Guiding Breathing at the Resonance Frequency with Haptic Sensors Potentiates Cardiac Coherence
Abstract
1. Introduction
2. Methods
2.1. Participants
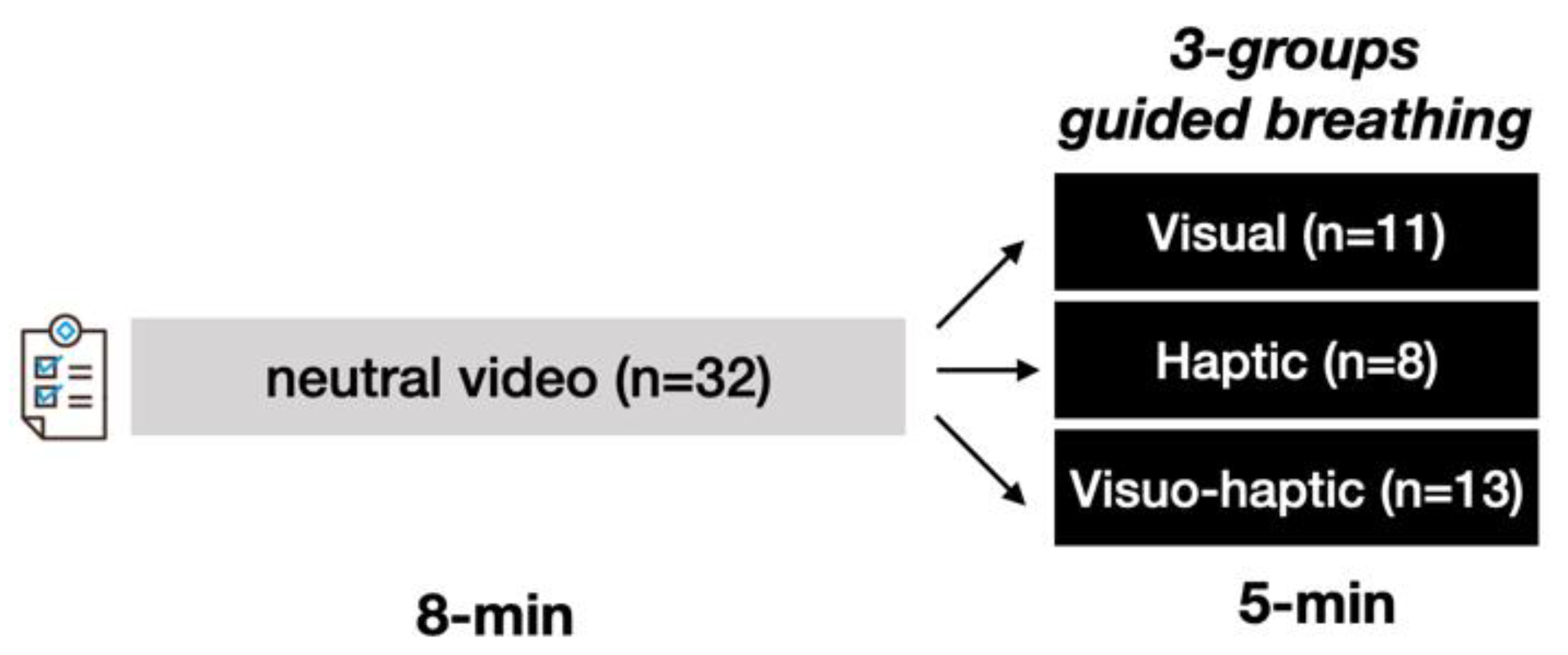
2.2. Protocol
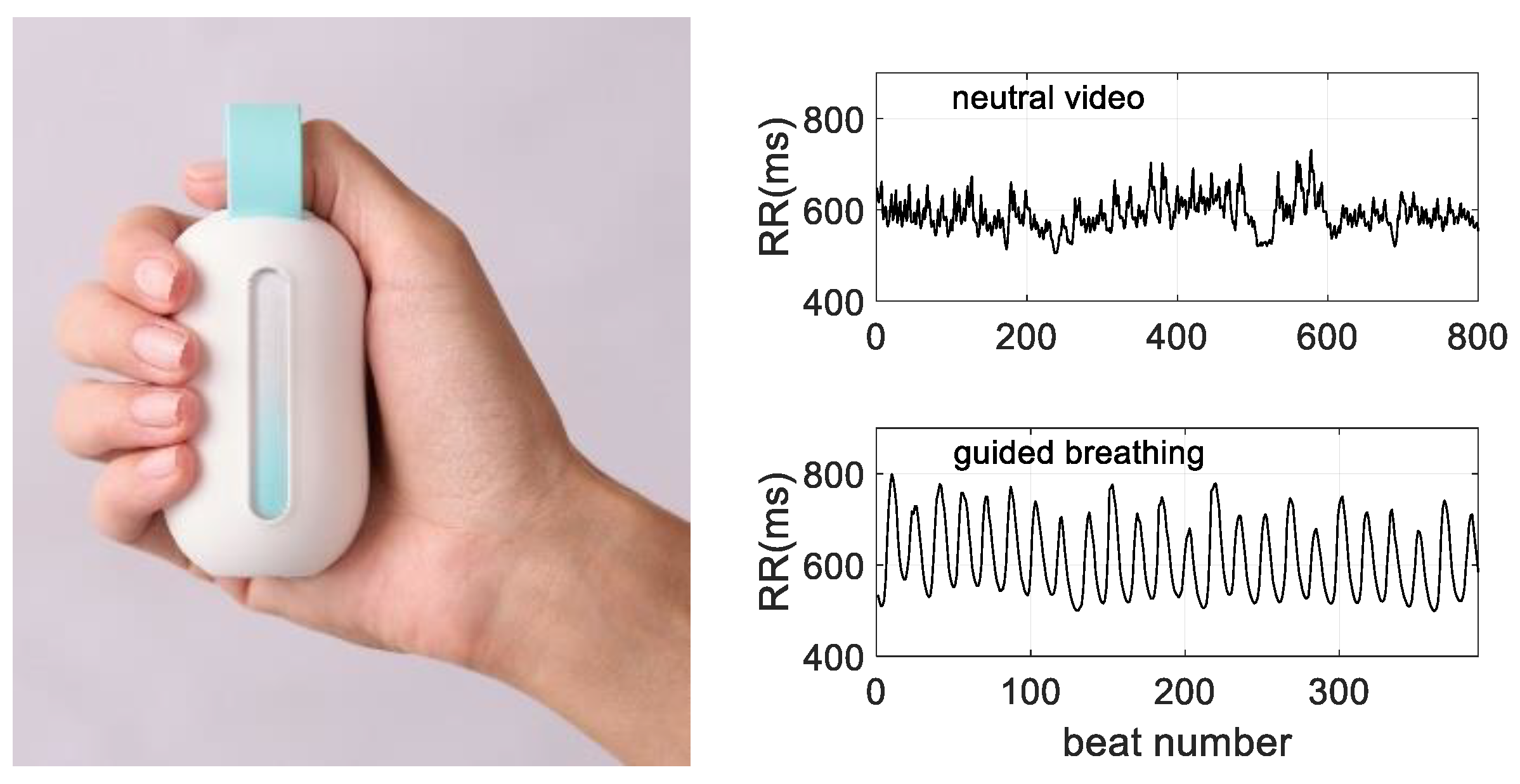
2.3. The Device
2.4. Measurement of Heart Rate Variability
2.5. Measurement of the Breathing Rate
2.6. Analyses of HRV Dynamics
2.7. Analyses of the Breathing Rate
2.8. Statistical Analysis
3. Results
3.1. HRV Dynamics during Guided Breathing
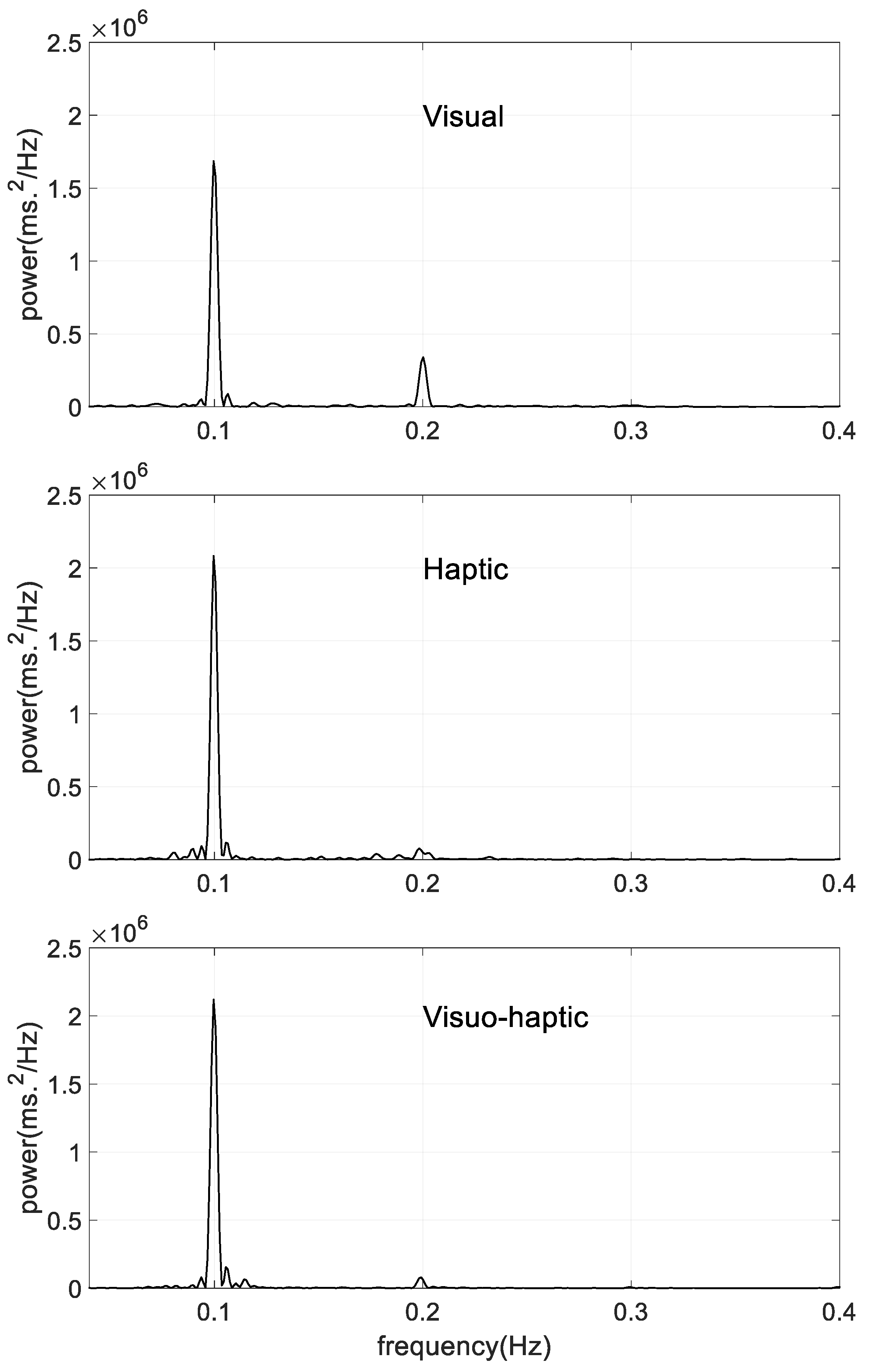
3.2. Power Spectral Density
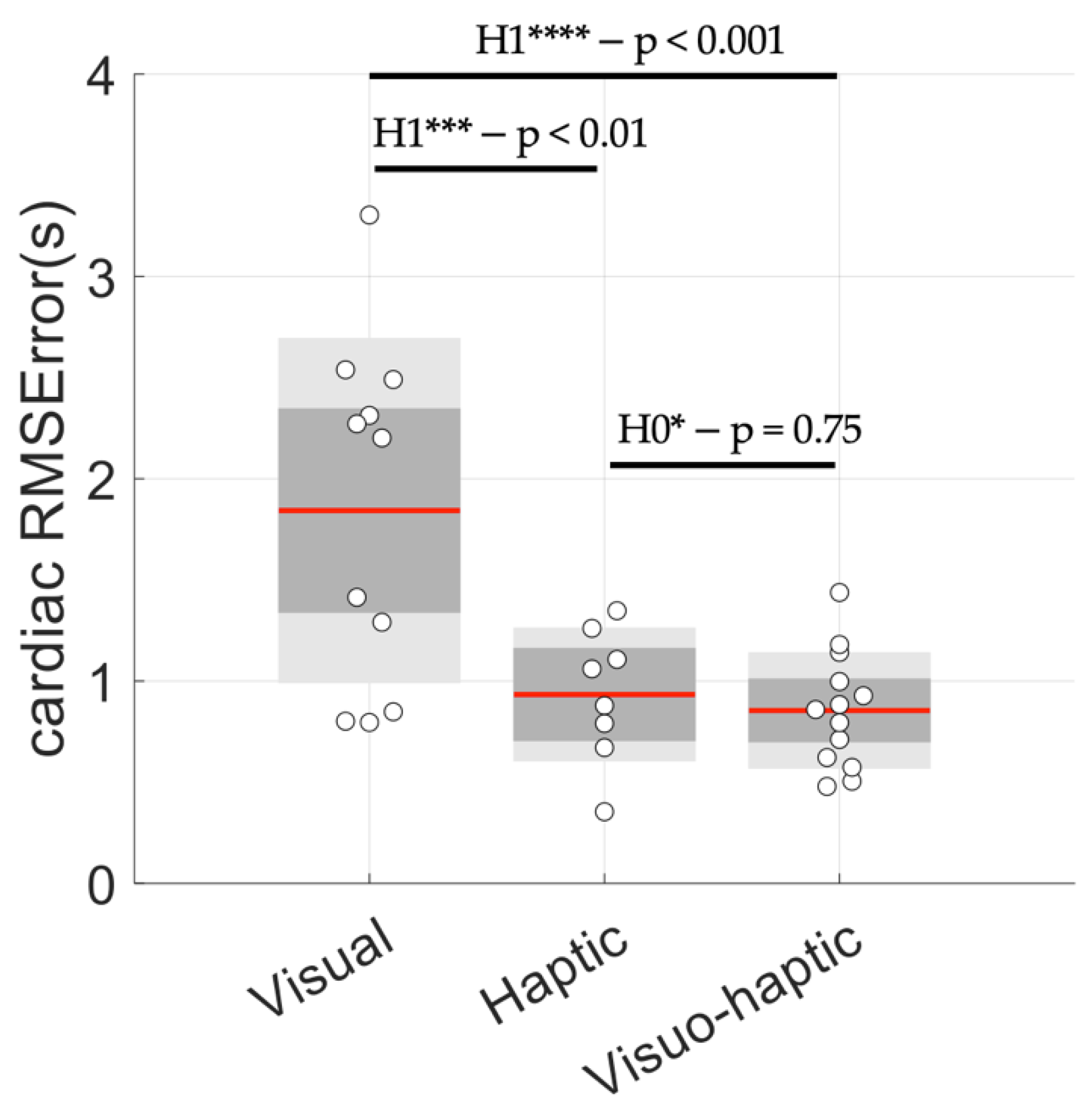
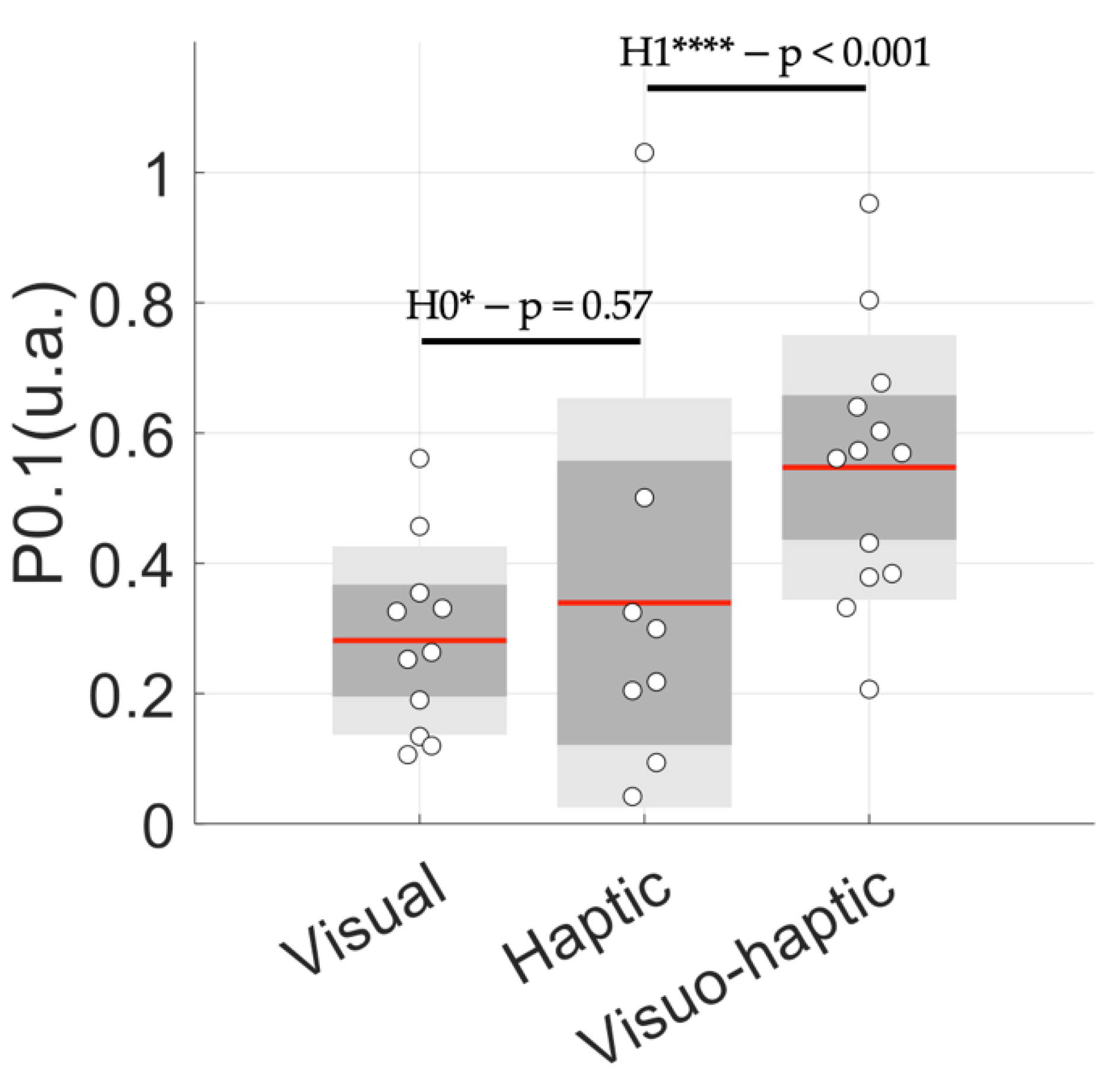
3.3. Cardiac Coherence Assessed by P0.1
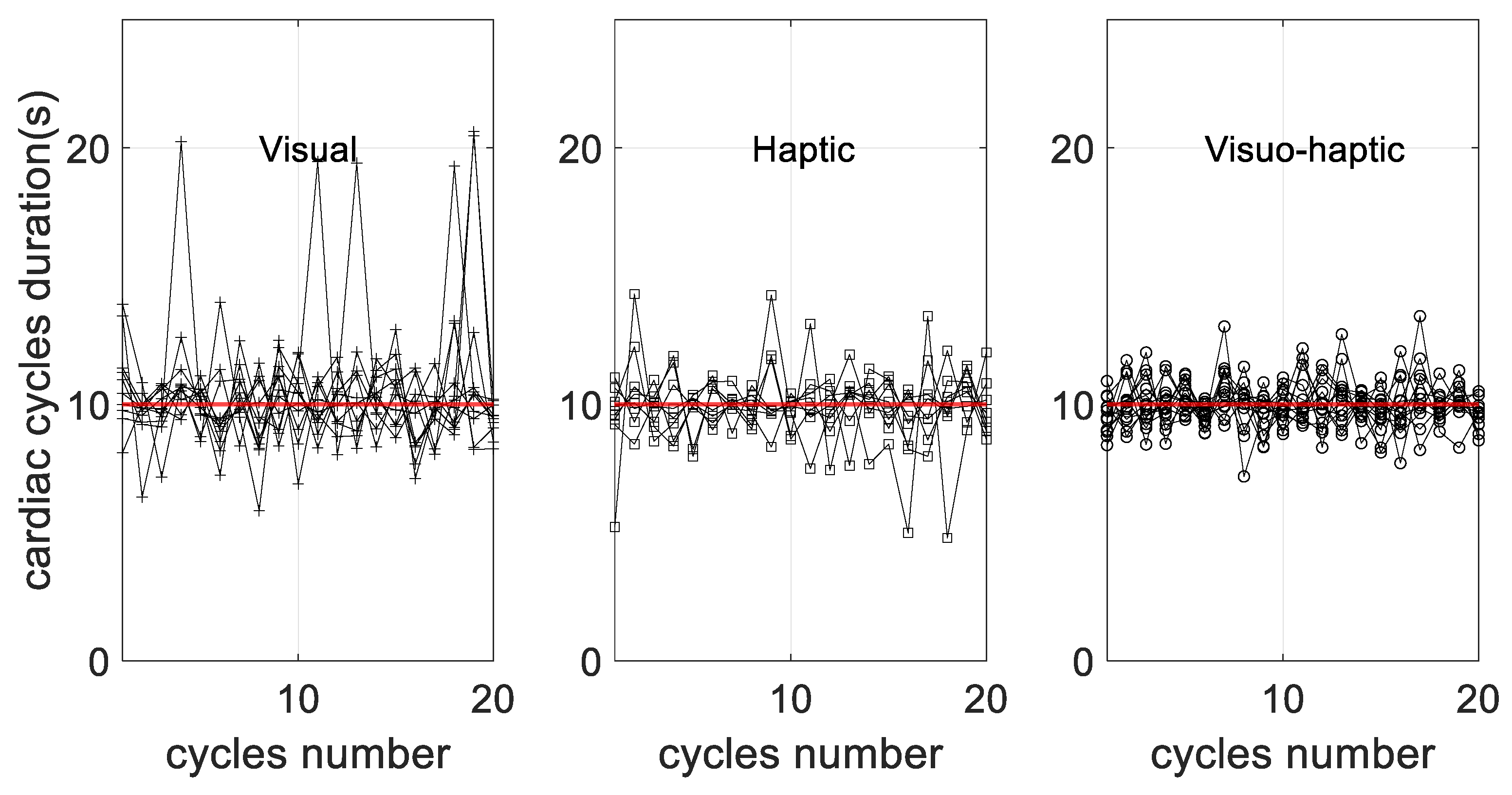
3.4. Control of Breathing Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B. Resonant Frequency Biofeedback Training to Increase Cardiac Variability: Rationale and Manual for Training. Appl. Psychophysiol. Biofeedback 2000, 25, 177–191. [Google Scholar] [CrossRef]
- Vaschillo, E.G.; Vaschillo, B.; Lehrer, P.M. Characteristics of Resonance in Heart Rate Variability Stimulated by Biofeedback. Appl. Psychophysiol. Biofeedback 2006, 31, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B.; Lu, S.-E.; Eckberg, D.L.; Edelberg, R.; Shih, W.J.; Lin, Y.; Kuusela, T.A.; Tahvanainen, K.U.O.; et al. Heart Rate Variability Biofeedback Increases Baroreflex Gain and Peak Expiratory Flow. Psychosom. Med. 2003, 65, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Vaschillo, E.; Lehrer, P.; Rishe, N.; Konstantinov, M. Heart Rate Variability Biofeedback as a Method for Assessing Baroreflex Function: A Preliminary Study of Resonance in the Cardiovascular System. Appl. Psychophysiol. Biofeedback 2002, 27, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Deschodt-Arsac, V.; Blons, E.; Gilfriche, P.; Spiluttini, B.; Arsac, L.M. Entropy in Heart Rate Dynamics Reflects How HRV-Biofeedback Training Improves Neurovisceral Complexity during Stress-Cognition Interactions. Entropy 2020, 22, 317. [Google Scholar] [CrossRef]
- McCraty, R.; Zayas, M.A. Cardiac Coherence, Self-Regulation, Autonomic Stability, and Psychosocial Well-Being. Front. Psychol. 2014, 5, 1090. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Tomasino, D. Emotional Stress, Positive Emotions, and Psychophysiological Coherence. In Stress in Health and Disease; Wiley-VCH Veriag GmbH & Co KGaA: Weinheim, Germany, 2006; pp. 342–365. ISBN 978-3-527-31221-4. [Google Scholar]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The Effect of Heart Rate Variability Biofeedback Training on Stress and Anxiety: A Meta-Analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef]
- Blase, K.L.; van Dijke, A.; Cluitmans, P.J.M.; Vermetten, E. Efficacy of HRV-biofeedback as additional treatment of depression and PTSD. Tijdschr. Voor Psychiatr. 2016, 58, 292–300. [Google Scholar]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef]
- Chen, S.; Sun, P.; Wang, S.; Lin, G.; Wang, T. Effects of Heart Rate Variability Biofeedback on Cardiovascular Responses and Autonomic Sympathovagal Modulation Following Stressor Tasks in Prehypertensives. J. Hum. Hypertens. 2016, 30, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, G.E.; Derman, W.E.; Lambert, M.I.; Rauch, H.G.L. The Effect of a Single Episode of Short Duration Heart Rate Variability Biofeedback on Measures of Anxiety and Relaxation States. Int. J. Stress Manag. 2013, 20, 391–411. [Google Scholar] [CrossRef]
- Prinsloo, G.E.; Rauch, H.G.L.; Lambert, M.I.; Muench, F.; Noakes, T.D.; Derman, W.E. The Effect of Short Duration Heart Rate Variability (HRV) Biofeedback on Cognitive Performance during Laboratory Induced Cognitive Stress. Appl. Cogn. Psychol. 2011, 25, 792–801. [Google Scholar] [CrossRef]
- Rosaura Polak, A.; Witteveen, A.B.; Denys, D.; Olff, M. Breathing Biofeedback as an Adjunct to Exposure in Cognitive Behavioral Therapy Hastens the Reduction of PTSD Symptoms: A Pilot Study. Appl. Psychophysiol. Biofeedback 2015, 40, 25–31. [Google Scholar] [CrossRef]
- Jester, D.J.; Rozek, E.K.; McKelley, R.A. Heart Rate Variability Biofeedback: Implications for Cognitive and Psychiatric Effects in Older Adults. Aging Ment. Health 2019, 23, 574–580. [Google Scholar] [CrossRef]
- Deschodt-Arsac, V.; Lalanne, R.; Spiluttini, B.; Bertin, C.; Arsac, L.M. Effects of Heart Rate Variability Biofeedback Training in Athletes Exposed to Stress of University Examinations. PLoS ONE 2018, 13, e0201388. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Morgan, S.; Molina Mora, J.A. Effect of Heart Rate Variability Biofeedback on Sport Performance, a Systematic Review. Appl. Psychophysiol. Biofeedback 2017, 42, 235–245. [Google Scholar] [CrossRef]
- Benarroch, E.E. The Central Autonomic Network: Functional Organization, Dysfunction, and Perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Valenza, G.; Sclocco, R.; Duggento, A.; Passamonti, L.; Napadow, V.; Barbieri, R.; Toschi, N. The Central Autonomic Network at Rest: Uncovering Functional MRI Correlates of Time-Varying Autonomic Outflow. NeuroImage 2019, 197, 383–390. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Thayer, J.F. Heart Rate Variability as a Transdiagnostic Biomarker of Psychopathology. Int. J. Psychophysiol. 2015, 98, 338–350. [Google Scholar] [CrossRef]
- Jarczok, M.N.; Koenig, J.; Wittling, A.; Fischer, J.E.; Thayer, J.F. First Evaluation of an Index of Low Vagally-Mediated Heart Rate Variability as a Marker of Health Risks in Human Adults: Proof of Concept. J. Clin. Med. 2019, 8, 1940. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-Regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Thayer, J. How Heart Rate Variability Affects Emotion Regulation Brain Networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Gevirtz, R. Heart Rate Variability Biofeedback: How and Why Does It Work? Front. Psychol. 2014, 5, 756. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Ranta-aho, P.O.; Karjalainen, P.A. An Advanced Detrending Method with Application to HRV Analysis. IEEE Trans. Biomed. Eng. 2002, 49, 172–175. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Pub. Co.: Reading, MA, USA, 1977; ISBN 978-0-201-07616-5. [Google Scholar]
- Bouny, P.; Arsac, L.M.; Touré Cuq, E.; Deschodt-Arsac, V. Entropy and Multifractal-Multiscale Indices of Heart Rate Time Series to Evaluate Intricate Cognitive-Autonomic Interactions. Entropy 2021, 23, 663. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.-J. Using Bayes Factor Hypothesis Testing in Neuroscience to Establish Evidence of Absence. Nat. Neurosci. 2020, 23, 788–799. [Google Scholar] [CrossRef]
- Rouder, J.N.; Speckman, P.L.; Sun, D.; Morey, R.D.; Iverson, G. Bayesian t Tests for Accepting and Rejecting the Null Hypothesis. Psychon. Bull. Rev. 2009, 16, 225–237. [Google Scholar] [CrossRef]
- Lehrer, P.; Vaschillo, E.; Lu, S.-E.; Eckberg, D.; Vaschillo, B.; Scardella, A.; Habib, R. Heart Rate Variability Biofeedback: Effects of Age on Heart Rate Variability, Baroreflex Gain, and Asthma. Chest 2006, 129, 278–284. [Google Scholar] [CrossRef]
- Tabor, A.; Bateman, S.; Scheme, E.J.; Schraefel, M.C. Comparing Heart Rate Variability Biofeedback and Simple Paced Breathing to Inform the Design of Guided Breathing Technologies. Front. Comput. Sci. 2022, 4, 926649. [Google Scholar] [CrossRef]
- Feygin, D.; Keehner, M.; Tendick, R. Haptic Guidance: Experimental Evaluation of a Haptic Training Method for a Perceptual Motor Skill. In Proceedings of the 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, HAPTICS 2002, Orlando, FL, USA, 24–25 March 2002; pp. 40–47. [Google Scholar]
- Reuter, E.-M.; Voelcker-Rehage, C.; Vieluf, S.; Godde, B. Touch Perception throughout Working Life: Effects of Age and Expertise. Exp. Brain Res. 2012, 216, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Rassler, B.; Schwerdtfeger, A.R.; Klimesch, W.; Andrade, A.; Schwarz, G.; Thayer, J.F. “Switch-Off” of Respiratory Sinus Arrhythmia May Be Associated with the Activation of an Oscillatory Source (Pacemaker) in the Brain Stem. Front. Physiol. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
| Visual | Haptic | Visuo-Haptic | |
|---|---|---|---|
| Age (years ± SD) | 22 ± 4 | 21 ± 1 | 20 ± 2 |
| Female (%) | 45 | 25 | 69 |
| Visual | Haptic | Visuo-Haptic | |
|---|---|---|---|
| Peak location (Hz) | 0.100 ± 0.001 | 0.100 ± 0.001 | 0.100 ± 0.001 |
| Peak height (ms2 × 106) | 1.97 ± 1.61 | 1.60 ± 0.48 | 2.31 ± 1.27 |
| MPHW (Hz) | 0.0120 ± 0.0003 | 0.0121 ± 0.0002 | 0.0121 ± 0.0003 |
| Visual | Haptic | Visuo-Haptic | |
|---|---|---|---|
| Peak location (Hz) | 0.100 ± 0.000 | 0.100 ± 0.000 | 0.100 ± 0.000 |
| RMSError (s) | 0.82 ± 0.19 * | 0.34 ± 0.15 c | 0.45 ± 0.17 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouny, P.; Arsac, L.M.; Guérin, A.; Nerincx, G.; Deschodt-Arsac, V. Guiding Breathing at the Resonance Frequency with Haptic Sensors Potentiates Cardiac Coherence. Sensors 2023, 23, 4494. https://doi.org/10.3390/s23094494
Bouny P, Arsac LM, Guérin A, Nerincx G, Deschodt-Arsac V. Guiding Breathing at the Resonance Frequency with Haptic Sensors Potentiates Cardiac Coherence. Sensors. 2023; 23(9):4494. https://doi.org/10.3390/s23094494
Chicago/Turabian StyleBouny, Pierre, Laurent M. Arsac, Antoine Guérin, Guillam Nerincx, and Veronique Deschodt-Arsac. 2023. "Guiding Breathing at the Resonance Frequency with Haptic Sensors Potentiates Cardiac Coherence" Sensors 23, no. 9: 4494. https://doi.org/10.3390/s23094494
APA StyleBouny, P., Arsac, L. M., Guérin, A., Nerincx, G., & Deschodt-Arsac, V. (2023). Guiding Breathing at the Resonance Frequency with Haptic Sensors Potentiates Cardiac Coherence. Sensors, 23(9), 4494. https://doi.org/10.3390/s23094494






