Characterization of a Novel Approach for Neonatal Hematocrit Screening Based on Penetration Velocity in Lateral Flow Test Strip
Abstract
1. Introduction
2. Materials and Methods
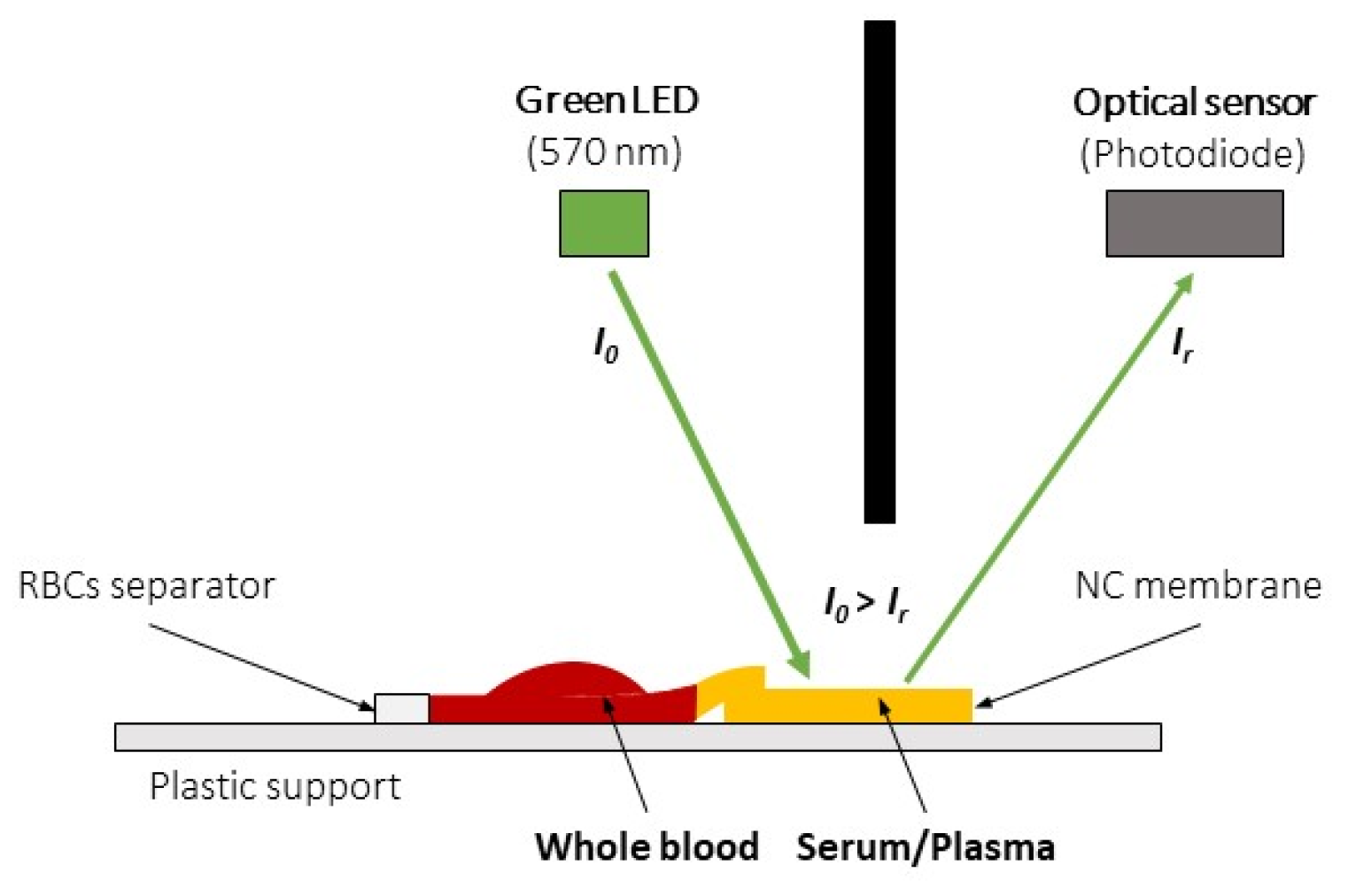
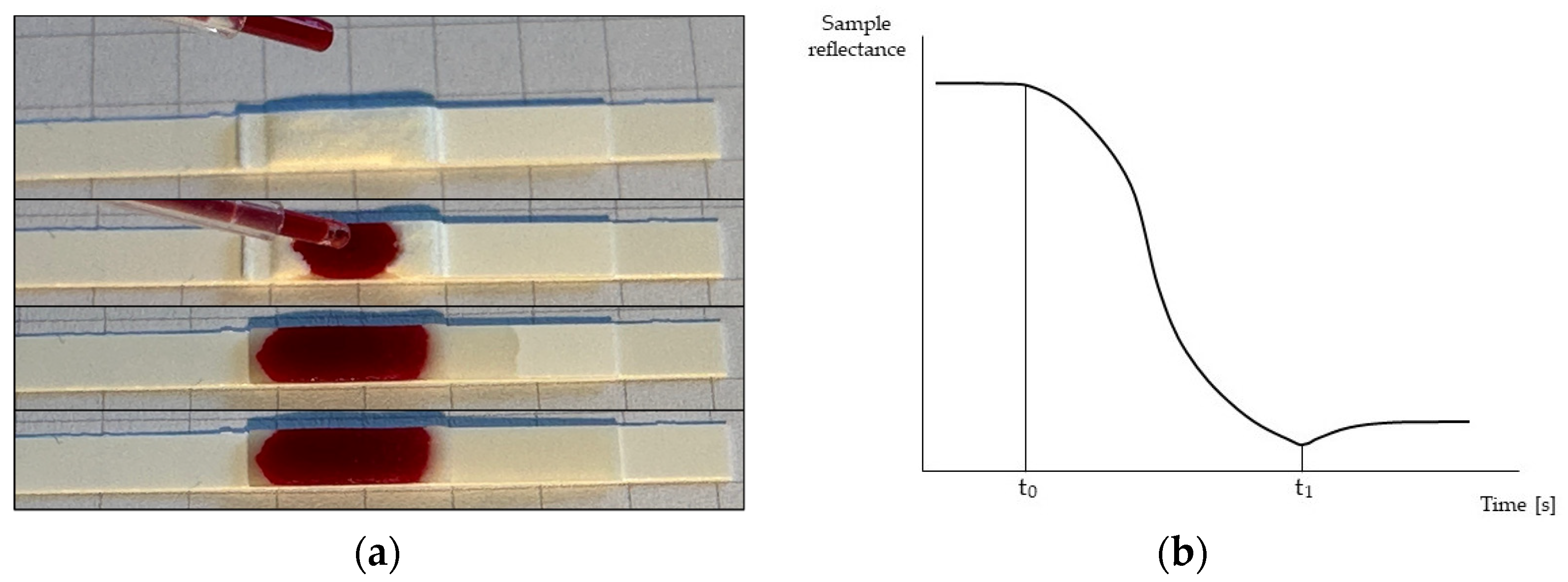
2.1. HCT Estimation Method
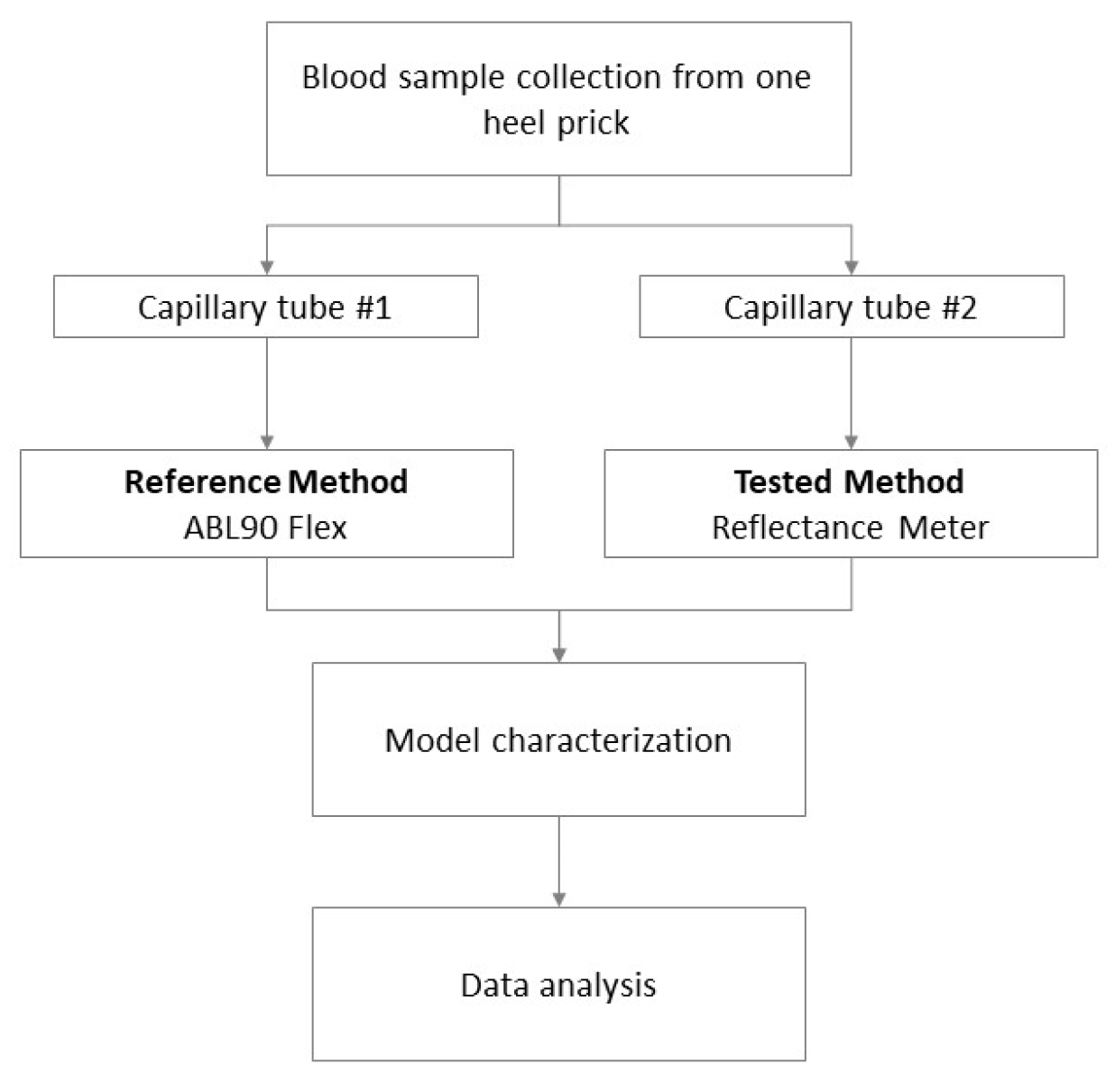
2.2. Data Acquisition, Calibration, and Clinical Test
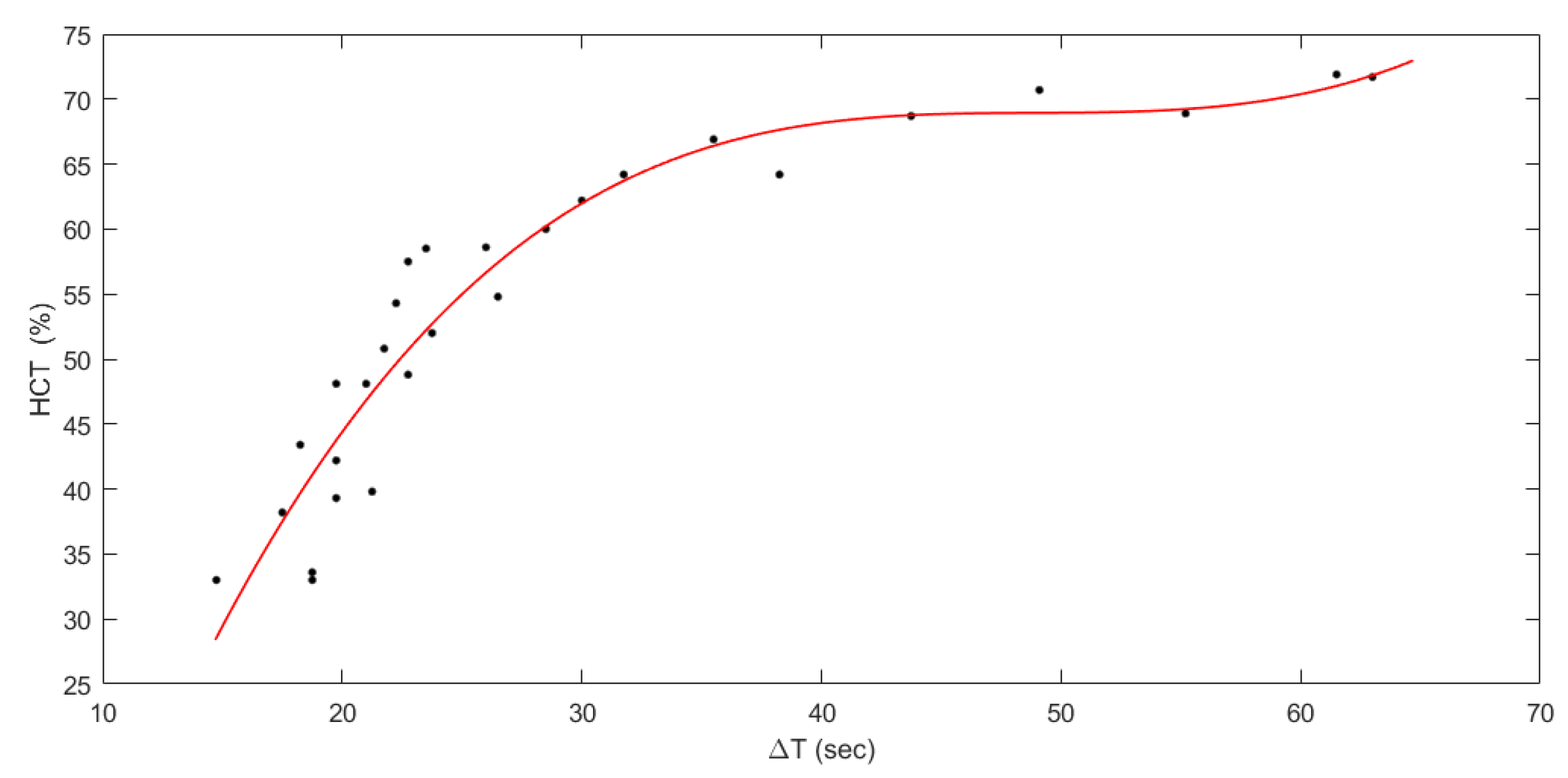
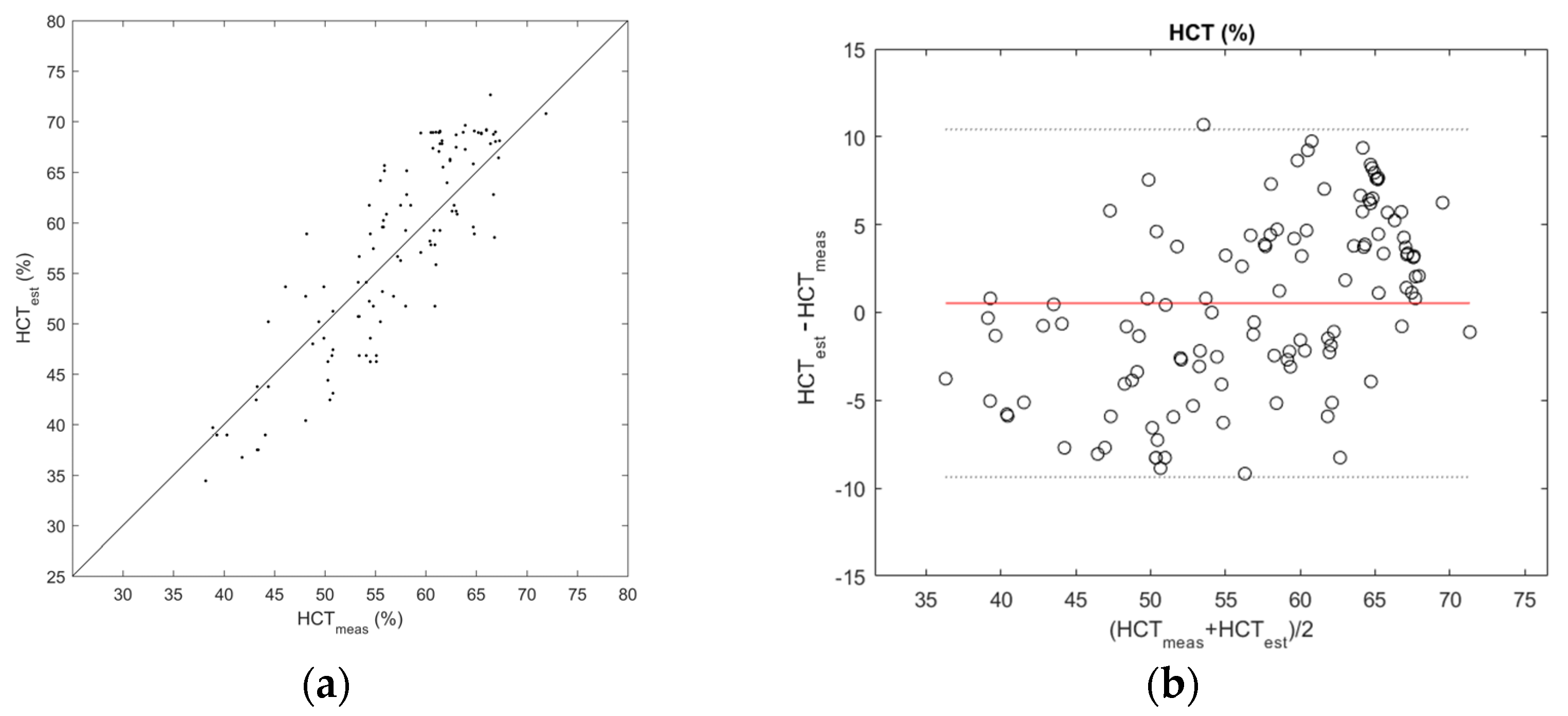
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jopling, J.; Henry, E.; Wiedmeier, S.E.; Christensen, R.D. Reference Ranges for Hematocrit and Blood Hemoglobin Concentration during the Neonatal Period: Data from a Multihospital Health Care System. Pediatrics 2009, 123, e333–e337. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Ostojic, D.; Isler, H.; Bassler, D.; Wolf, M.; Karen, T. Reference Ranges for Hemoglobin and Hematocrit Levels in Neonates as a Function of Gestational Age (22–42 Weeks) and Postnatal Age (0–29 Days): Mathematical Modeling. Children 2019, 6, 38. [Google Scholar] [CrossRef]
- Bosshart, M.; Stover, J.F.; Stocker, R.; Asmis, L.M.; Feige, J.; Neff, T.A.; Schuepbach, R.A.; Cottini, S.R.; Béchir, M. Two different hematocrit detection methods: Different methods, different results? BMC Res. Notes 2010, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejewska-Szczerska, M.; Gnyba, M. Optical Investigation of Hematocrit Level in Human Blood. Acta Phys. Pol. A 2011, 4, 642–646. [Google Scholar] [CrossRef]
- Colombatti, R.; Sainati, L.; Trevisanuto, D. Anemia and transfusion in the neonate. Semin. Fetal Neonatal Med. 2016, 21, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.G.; Tansey, E.A.; Johnson, C.D.; Roe, S.M.; Montgomery, L.E.A. Blood: Tests used to assess the physiological and immunological properties of blood. Adv. Physiol. Educ. 2016, 40, 165–175. [Google Scholar] [CrossRef]
- Livshits, L.; Bilu, T.; Peretz, S.; Bogdanova, A.; Gassmann, M.; Eitam, H.; Koren, A.; Levin, C. Back to the “Gold Standard”: How Precise Is Hematocrit Detection Today? Novel ImageJ-based approach for the precise hematocrit measurement. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022049. [Google Scholar] [CrossRef]
- Lamola, A.A.; Bhutani, V.K.; Wong, R.J.; Stevenson, D.K.; McDonagh, A.F. The effect of hematocrit on the efficacy of phototherapy for neonatal jaundice. Pediatr. Res. 2013, 74, 54–60. [Google Scholar] [CrossRef]
- Kemper, A.R.; Newman, T.B.; Slaughter, J.L.; Maisels, M.J.; Watchko, J.F.; Downs, S.M.; Grout, R.W.; Bundy, D.G.; Stark, A.R.; Bogen, D.L.; et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2022, 150, e2022058859. [Google Scholar] [CrossRef]
- Gebretsadkan, G.; Ambachew, H. The Comparison between Microhematocrit and Automated Methods for Hematocrit Determination. Int. J. Blood Res. Disord. 2015, 2. [Google Scholar] [CrossRef]
- Mondal, H.; Lotfollahzadeh, S. Hematocrit. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Verbrugge, S.E.; Huisman, A. Verification and Standardization of Blood Cell Counters for Routine Clinical Laboratory Tests. Clin. Lab. Med. 2015, 35, 183–196. [Google Scholar] [CrossRef]
- Buttarello, M. Laboratory diagnosis of anemia: Are the old and new red cell parameters useful in classification and treatment, how? Int. J. Lab. Hematol. 2016, 38, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gavala, A.; Myrianthefs, P. Comparison of point-of-care versus central laboratory measurement of hematocrit, hemoglobin, and electrolyte concentrations. Heart Lung 2017, 46, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Woldie, M.; Feyissa, G.T.; Admasu, B.; Hassen, K.; Mitchell, K.; Mayhew, S.; McKee, M.; Balabanova, D. Community health volunteers could help improve access to and use of essential health services by communities in LMICs: An umbrella review. Health Policy Plan. 2018, 33, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Dieleman, M.; Martineau, T. Staffing remote rural areas in middle- and low-income countries: A literature review of attraction and retention. BMC Health Serv. Res. 2008, 8, 19. [Google Scholar] [CrossRef]
- Caldwell, A.; Young, A.; Gomez-Marquez, J.; Olson, K.R. Global Health Technology 2.0. IEEE Pulse 2011, 2, 63–67. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Xiu, M.; Zhou, C.; Li, L.; Zhang, J.; Hao, S.; Zhang, L.; Ge, S.; Huang, Y.; et al. In situ growth of WO3/BiVO4 nanoflowers onto cellulose fibers to construct photoelectrochemical/colorimetric lab-on-paper devices for the ultrasensitive detection of AFP. J. Mater. Chem. B 2022, 10, 4031–4039. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, K.; Liu, Y.; Li, L.; Zhang, L.; Hao, S.; Ge, S.; Yu, J. Bi2S3@MoS2 Nanoflowers on Cellulose Fibers Combined with Octahedral CeO2 for Dual-Mode Microfluidic Paper-Based MiRNA-141 Sensors. ACS Appl. Mater. Interfaces 2021, 13, 32780–32789. [Google Scholar] [CrossRef]
- Waller, A.W.; Toc, M.; Rigsby, D.J.; Gaytán-Martínez, M.; Andrade, J.E. Development of a Paper-Based Sensor Compatible with a Mobile Phone for the Detection of Common Iron Formulas Used in Fortified Foods within Resource-Limited Settings. Nutrients 2019, 11, 1673. [Google Scholar] [CrossRef]
- Bender, A.T.; Sullivan, B.P.; Zhang, J.Y.; Juergens, D.C.; Lillis, L.; Boyle, D.S.; Posner, J.D. HIV detection from human serum with paper-based isotachophoretic RNA extraction and reverse transcription recombinase polymerase amplification. Analyst 2021, 146, 2851–2861. [Google Scholar] [CrossRef]
- Smith, S.; Land, K.; Joubert, T.-H. Emerging Technology Solutions Towards REASSURED Point-of-Need Diagnostics. In Proceedings of the 2021 IEEE 21st International Conference on Nanotechnology (NANO), Montreal, QC, Canada, 28–30 July 2021; pp. 265–268. [Google Scholar] [CrossRef]
- Murray, L.P.; Mace, C.R. Usability as a guiding principle for the design of paper-based, point-of-care devices—A review. Anal. Chim. Acta 2020, 1140, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.B.; Fernandes, S.C.; Rajaratnam, A.; DeChiara, N.S.; Mace, C.R. Measurement of the hematocrit using paper-based microfluidic devices. Lab Chip 2016, 16, 3689–3694. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.; Baillargeon, K.R.; Mace, C.R. Reduction of blood volume required to perform paper-based hematocrit assays guided by device design. Anal. Methods 2019, 11, 2057–2063. [Google Scholar] [CrossRef]
- Ben, F.D.; Biasizzo, J.; Curcio, F. A fast, nondestructive, low-cost method for the determination of hematocrit of dried blood spots using image analysis. Clin. Chem. Lab. Med. (CCLM) 2019, 57, e81–e82. [Google Scholar] [CrossRef]
- Miller, J.H., IV. An On-card Approach for Assessment of Hematocrit on Dried Blood Spots which Allows for Correction of Sample Volume. J. Anal. Bioanal. Tech. 2013, 4, 162. [Google Scholar] [CrossRef]
- Punter-Villagrasa, J.; Cid, J.; Páez-Avilés, C.; Rodríguez-Villarreal, I.; Juanola-Feliu, E.; Colomer-Farrarons, J.; Miribel-Català, P.L. An Instantaneous Low-Cost Point-of-Care Anemia Detection Device. Sensors 2015, 15, 4564–4577. [Google Scholar] [CrossRef]
- Cohen, E.; Kramer, M.; Shochat, T.; Goldberg, E.; Krause, I. Relationship between hematocrit levels and intraocular pressure in men and women: A population-based cross-sectional study. Medicine 2017, 96, e8290. [Google Scholar] [CrossRef]
- Greco, C.; Iskander, I.F.; Houchi, S.Z.E.; Rohsiswatmo, R.; Rundjan, L.; Ogala, W.N.; Ofakunrin, A.O.D.; Moccia, L.; Hoi, N.T.X.; Bedogni, G.; et al. Diagnostic Performance Analysis of the Point-of-Care Bilistick System in Identifying Severe Neonatal Hyperbilirubinemia by a Multi-Country Approach. eClinicalMedicine 2018, 1, 14–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zucchini, L.; Ajčević, M.; Coda Zabetta, C.D.; Greco, C.; Fernetti, C.; Moretto, C.; Pennini, S.; Accardo, A. Characterization of a Novel Approach for Neonatal Hematocrit Screening Based on Penetration Velocity in Lateral Flow Test Strip. Sensors 2023, 23, 2813. https://doi.org/10.3390/s23052813
Zucchini L, Ajčević M, Coda Zabetta CD, Greco C, Fernetti C, Moretto C, Pennini S, Accardo A. Characterization of a Novel Approach for Neonatal Hematocrit Screening Based on Penetration Velocity in Lateral Flow Test Strip. Sensors. 2023; 23(5):2813. https://doi.org/10.3390/s23052813
Chicago/Turabian StyleZucchini, Lorenzo, Miloš Ajčević, Carlos Daniel Coda Zabetta, Chiara Greco, Cristina Fernetti, Carlo Moretto, Simone Pennini, and Agostino Accardo. 2023. "Characterization of a Novel Approach for Neonatal Hematocrit Screening Based on Penetration Velocity in Lateral Flow Test Strip" Sensors 23, no. 5: 2813. https://doi.org/10.3390/s23052813
APA StyleZucchini, L., Ajčević, M., Coda Zabetta, C. D., Greco, C., Fernetti, C., Moretto, C., Pennini, S., & Accardo, A. (2023). Characterization of a Novel Approach for Neonatal Hematocrit Screening Based on Penetration Velocity in Lateral Flow Test Strip. Sensors, 23(5), 2813. https://doi.org/10.3390/s23052813





