Portable Surface Plasmon Resonance Detector for COVID-19 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Evaluation of Protein–Antibody System
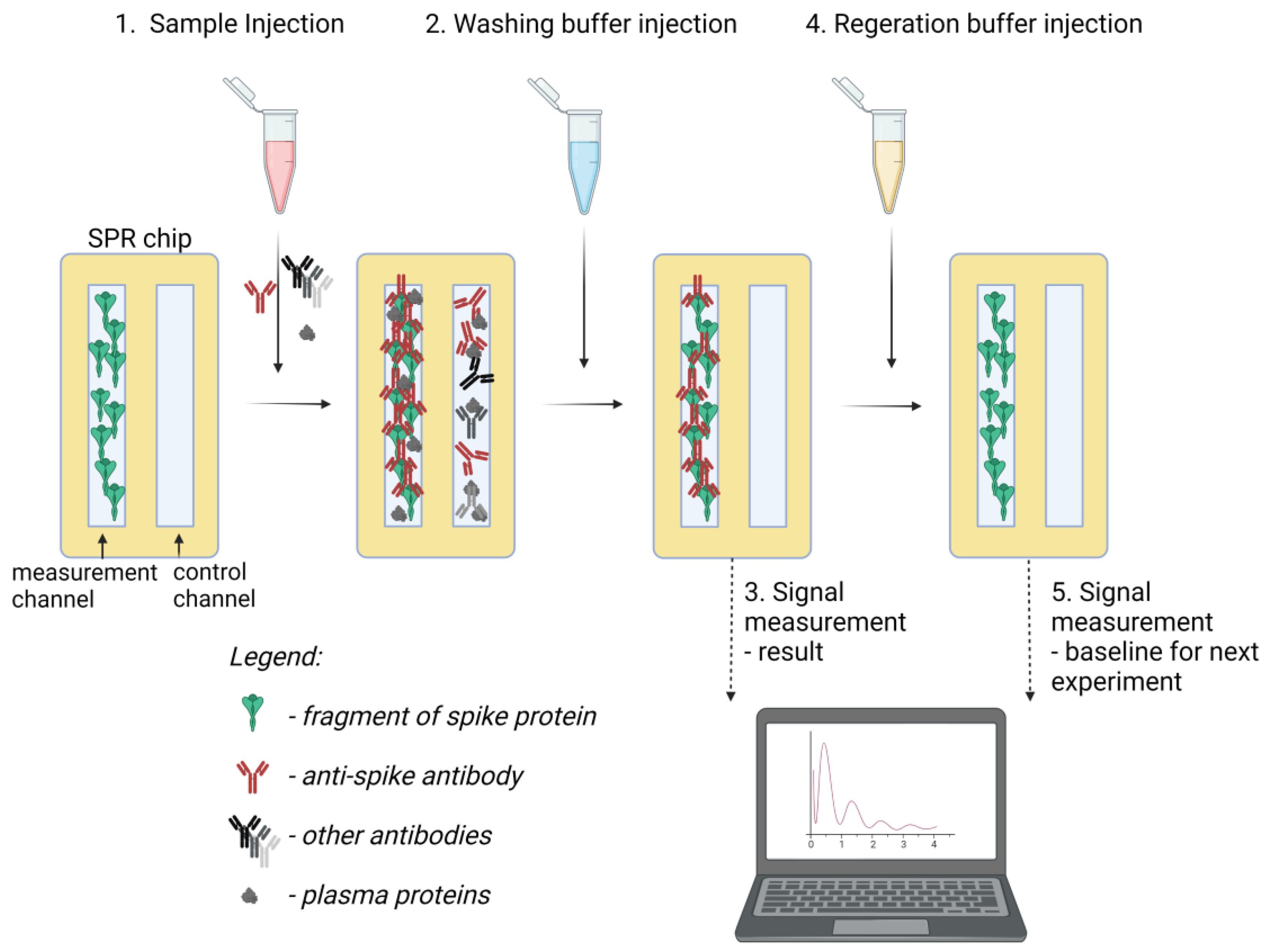
2.4. Preparation of S2k Sensing Chips
2.5. Detection of Plasma Samples
3. Results
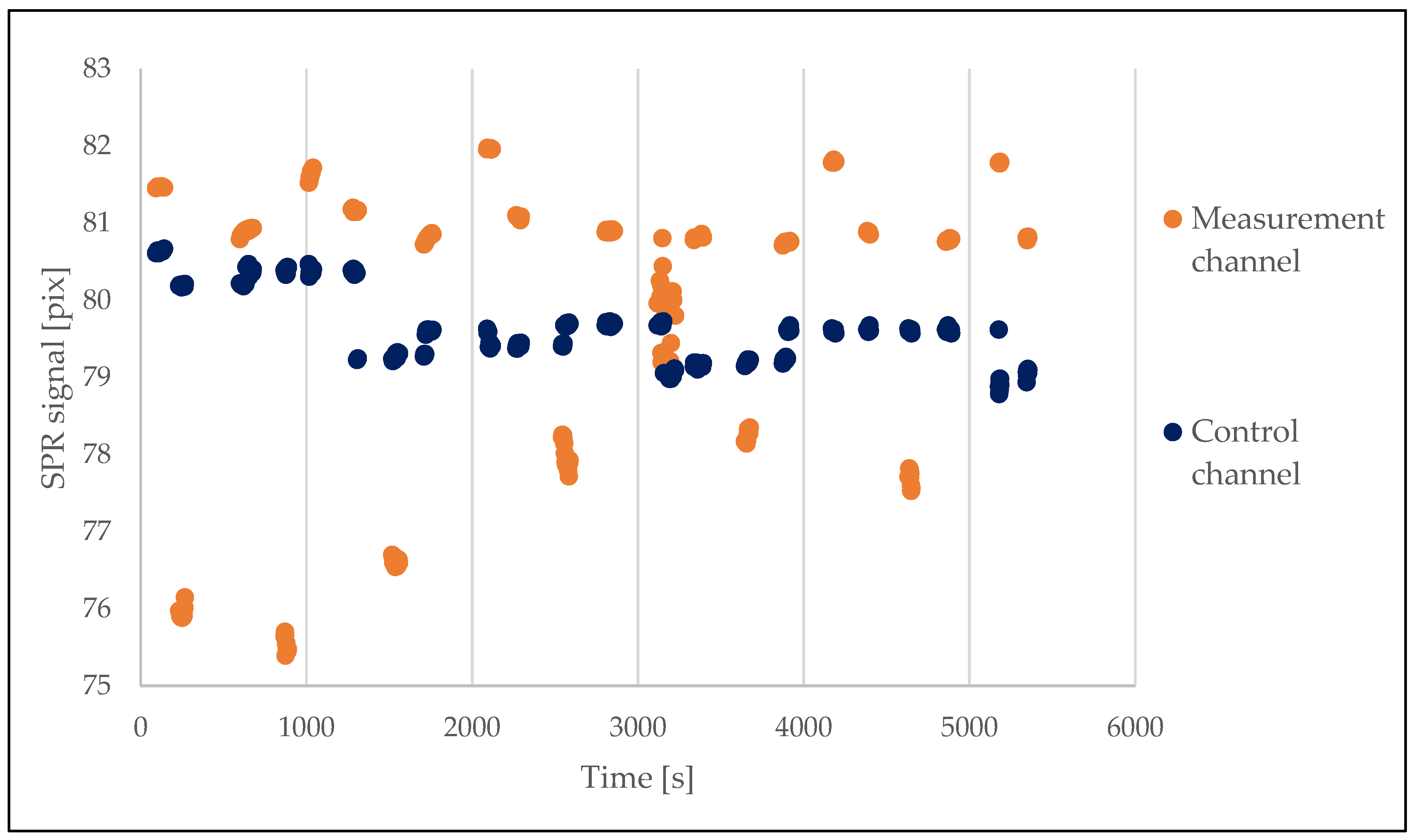
3.1. Initial SPR Experiments
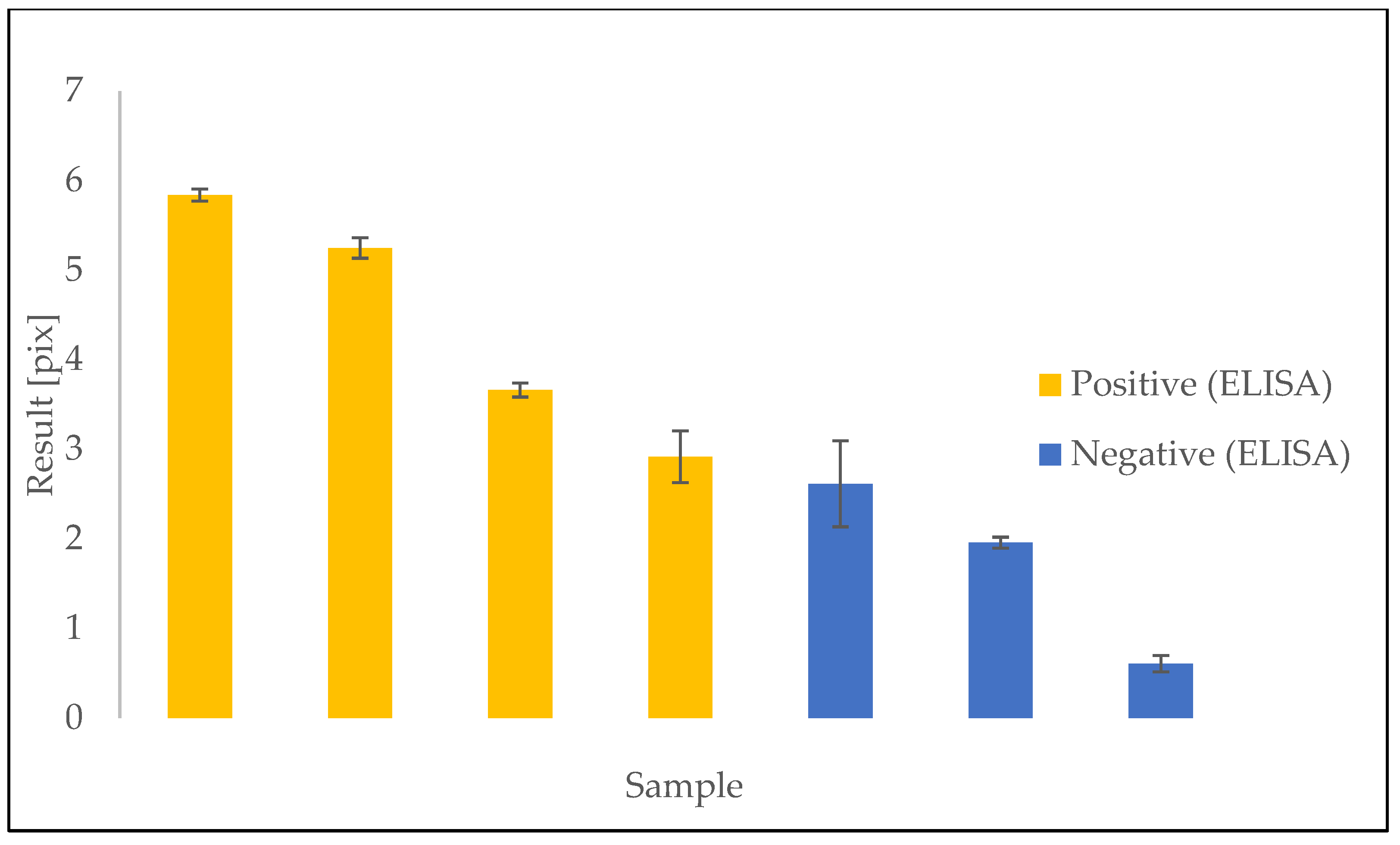
3.2. Results of Plasma Sample Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Kupferschmidt, K.; Cohen, J. Will novel virus go pandemic or be contained? Science 2020, 367, 610–611. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 March 2023).
- Anthony, S.J.; Johnson, C.K.; Greig, D.J.; Kramer, S.; Che, X.; Wells, H.; Hicks, A.L.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; et al. Global patterns in coronavirus diversity. Virus Evol. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Nianshuang, W.; Kizzmekia, S.C.; Jory, A.G.; Ching-Lin, H.; Olubukola, A.; Barney, S.G.; Jason, S.M. Cryo-EM structure of the 2019-nCoV Spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680.e2. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor binding domain (RDB) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef]
- Liu, G.; Rusling, J.F. COVID-19 Antibody Tests and Their Limitations. ACS Sens. 2021, 6, 593–612. [Google Scholar] [CrossRef]
- Younes, N.; Al-Sadeq, D.W.; AL-Jighefee, H.; Younes, S.; Al-Jamal, O.; Daas, H.I.; Yassine, H.M.; Nasrallah, G.K. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.Y.; Wu, Y.J.; Liu, H. Evolving status of the 2019 novel coronavirus infection: Proposal of conventional serologic assays for disease diagnosis and infection monitoring. J. Med. Virol. 2020, 92, 464–467. [Google Scholar] [CrossRef]
- Pisanic, N.; Randad, P.R.; Kruczynski, K.; Manabe, Y.C.; Thomas, D.L.; Pekosz, A.; Klein, S.L.; Betenbaugh, M.J.; Clarke, W.A.; Laeyendecker, O. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J. Clin. Microbiol. 2020, 59, e02204-20. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Jiang, T.; Ma, Y.; To, D.; Wang, S.; Chen, J. A Portable, low-cost and high-throughput electrochemical impedance spectroscopy device for point-of-care biomarker detection. Biosens. Bioelectron. X 2023, 13, 100301. [Google Scholar] [CrossRef]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00461–e004620. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.; Tam, A.R.; Wu, T.; Lung, D.; Yip, C.C.; Cai, J.; Chan, J.; Chik, T.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gao, Q.; Wang, T.; Ke, Y.; Mo, F.; Jia, R.; Liu, W.; Liu, L.; Zheng, S.; Liu, Y.; et al. Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef]
- Atyeo, C.; Fischinger, S.; Zohar, T.; Slein, M.D.; Burke, J.; Loos, C.; McCulloch, D.J.; Newman, K.L.; Wolf, C.; Yu, J.; et al. Distinct Early Serological Signatures Track with SARS-CoV-2 Survival. Immunity 2020, 53, 524–532.e4. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of SARS-CoV-2 specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2020, 34, 108728. [Google Scholar] [CrossRef]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Homola, J.; Jiang, S. Surface Plasmon Resonance (SPR) Sensors for the Detection of Bacterial Pathogens, In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; pp. 83–108. [Google Scholar] [CrossRef]
- Yano, T.; Kajisa, T.; Ono, M.; Miyasaka, Y.; Hasegawa, Y.; Saito, A.; Otsuka, K.; Sakane, A.; Sasaki, T.; Yasutomo, K.; et al. Ultrasensitive detection of SARS-CoV-2 nucleocapsid protein using large gold nanoparticle-enhanced surface plasmon resonance. Sci. Rep. 2022, 12, 1060. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, T.; Han, C.; Liu, L.; Zhang, T.; Kang, Q.; Wang, P.; Zhou, F. Regenerable and high-throughput surface plasmon resonance assay for rapid screening of anti-SARS-CoV-2 antibody in serum samples. Anal. Chim. Acta 2022, 1208, 339830. [Google Scholar] [CrossRef] [PubMed]
- Djaileb, A.; Hojjat Jodaylami, M.; Coutu, J.; Ricard, P.; Lamarre, M.; Rochet, L.; Cellier-Goetghebeur, S.; Macaulay, D.; Charron, B.; Lavallée, É.; et al. Cross-validation of ELISA and a portable surface plasmon resonance instrument for IgG antibody serology with SARS-CoV-2 positive individuals. Analyst 2021, 146, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Trzaskowski, M.; Mizak, L.; Gryko, R.; Ciach, T. SPR System for On-Site Detection of Biological Warfare. Curr. Anal. Chem. 2017, 13, 144–149. [Google Scholar] [CrossRef]
- Trzaskowski, M.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Ciach, T. Detection of tuberculosis in patients with the use of portable SPR device. Sens. Actuators B 2018, 260, 786–792. [Google Scholar] [CrossRef]
- Cady, N.C. Multiplexed detection and quantification of human antibody response to COVID-19 infection using a plasmon enhanced biosensor platform. Biosens. Bioelectron. 2021, 171, 112679. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Leirs, K.; Maes, W.; Imbrechts, M.; Callewaert, N.; Lagrou, K.; Geukens, N.; Lammertyn, J.; Spasic, D. Innovative FO-SPR Label-free Strategy for Detecting Anti-RBD Antibodies in COVID-19 Patient Serum and Whole Blood. ACS Sens. 2022, 7, 477–487. [Google Scholar] [CrossRef]
- SARS-CoV-2 IgG/IgM Rapid Test Kit (TRFIA)_POCT Devices, Rapid Diagnose—Lumigenex. Available online: https://www.lumigenex.com/product/52.html (accessed on 28 March 2023).
- Cellex qSARS-CoV-2 IgG/IgM Rapid Test. Available online: https://www.cellexcovid.com/ (accessed on 28 March 2023).
- Battling Coronavirus_Beijing Hot View Biology. Available online: https://www.hotgen.com.cn/ky/upt.html (accessed on 28 March 2023).
- Wondfo 2019-nCoV Antibody Test. Available online: https://en.wondfo.com/pt/index77.html (accessed on 28 March 2023).
- The Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) (Lateral Flow). Available online: https://www.livzondiagnostics.com/en-us/info/17.html (accessed on 28 March 2023).
- TISENC Medical SARS-CoV-2 Products. Available online: https://www.tisenc.net/?post_type=products&page_id=57588 (accessed on 28 March 2023).
- Uzochukwu, V. BioCheck SARS-CoV-2 IgM and IgG Antibody Combo Test Kit Instructions for Use. Available online: https://www.axdx.com (accessed on 28 March 2023).
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- Ragnesola, B.; Jin, D.; Lamb, C.C.; Shaz, B.H.; Hillyer, C.D.; Luchsinger, L.L. COVID19 antibody detection using lateral flow assay tests in a cohort of convalescent plasma donors. BMC Res. Notes 2020, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Agrawal, A. Blood Plasma Microfluidic Device: Aiming for the Detection of COVID-19 Antibodies Using an On-Chip ELISA Platform. Trans. Indian Natl. Acad. Eng. 2020, 5, 217–220. [Google Scholar] [CrossRef]
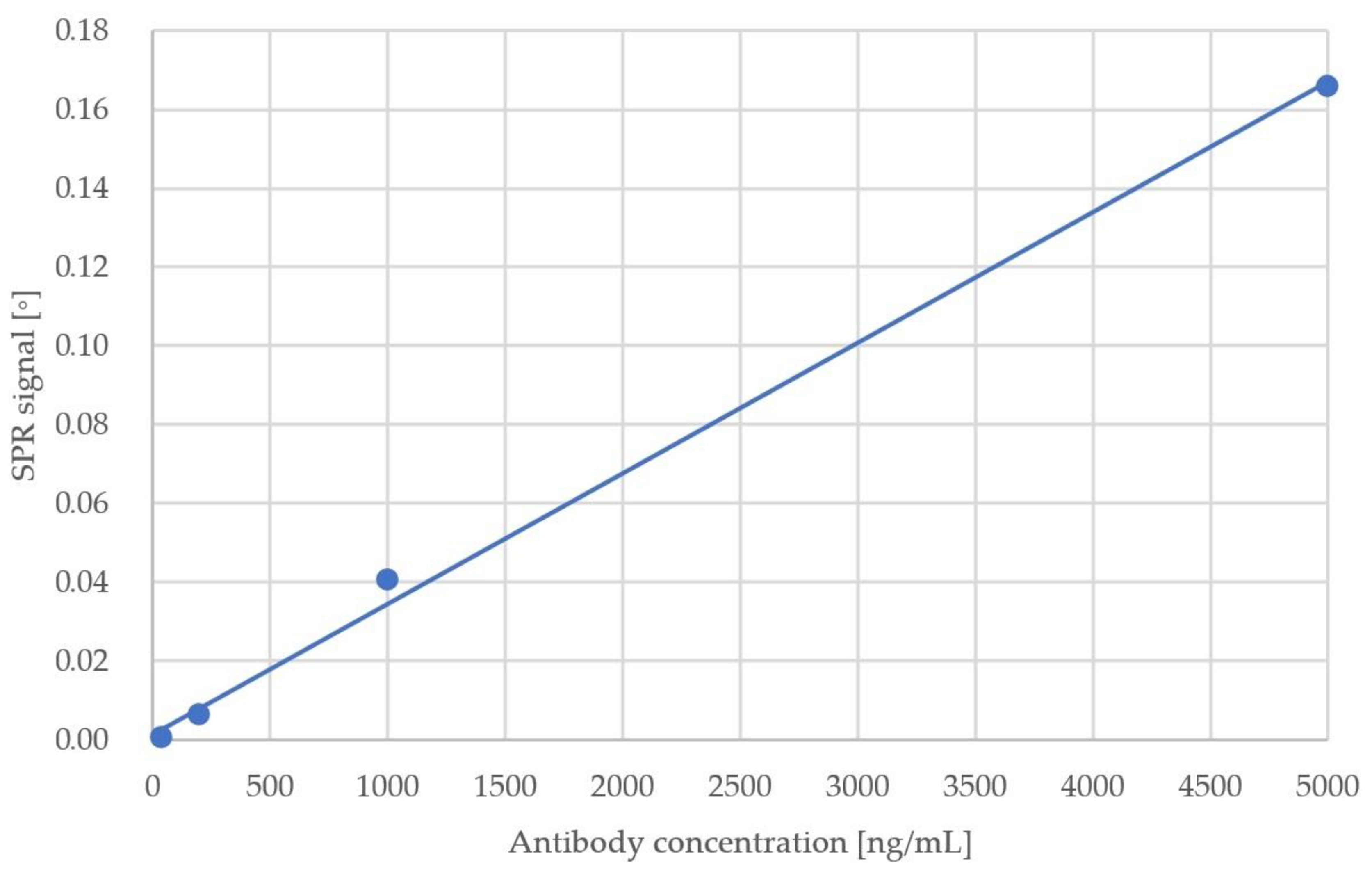
| Sample No. | Antibody Concentration (ng/mL) | SPR Response (°) |
|---|---|---|
| 1 | 8 | 3.10 × 10−5 |
| 2 | 40 | 4.35 × 10−4 |
| 3 | 200 | 6.17 × 10−3 |
| 4 | 1000 | 4.05 × 10−2 |
| 5 | 5000 | 1.66 × 10−1 |
| Reference | Detection Limit | Reported Time to Result (min) | Comments |
|---|---|---|---|
| Large gold nanoparticle-enhanced surface plasmon resonance [28] | 4 pg/mL | Not specified | Sandwich assay |
| Multiplexed grating-coupled fluorescent plasmonics (GC-FP) biosensor platform [33] | Not specified | <30 min | - |
| Fibre optic surface plasmon resonance [34] | <8 µg/mL | 30 min or 67 min depending on the mode | Two modes of operation: label-free and sandwich assay |
| Portable surface plasmon resonance detector | 40 ng/mL | <10 | - |
| Test Name | Producer | Reported Time to Result (min) 1 |
|---|---|---|
| Elecsys® Anti-SARS-CoV-2 [17] | Roche Diagnostics Corporation Indianapolis, IN, USA | 18 |
| BioCheck SARS-CoV-2 IgG and IgM Combo test [41] | BioCheck, Inc., South San Francisco, CA, USA | 30 |
| Cellex qSARS-CoV-2 IgG/IgM Rapid Test [36] | Cellex Inc., Cary NC, USA | 15–20 |
| SARS-CoV-2 IgG IgM Antibody Rapid Test Kit [35] | Lumigenex Co., Ltd., Suzhou, China | 10 |
| Novel Coronavirus 2019-nCoVAntibody Test [37] | Beijing Hotgen Biotech Co., Ltd., Beijing, China | 15 |
| SARS-CoV-2 Antibody Test [38] | Guangzhou Wondfo Biotech Co., Ltd., Guangzhou, China | 15 |
| Accre 6 [40] | Shenzhen Tisenc Medical Devices Co., Ltd., Shenzhen, China | 22 |
| Diagnostic Kit for IgM/IgGAntibody to Coronavirus (SARS-CoV-2) [39] | Zhuhai Livzon Diagnostics Inc., Zhuhai, China | 15 |
| Portable Surface Plasmon Resonance Detector | - | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzaskowski, M.; Mazurkiewicz-Pisarek, A.; Trzciński, J.W.; Drozd, M.; Podgórski, R.; Zabost, A.; Augustynowicz-Kopeć, E. Portable Surface Plasmon Resonance Detector for COVID-19 Infection. Sensors 2023, 23, 3946. https://doi.org/10.3390/s23083946
Trzaskowski M, Mazurkiewicz-Pisarek A, Trzciński JW, Drozd M, Podgórski R, Zabost A, Augustynowicz-Kopeć E. Portable Surface Plasmon Resonance Detector for COVID-19 Infection. Sensors. 2023; 23(8):3946. https://doi.org/10.3390/s23083946
Chicago/Turabian StyleTrzaskowski, Maciej, Anna Mazurkiewicz-Pisarek, Jakub Waldemar Trzciński, Marcin Drozd, Rafał Podgórski, Anna Zabost, and Ewa Augustynowicz-Kopeć. 2023. "Portable Surface Plasmon Resonance Detector for COVID-19 Infection" Sensors 23, no. 8: 3946. https://doi.org/10.3390/s23083946
APA StyleTrzaskowski, M., Mazurkiewicz-Pisarek, A., Trzciński, J. W., Drozd, M., Podgórski, R., Zabost, A., & Augustynowicz-Kopeć, E. (2023). Portable Surface Plasmon Resonance Detector for COVID-19 Infection. Sensors, 23(8), 3946. https://doi.org/10.3390/s23083946









