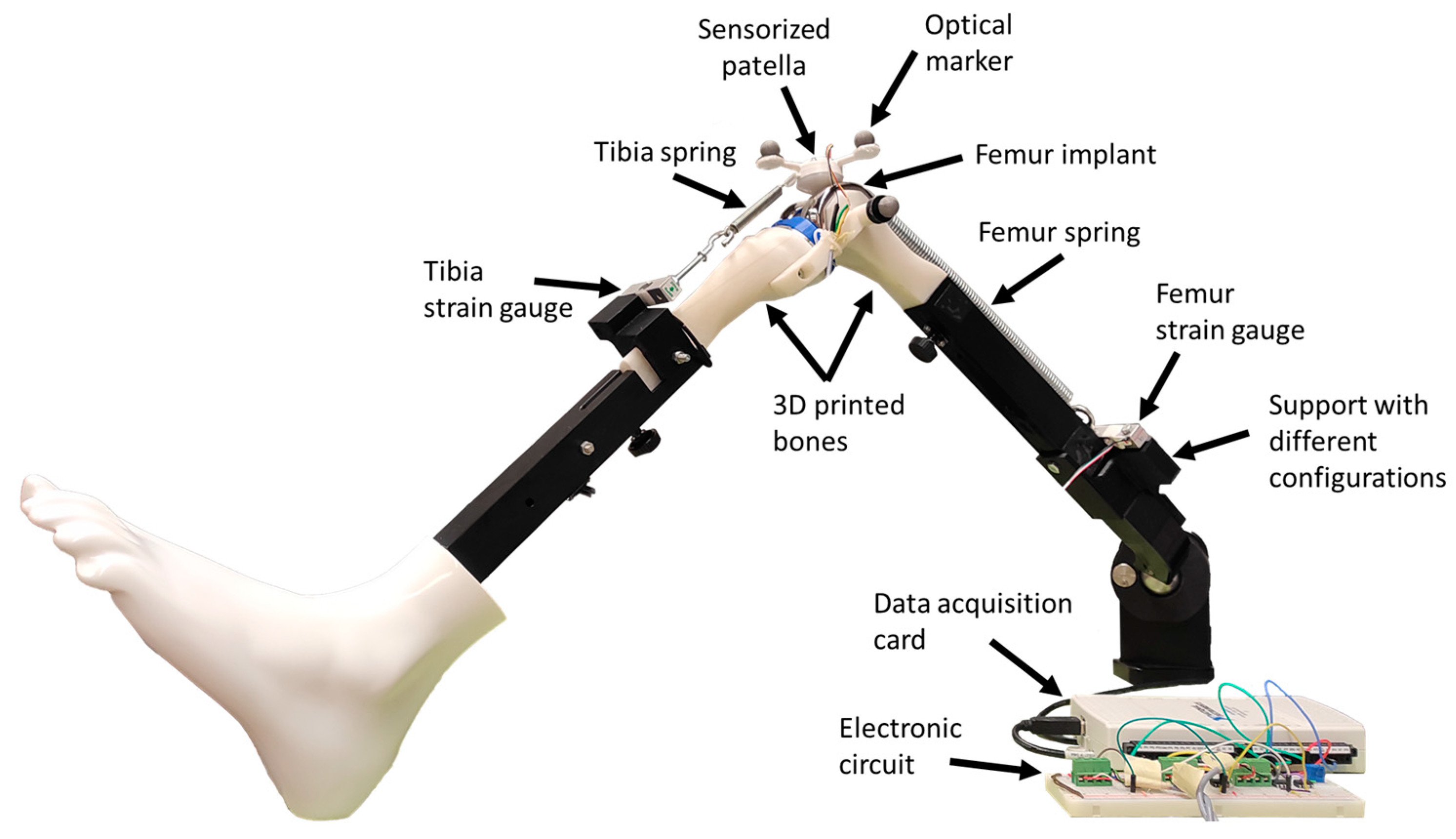
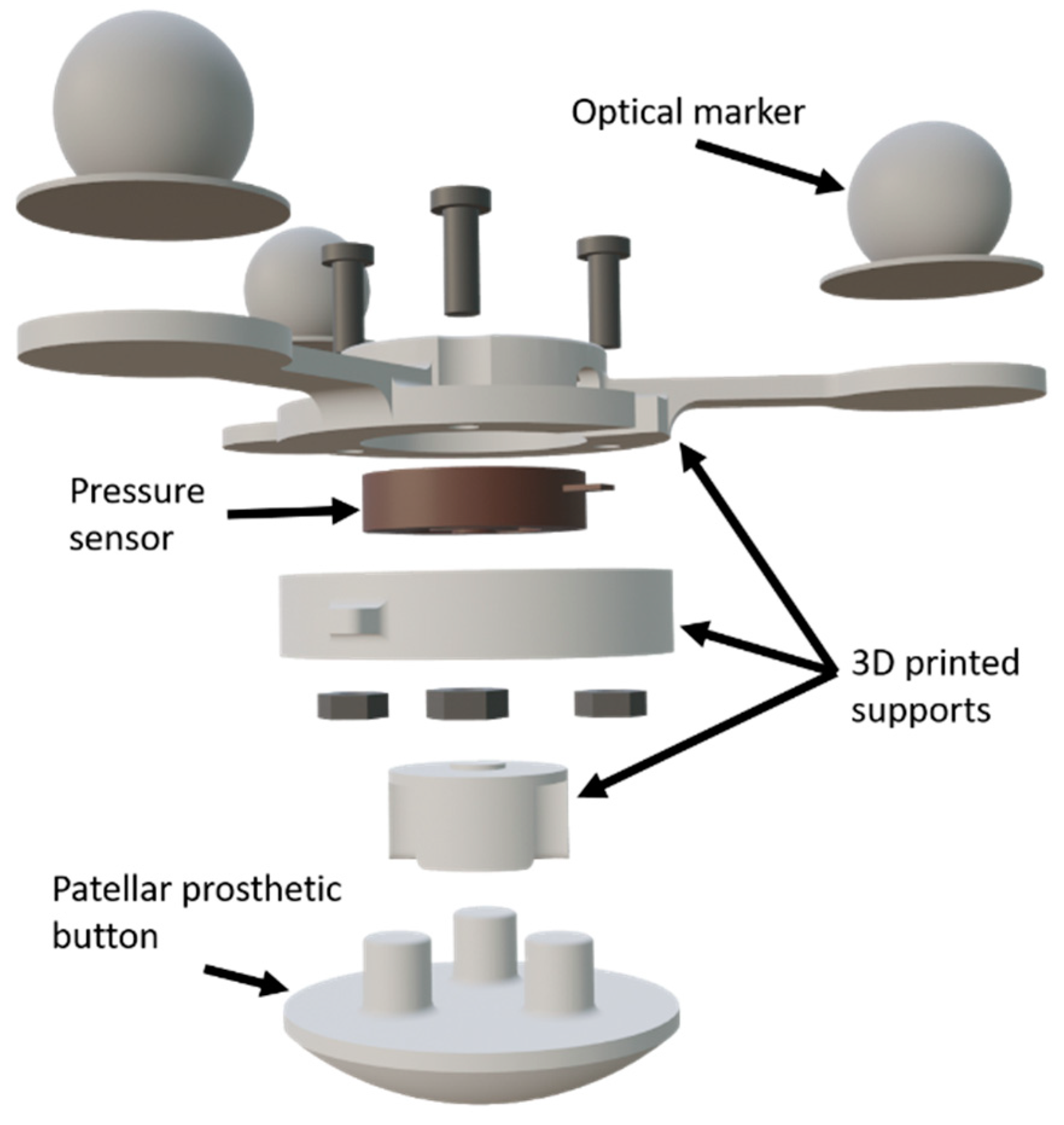
2.1. Test Bench
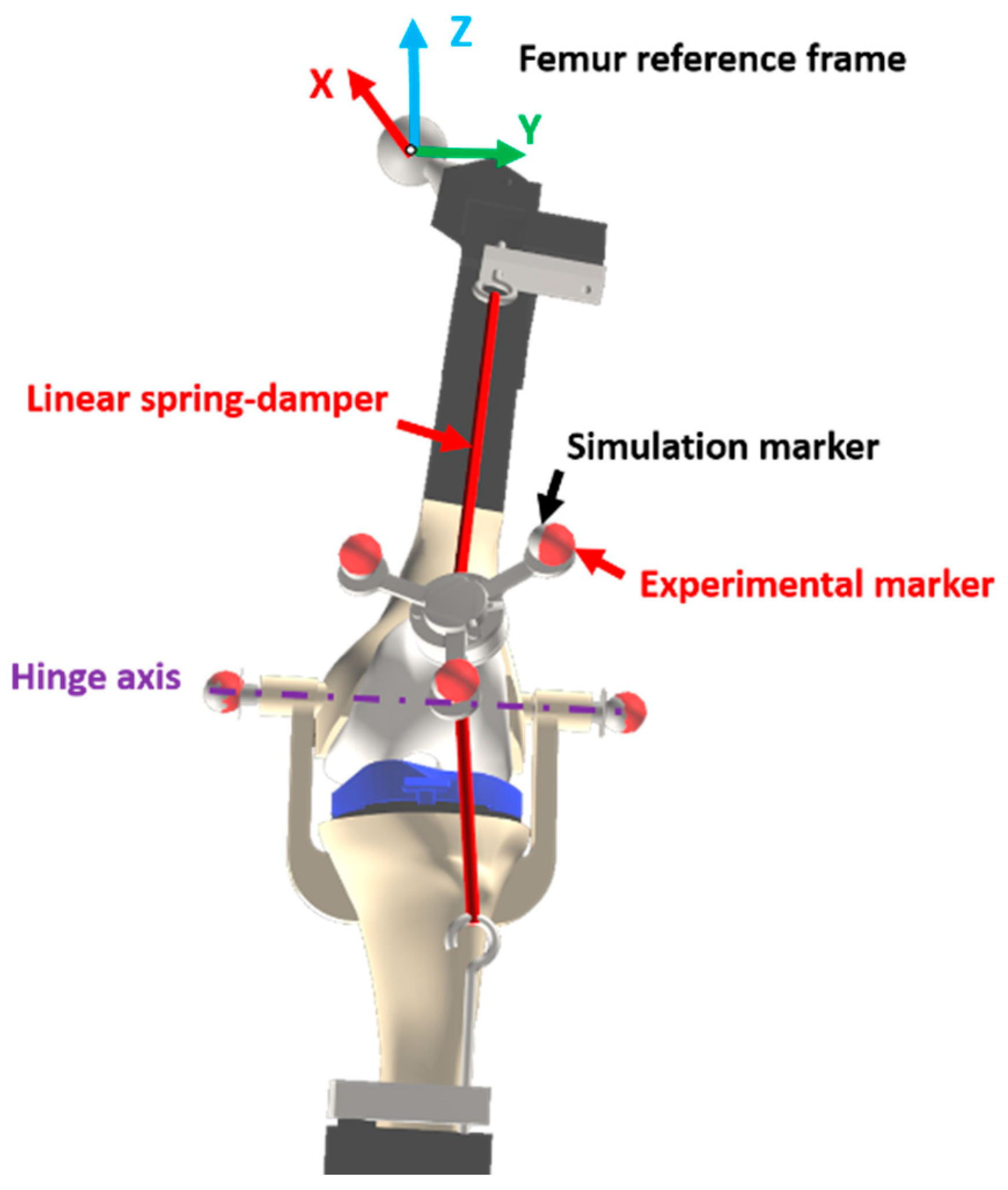
2.2. Movement and Experimental Data Collection
2.3. Computational Model
2.4. Simulation
2.4.1. Guiding
2.4.2. Formulation
2.4.3. Static Equilibrium
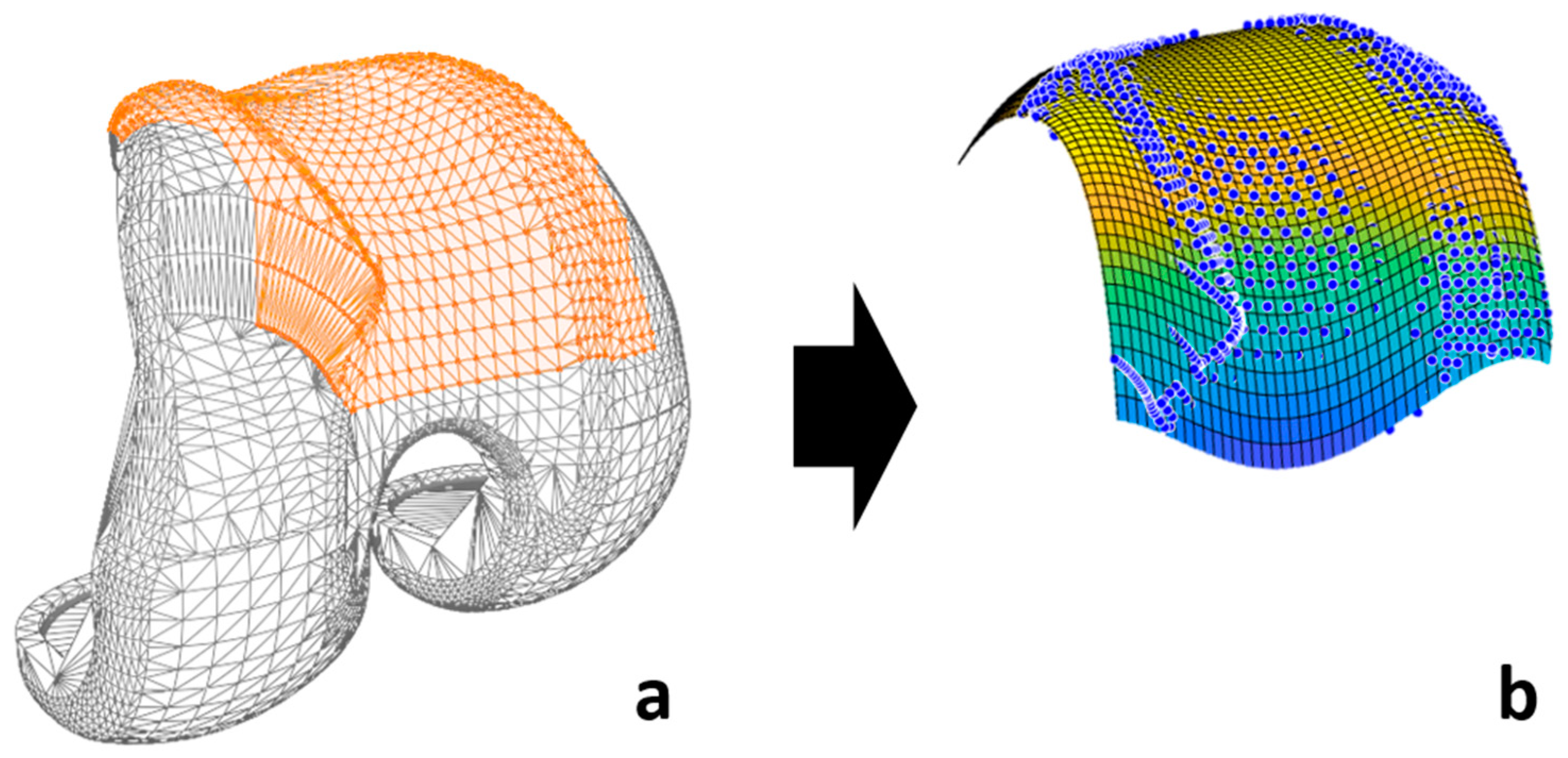
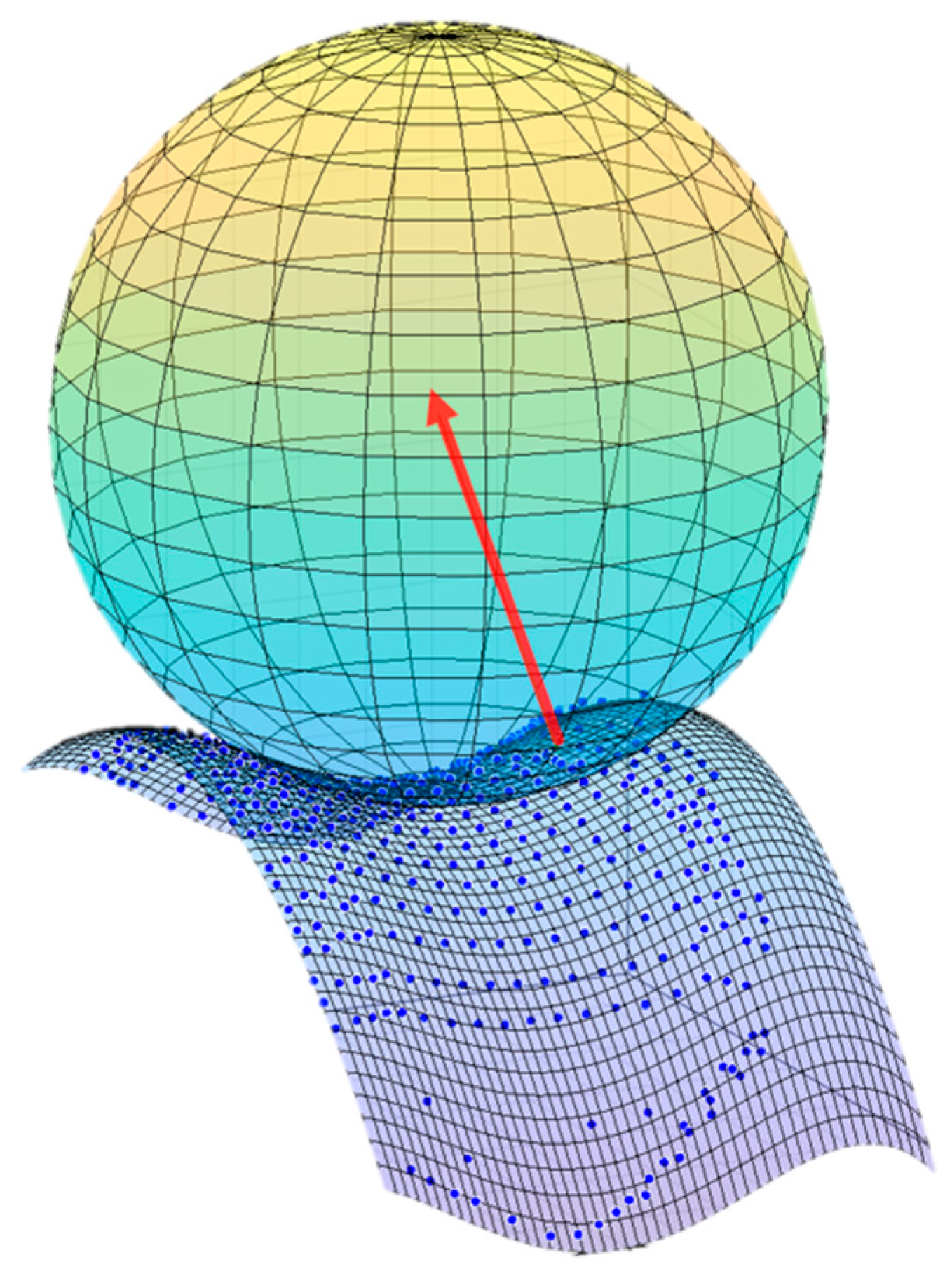
2.4.4. Contact Model and Detection
2.4.5. Experimental Validation
2.4.6. Computational Details
2.4.7. Optimization
3. Results
3.1. Sensorized 3D-Printed Knee Test Rig and Sensorized Patella
3.2. Computational Time
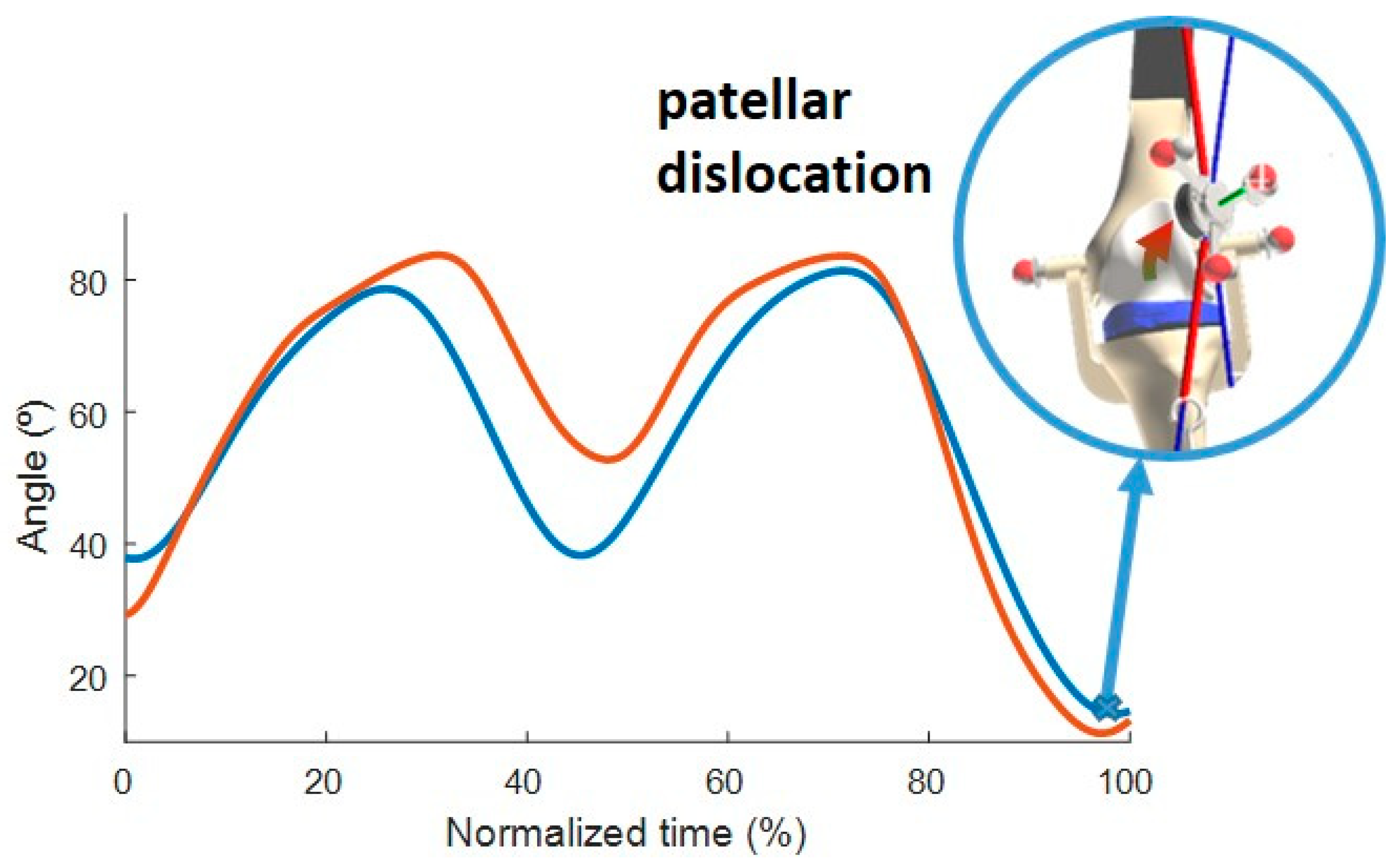
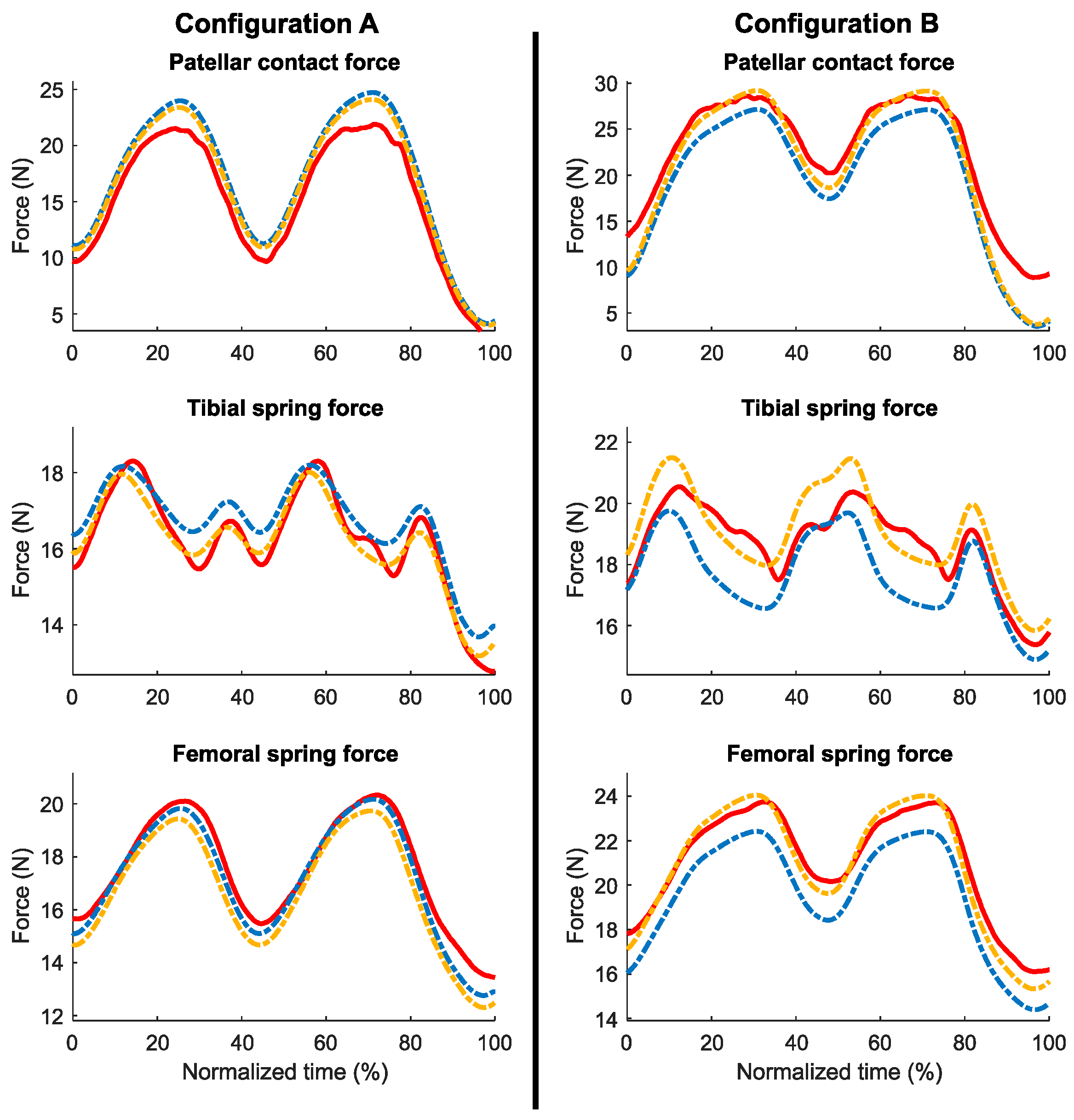
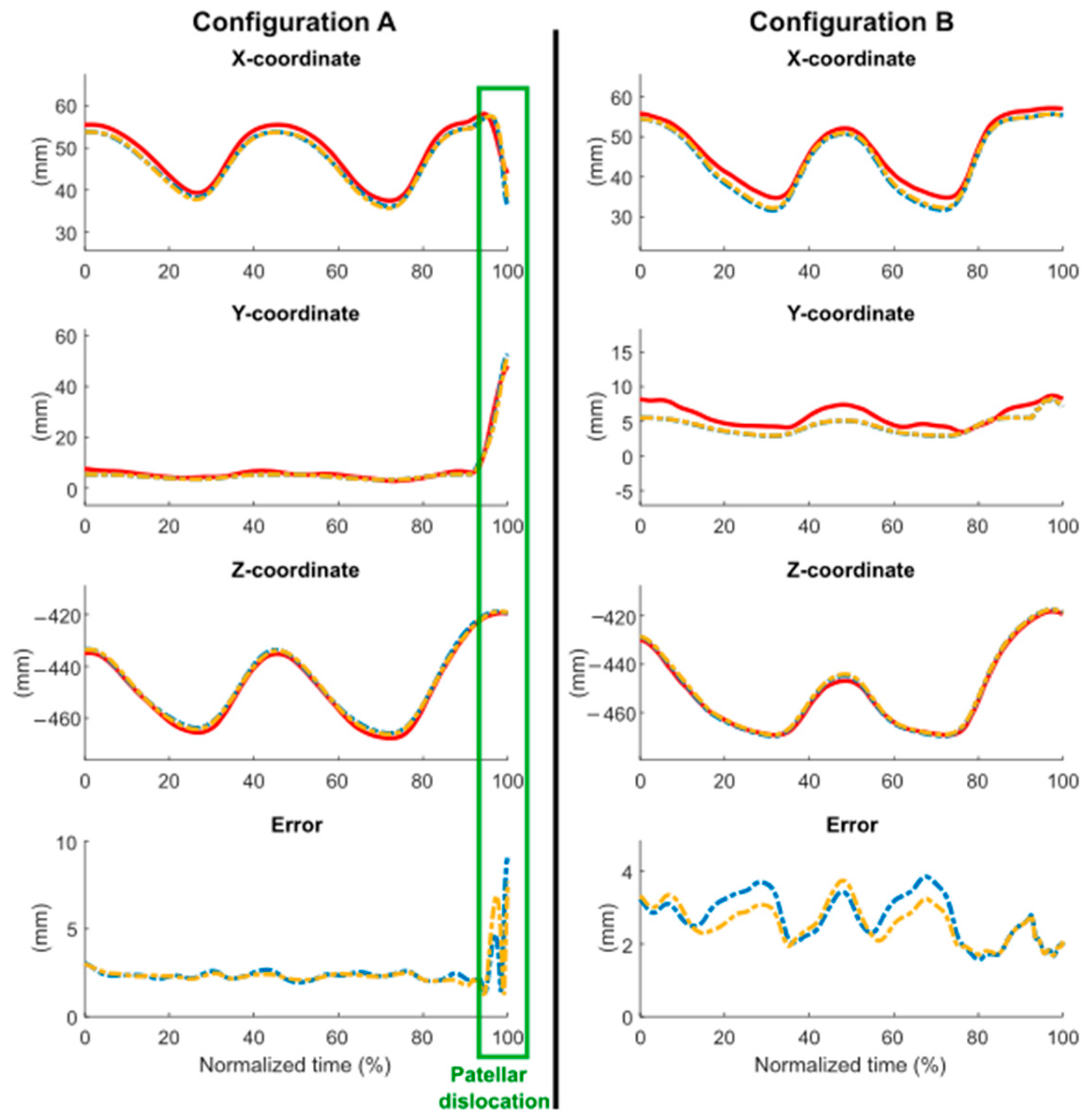
3.3. Comparison with Experimental Data
4. Discussion
4.1. Sensorized 3D-Printed Knee Test Rig and Sensorized Patella
4.2. Computational Time
4.3. Comparison with Experimental Data
4.4. Limitations
4.5. Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putman, S.; Boureau, F.; Girard, J.; Migaud, H.; Pasquier, G. Patellar complications after total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2019, 105, S43–S51. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Matar, W.Y.; Parvizi, J. Assessing patellar tracking during total knee arthroplasty: A technical note. Am. J. Orthop. 2012, 41, 450–451. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23376987 (accessed on 19 March 2024).
- Fregly, B.J.; Besier, T.F.; Lloyd, D.G.; Delp, S.L.; Banks, S.A.; Pandy, M.G.; D’Lima, D.D. Grand challenge competition to predict in vivo knee loads. J. Orthop. Res. 2012, 30, 503–513. [Google Scholar] [CrossRef]
- Kebbach, M.; Darowski, M.; Krueger, S.; Schilling, C.; Grupp, T.M.; Bader, R.; Geier, A. Musculoskeletal multibody simulation analysis on the impact of patellar component design and positioning on joint dynamics after unconstrained total knee arthroplasty. Materials 2020, 13, 2365. [Google Scholar] [CrossRef]
- Geier, A.; Tischer, T.; Bader, R. Simulation of varying femoral attachment sites of medial patellofemoral ligament using a musculoskeletal multi-body model. Curr. Dir. Biomed. Eng. 2015, 1, 547–551. [Google Scholar] [CrossRef]
- Pitto, L.; Kainz, H.; Falisse, A.; Wesseling, M.; Van Rossom, S.; Hoang, H.; Papageorgiou, E.; Hallemans, A.; Desloovere, K.; Molenaers, G.; et al. SimCP: A Simulation Platform to Predict Gait Performance Following Orthopedic Intervention in Children With Cerebral Palsy. Front. Neurorobot. 2019, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ao, D.; Vega, M.M.; Zandiyeh, P.; Chang, S.-H.; Penny, A.N.; Lewis, V.O.; Fregly, B.J. Changes in Walking Function and Neural Control following Pelvic Cancer Surgery with Reconstruction. Front. Bioeng. Biotechnol. 2024, 12. [Google Scholar] [CrossRef]
- Barberousse, A.; Franceschelli, S.; Imbert, C. Computer simulations as experiments. Synthese 2009, 169, 557–574. [Google Scholar] [CrossRef]
- Bentley, P.J.; Lim, S.L. From evolutionary ecosystem simulations to computational models of human behavior. WIREs Cogn. Sci. 2022, 13, e1622. [Google Scholar] [CrossRef]
- Fregly, B.J. A Conceptual Blueprint for Making Neuromusculoskeletal Models Clinically Useful. Appl. Sci. 2021, 11, 2037. [Google Scholar] [CrossRef]
- Dopico, D.; Luaces, A.; Saura, M.; Cuadrado, J.; Vilela, D. Simulating the anchor lifting maneuver of ships using contact detection techniques and continuous contact force models. Multibody Syst. Dyn. 2019, 46, 147–179. [Google Scholar] [CrossRef]
- Bei, Y.; Fregly, B.J. Multibody dynamic simulation of knee contact mechanics. Med. Eng. Phys. 2004, 26, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.J.; Kelly, M.J.; Smith, K.E.; Gall, K.A.; Farr, J. Dynamic Simulation of the Effects of Graft Fixation Errors During Medial Patellofemoral Ligament Reconstruction. Orthop. J. Sport. Med. 2016, 4, 232596711666508. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.D.; Blankevoort, L.; Ateshian, G.A. A Mathematical Formulation for 3D Quasi-Static Multibody Models of Diarthrodial Joints. Comput. Methods Biomech. Biomed. Engin. 2000, 3, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Keyak, J.H.; Powers, C.M. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: A finite element analysis study. Osteoarthr. Cartil. 2011, 19, 287–294. [Google Scholar] [CrossRef]
- Aksahin, E.; Kocadal, O.; Aktekin, C.N.; Kaya, D.; Pepe, M.; Yılmaz, S.; Yuksel, H.Y.; Bicimoglu, A. The effects of the sagittal plane malpositioning of the patella and concomitant quadriceps hypotrophy on the patellofemoral joint: A finite element analysis. Knee Surgery Sport. Traumatol. Arthrosc. 2016, 24, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Duke, K.; Mustafy, T.; Adeeb, S.M.; Ronsky, J.L.; El-Rich, M. A geometric approach to study the contact mechanisms in the patellofemoral joint of normal versus patellofemoral pain syndrome subjects. Comput. Methods Biomech. Biomed. Engin. 2015, 18, 391–400. [Google Scholar] [CrossRef]
- Fischer, M.; De Pieri, E.; Lund, M.; Damm, P.; Ferguson, S.; Radermacher, K. Impact of Underlying Cadaver Data on the Validity of Musculoskeletal Multibody Simulations. In Proceedings of the 25th Congress of the European Society of Biomechanics, Vienna, Austria, 7–10 July 2019; pp. 1–2. [Google Scholar]
- Tesfaye, S.; Hamba, N.; Kebede, W.; Bajiro, M.; Debela, L.; Nigatu, T.A.; Gerbi, A. Assessment of Ethical Compliance of Handling and Usage of the Human Body in Anatomical Facilities of Ethiopian Medical Schools. Pragmatic Obs. Res. 2021, 12, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Mita. LEFT LEG Collateral Ligament Release Workstation with Locking Foot. Available online: https://www.medical-models.com/left-leg-collateral-ligament-release-workstation-with-locking-foot-c2x14108433 (accessed on 23 May 2023).
- Dopico, D. MBSLIM: Multibody Systems en Laboratorio de Ingeniería Mecánica. 2016. Available online: https://lim.ii.udc.es/MBSLIM (accessed on 19 March 2024).
- Katchburian, M.V.; Bull, A.M.J.; Shih, Y.F.; Heatley, F.W.; Amis, A.A. Measurement of patellar tracking: Assessment and analysis of the literature. Clin. Orthop. Relat. Res. 2003, 412, 241–259. [Google Scholar] [CrossRef]
- Best, M.J.; Tanaka, M.J.; Demehri, S.; Cosgarea, A.J. Accuracy and Reliability of the Visual Assessment of Patellar Tracking. Am. J. Sports Med. 2020, 48, 370–375. [Google Scholar] [CrossRef]
- Cuadrado, J.; Michaud, F.; Lugrís, U.; Pérez Soto, M. Using Accelerometer Data to Tune the Parameters of an Extended Kalman Filter for Optical Motion Capture: Preliminary Application to Gait Analysis. Sensors 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Alonso, F.J.J.; Cubero, J.; Galán-marín, G. An automatic SSA-based de-noising and smoothing technique for surface electromyography signals. Biomed. Signal Process. Control 2015, 18, 317–324. [Google Scholar] [CrossRef]
- Khasawneh, R.R.; Allouh, M.Z.; Abu-El-Rub, E. Measurement of the quadriceps (Q) angle with respect to various body parameters in young Arab population. PLoS ONE 2019, 14, e0218387. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Orozco, G.A.; Korhonen, R.K.; García, J.J.; Mononen, M.E. Expediting Finite Element Analyses for Subject-Specific Studies of Knee Osteoarthritis: A Literature Review. Appl. Sci. 2021, 11, 11440. [Google Scholar] [CrossRef]
- Vaughan, C.L.; Davis, B.L.; O’Connor, J.C. Dynamics of Human Gait, 2nd ed.; Kiboho Publishers: Cape Town, South Africa, 1999; ISBN 0-620-23558-6. [Google Scholar]
- Dopico, D.; González, F.; Cuadrado, J.; Kövecses, J. Determination of Holonomic and Nonholonomic Constraint Reactions in an Index-3 Augmented Lagrangian Formulation With Velocity and Acceleration Projections. J. Comput. Nonlinear Dyn. 2014, 9, 041006. [Google Scholar] [CrossRef]
- Cuadrado, J.; Gutiérrez, R.; Naya, M.A.; Morer, P. A comparison in terms of accuracy and efficiency between a MBS dynamic formulation with stress analysis and a non-linear FEA code. Int. J. Numer. Methods Eng. 2001, 51, 1033–1052. [Google Scholar] [CrossRef]
- Bayo, E.; Ledesma, R. Augmented lagrangian and mass-orthogonal projection methods for constrained multibody dynamics. Nonlinear Dyn. 1996, 9, 113–130. [Google Scholar] [CrossRef]
- Gavrea, B.; Negrut, D.; Potra, F.A. The Newmark Integration Method for Simulation of Multibody Systems: Analytical Considerations. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Orlando, FL, USA, 5–11 November 2005; pp. 1079–1092. [Google Scholar]
- Flores, P.; Machado, M.; Silva, M.T.; Martins, J.M. On the continuous contact force models for soft materials in multibody dynamics. Multibody Syst. Dyn. 2011, 25, 357–375. [Google Scholar] [CrossRef]
- Dopico, D.; Luaces, A.; Gonzalez, M.; Cuadrado, J. Dealing with multiple contacts in a human-in-the-loop application. Multibody Syst. Dyn. 2011, 25, 167–183. [Google Scholar] [CrossRef]
- Yastrebov, V.A.; Breitkopf, P. (Eds.) Numerical Methods in Contact Mechanics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 9781118647974. [Google Scholar]
- Maletsky, L.P.; Hillberry, B.M. Simulating Dynamic Activities Using a Five-Axis Knee Simulator. J. Biomech. Eng. 2005, 127, 123–133. [Google Scholar] [CrossRef]
- Halloran, J.P.; Clary, C.W.; Maletsky, L.P.; Taylor, M.; Petrella, A.J.; Rullkoetter, P.J. Verification of Predicted Knee Replacement Kinematics During Simulated Gait in the Kansas Knee Simulator. J. Biomech. Eng. 2010, 132, 081010. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Vakanski, A.; Xian, M.; Paul, D.; Baker, R. A review of computational approaches for evaluation of rehabilitation exercises. Comput. Biol. Med. 2020, 119, 103687. [Google Scholar] [CrossRef] [PubMed]
- Michaud, F.; Luaces, A.; Mouzo, F.; Cuadrado, J. Use of patellofemoral digital twins for patellar tracking and treatment prediction: Comparison of 3D models and contact detection algorithms. Front. Bioeng. Biotechnol. 2024, 12, 1347720. [Google Scholar] [CrossRef] [PubMed]
- Lugrís, U.; Pérez-Soto, M.; Michaud, F.; Cuadrado, J. Human motion capture, reconstruction, and musculoskeletal analysis in real time. Multibody Syst. Dyn. 2023, 60, 3–25. [Google Scholar] [CrossRef]


| Configuration A | Configuration B | ||||
| Original | Optimized | Original | Optimized | ||
| Bias | Contact Force (N) | −1.82 | −1.38 | 2.83 | 1.40 |
| Tibial Spring Force (N) | −0.46 | 0.04 | 1.03 | −0.40 | |
| Femoral Spring Force (N) | 0.36 | 0.80 | 1.47 | 0.12 | |
| X-coord. (mm) | 1.51 | 1.58 | 2.04 | 1.65 | |
| Y-coord. (mm) | 0.59 | 0.72 | 1.37 | 1.33 | |
| Z-coord. (mm) | −1.29 | −1.09 | −0.51 | −1.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaud, F.; Mouzo, F.; Dopico, D.; Cuadrado, J. A Sensorized 3D-Printed Knee Test Rig for Preliminary Experimental Validation of Patellar Tracking and Contact Simulation. Sensors 2024, 24, 3042. https://doi.org/10.3390/s24103042
Michaud F, Mouzo F, Dopico D, Cuadrado J. A Sensorized 3D-Printed Knee Test Rig for Preliminary Experimental Validation of Patellar Tracking and Contact Simulation. Sensors. 2024; 24(10):3042. https://doi.org/10.3390/s24103042
Chicago/Turabian StyleMichaud, Florian, Francisco Mouzo, Daniel Dopico, and Javier Cuadrado. 2024. "A Sensorized 3D-Printed Knee Test Rig for Preliminary Experimental Validation of Patellar Tracking and Contact Simulation" Sensors 24, no. 10: 3042. https://doi.org/10.3390/s24103042
APA StyleMichaud, F., Mouzo, F., Dopico, D., & Cuadrado, J. (2024). A Sensorized 3D-Printed Knee Test Rig for Preliminary Experimental Validation of Patellar Tracking and Contact Simulation. Sensors, 24(10), 3042. https://doi.org/10.3390/s24103042









