Protocols Targeting Afferent Pathways via Neuromuscular Electrical Stimulation for the Plantar Flexors: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Collection and Selection Process
2.4. Data Extraction Process
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
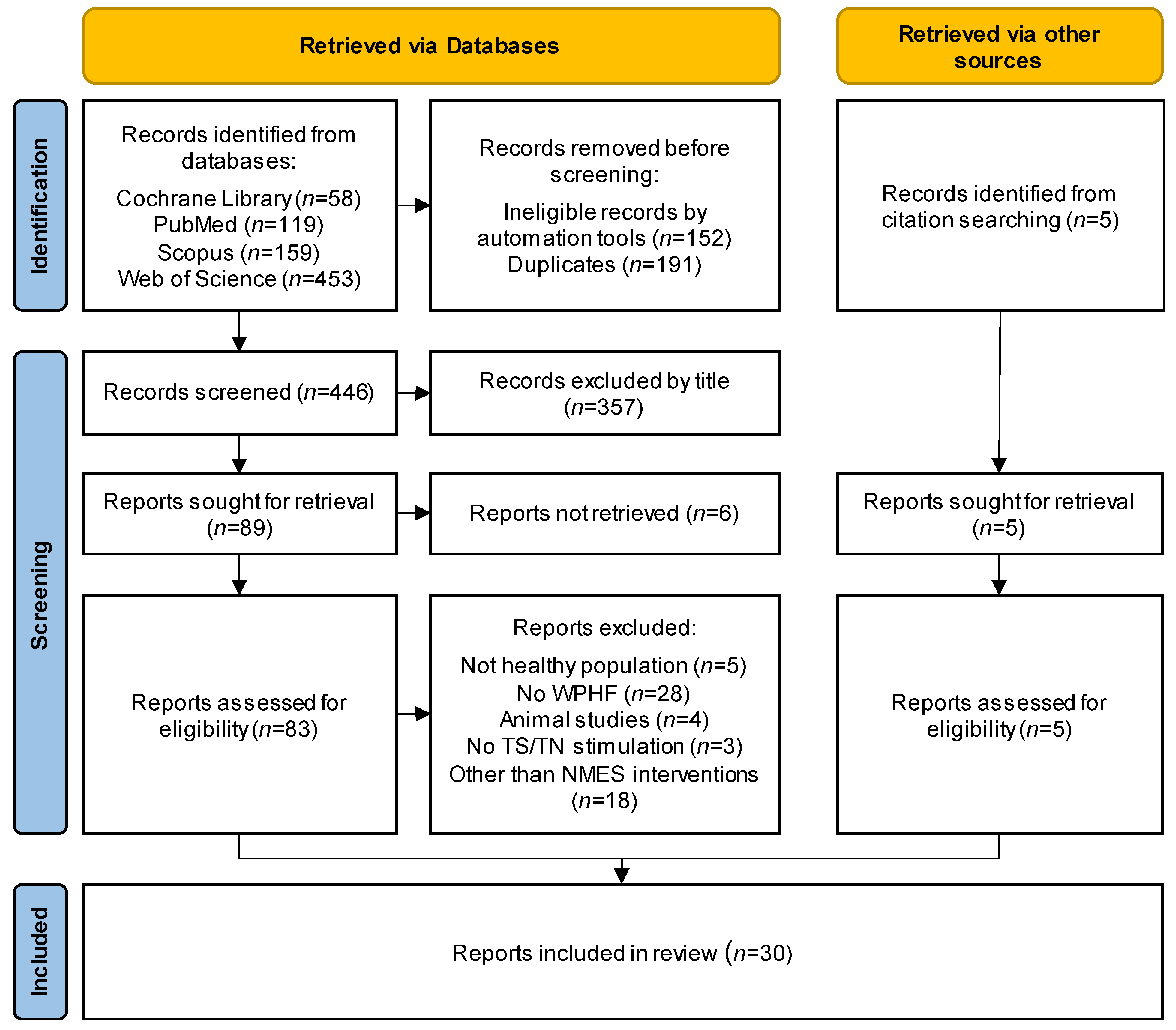
3.1. Study Selection
3.2. Risk of Bias within Studies
3.3. Participants
3.4. Protocols
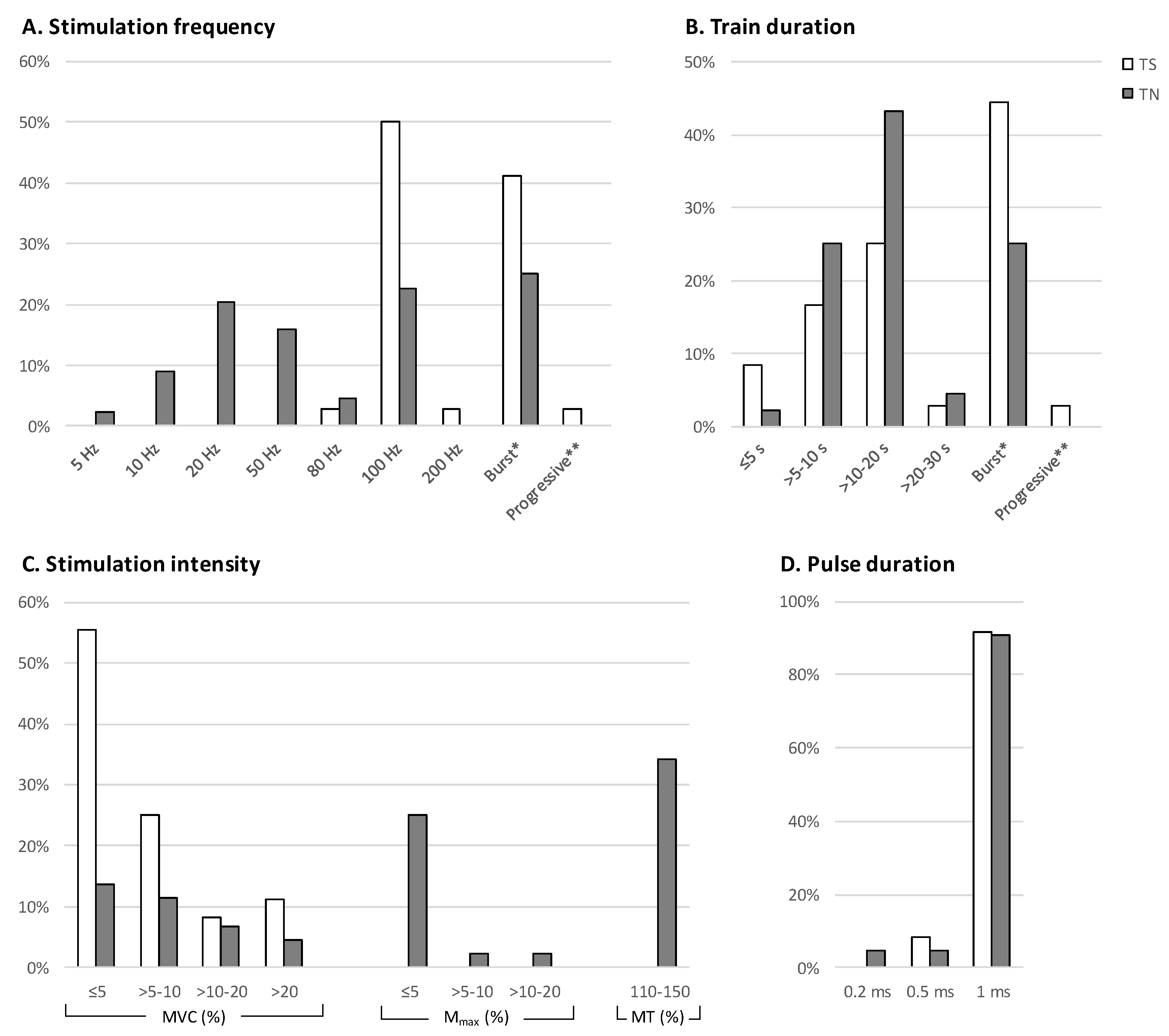
3.5. Stimulation Frequency
3.6. Train Duration
3.7. Stimulation Intensity
3.8. Pulse Width
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanderthommen, M.; Duchateau, J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc. Sport Sci. Rev. 2007, 35, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Excitability and inhibitability of motoneurons of different sizes. J. Neurophysiol. 1965, 28, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.M.; Bickel, C.S. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys. Ther. 2005, 85, 358–364. [Google Scholar] [CrossRef]
- Collins, D.F.; Burke, D.; Gandevia, S.C. Sustained contractions produced by plateau-like behaviour in human motoneurones. J. Physiol. 2002, 538, 289–301. [Google Scholar] [CrossRef]
- Hultman, E.; Sjöholm, H.; Jäderholm-Ek, I.; Krynicki, J. Evaluation of methods for electrical stimulation of human skeletal muscle in situ. Pflüg. Arch. Eur. J. Physiol. 1983, 398, 139–141. [Google Scholar] [CrossRef]
- Neyroud, D.; Grosprêtre, S.; Gondin, J.; Kayser, B.; Place, N. Test-retest reliability of wide-pulse high-frequency neuromuscular electrical stimulation evoked force. Muscle Nerve 2018, 57, E70–E77. [Google Scholar] [CrossRef]
- Dean, J.C.; Yates, L.M.; Collins, D.F. Turning on the central contribution to contractions evoked by neuromuscular electrical stimulation. J. Appl. Physiol. 2007, 103, 170–176. [Google Scholar] [CrossRef]
- Collins, D.F. Central Contributions to Contractions Evoked by Neuromuscular Electrical Stimulation. J. Appl. Physiol. 2007, 9, 102–109. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Lin, C.S.Y.; Burke, D. Differences in activity-dependent hyperpolarization in human sensory and motor axons. J. Physiol. 2004, 558, 341–349. [Google Scholar] [CrossRef]
- Lagerquist, O.; Collins, D.F. Influence of stimulus pulse width on M-waves, H-reflexes, and torque during tetanic low-intensity neuromuscular stimulation. Muscle Nerve 2010, 42, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Lagerquist, O.; Mang, C.S.; Collins, D.F. Changes in spinal but not cortical excitability following combined electrical stimulation of the tibial nerve and voluntary plantar-flexion. Exp. Brain Res. 2012, 222, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Vitry, F.; Martin, A.; Deley, G.; Papaiordanidou, M. Effect of reflexive activation of motor units on torque development during electrically-evoked contractions of the triceps surae muscle. J. Appl. Physiol. 2019, 126, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Langeard, A.; Bigot, L.; Chastan, N.; Gauthier, A. Does neuromuscular electrical stimulation training of the lower limb have functional effects on the elderly?: A systematic review. Exp. Gerontol. 2017, 91, 88–98. [Google Scholar] [CrossRef]
- Wegrzyk, J.; Ranjeva, J.P.; Fouré, A.; Kavounoudias, A.; Vilmen, C.; Mattei, J.P.; Guye, M.; Maffiuletti, N.A.; Place, N.; Bendahan, D.; et al. Specific brain activation patterns associated with two neuromuscular electrical stimulation protocols. Sci. Rep. 2017, 7, 2742. [Google Scholar] [CrossRef]
- Neyroud, D.; Gonzalez, M.; Mueller, S.; Agostino, D.; Grosprêtre, S.; Maffiuletti, N.A.; Kayser, B.; Place, N. Neuromuscular adaptations to wide-pulse high-frequency neuromuscular electrical stimulation training. Eur. J. Appl. Physiol. 2019, 119, 1105–1116. [Google Scholar] [CrossRef]
- Bergquist, A.J.; Clair, J.M.; Collins, D.F. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: Triceps surae. J. Appl. Physiol. 2011, 110, 627–637. [Google Scholar] [CrossRef]
- Baldwin, E.R.L.; Klakowicz, P.M.; Collins, D.F. Wide-pulse-width, high-frequency neuromuscular stimulation: Implications for functional electrical stimulation. J. Appl. Physiol. 2006, 101, 228–240. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Lloyd, R.S.; Myer, G.D. Youth resistance training: Past practices, new perspectives, and future directions. Pediatr. Exerc. Sci. 2013, 25, 591–604. [Google Scholar] [CrossRef]
- Klakowicz, P.M.; Baldwin, E.R.L.; Collins, D.F. Contribution of M-waves and H-reflexes to contractions evoked by tetanic nerve stimulation in humans. J. Neurophysiol. 2006, 96, 1293–1302. [Google Scholar] [CrossRef]
- Doix, A.C.M.; Matkowski, B.; Martin, A.; Roeleveld, K.; Colson, S.S. Effect of neuromuscular electrical stimulation intensity over the tibial nerve trunk on triceps surae muscle fatigue. Eur. J. Appl. Physiol. 2014, 114, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Zhou, S. Soleus H-reflex and its relation to static postural control. Gait Posture 2011, 33, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Gurfinkel, V.S. Human postural control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Grosprêtre, S.; Vilmen, C.; Guye, M.; Mattei, J.P.; Le Fur, Y.; Bendahan, D.; Gondin, J. The etiology of muscle fatigue differs between two electrical stimulation protocols. Med. Sci. Sports Exerc. 2016, 48, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Riitano, D. Constructing a search strategy and searching for evidence. Am. J. Nurs. 2014, 114, 49–56. [Google Scholar] [CrossRef]
- Gargon, E.; Williamson, P.R.; Clarke, M. Collating the knowledge base for core outcome set development: Developing and appraising the search strategy for a systematic review. BMC Med. Res. Methodol. 2015, 15, 26. [Google Scholar] [CrossRef]
- Bouguetoch, A.; Martin, A.; Grosprêtre, S. Insights into the combination of neuromuscular electrical stimulation and motor imagery in a training-based approach. Eur. J. Appl. Physiol. 2021, 121, 941–955. [Google Scholar] [CrossRef]
- Vitry, F.; Martin, A.; Papaiordanidou, M. Torque gains and neural adaptations following low-intensity motor nerve electrical stimulation training. J. Appl. Physiol. 2019, 127, 1469–1477. [Google Scholar] [CrossRef]
- Donnelly, C.; Stegmüller, J.; Blazevich, A.J.; Crettaz von Roten, F.; Kayser, B.; Neyroud, D.; Place, N. Modulation of torque evoked by wide-pulse, high-frequency neuromuscular electrical stimulation and the potential implications for rehabilitation and training. Sci. Rep. 2021, 11, 6399. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Almuklass, A.M.; Amiridis, I.G.; Enoka, R.M. Neuromuscular electrical stimulation can improve mobility in older adults but the time course varies across tasks: Double-blind, randomized trial. Exp. Gerontol. 2018, 108, 269–275. [Google Scholar] [CrossRef]
- Espeit, L.; Rozand, V.; Millet, G.Y.; Gondin, J.; Maffiuletti, N.A.; Lapole, T. Influence of wide-pulse neuromuscular electrical stimulation frequency and superimposed tendon vibration on occurrence and magnitude of extra torque. J. Appl. Physiol. 2021, 131, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyk, J.; Fouré, A.; Vilmen, C.; Ghattas, B.; Maffiuletti, N.A.; Mattei, J.P.; Place, N.; Bendahan, D.; Gondin, J. Extra Forces induced by wide-pulse, high-frequency electrical stimulation: Occurrence, magnitude, variability and underlying mechanisms. Clin. Neurophysiol. 2015, 126, 1400–1412. [Google Scholar] [CrossRef]
- Clair, J.M.; Anderson-Reid, J.M.; Graham, C.M.; Collins, D.F. Postactivation depression and recovery of reflex transmission during repetitive electrical stimulation of the human tibial nerve. J. Neurophysiol. 2011, 106, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Neyroud, D.; Kayser, B.; Westerblad, H.; Place, N. Intramuscular contributions to low-frequency force potentiation induced by a high-frequency conditioning stimulation. Front. Physiol. 2017, 8, 712. [Google Scholar] [CrossRef]
- Dean, J.C.; Yates, L.M.; Collins, D.F. Turning off the central contribution to contractions evoked by neuromuscular electrical stimulation. Muscle Nerve 2008, 38, 978–986. [Google Scholar] [CrossRef]
- Dean, J.C.; Clair-Auger, J.M.; Lagerquist, O.; Collins, D.F. Asynchronous recruitment of low-threshold motor units during repetitive, low-current stimulation of the human tibial nerve. Front. Hum. Neurosci. 2014, 8, 1002. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Gueugneau, N.; Martin, A.; Lepers, R. Central Contribution to Electrically Induced Fatigue depends on Stimulation Frequency. Med. Sci. Sports Exerc. 2017, 49, 1530–1540. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Gueugneau, N.; Martin, A.; Lepers, R. Presynaptic inhibition mechanisms may subserve the spinal excitability modulation induced by neuromuscular electrical stimulation. J. Electromyogr. Kinesiol. 2018, 40, 95–101. [Google Scholar] [CrossRef]
- Lagerquist, O.; Walsh, L.D.; Blouin, J.S.; Collins, D.F.; Gandevia, S.C. Effect of a peripheral nerve block on torque produced by repetitive electrical stimulation. J. Appl. Physiol. 2009, 107, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Dodd, D.; Gondin, J.; Maffiuletti, N.A.; Kayser, B.; Place, N. Wide-pulse-high-frequency neuromuscular stimulation of triceps surae induces greater muscle fatigue compared with conventional stimulation. J. Appl. Physiol. 2014, 116, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Papaiordanidou, M.; Billot, M.; Varray, A.; Martin, A. Neuromuscular fatigue is not different between constant and variable frequency stimulation. PLoS ONE 2014, 9, e84740. [Google Scholar] [CrossRef] [PubMed]
- Papaiordanidou, M.; Stevenot, J.D.; Mustacchi, V.; Vanoncini, M.; Martin, A. Electrically induced torque decrease reflects more than muscle fatigue. Muscle Nerve 2014, 50, 604–607. [Google Scholar] [CrossRef]
- Regina Dias Da Silva, S.; Neyroud, D.; Maffiuletti, N.A.; Gondin, J.; Place, N. Twitch potentiation induced by two different modalities of neuromuscular electrical stimulation: Implications for motor unit recruitment: NMES Parameters & Potentiation. Muscle Nerve 2015, 51, 412–418. [Google Scholar]
- Vitry, F.; Martin, A.; Papaiordanidou, M. Impact of stimulation frequency on neuromuscular fatigue. Eur. J. Appl. Physiol. 2019, 119, 2609–2616. [Google Scholar] [CrossRef]
- Wegrzyk, J.; Fouré, A.; Le Fur, Y.; Maffiuletti, N.A.; Vilmen, C.; Guye, M.; Mattei, J.P.; Place, N.; Bendahan, D.; Gondin, J. Responders to Wide-Pulse, High-Frequency Neuromuscular Electrical Stimulation Show Reduced Metabolic Demand: A 31P-MRS Study in Humans. PLoS ONE 2015, 10, e0143972. [Google Scholar] [CrossRef]
- Amiridis, I.G.; Mani, D.; Almuklass, A.; Matkowski, B.; Gould, J.R.; Enoka, R.M. Modulation of motor unit activity in biceps brachii by neuromuscular electrical stimulation applied to the contralateral arm. J. Appl. Physiol. 2015, 118, 1544–1552. [Google Scholar] [CrossRef]
- Gobbo, M.; Maffiuletti, N.A.; Orizio, C.; Minetto, M.A. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J. Neuroeng. Rehabil. 2014, 11, 17. [Google Scholar] [CrossRef]
- Botter, A.; Oprandi, G.; Lanfranco, F.; Allasia, S.; Maffiuletti, N.A.; Minetto, M.A. Atlas of the muscle motor points for the lower limb: Implications for electrical stimulation procedures and electrode positioning. Eur. J. Appl. Physiol. 2011, 111, 2461–2471. [Google Scholar] [CrossRef]
| First Author, Year (Reference Number) | Study Design Research Field | n/Females | Age (Years) | Dropouts/Discomfort (Rate) | Subject/Scope of the Study | Main Conclusions |
|---|---|---|---|---|---|---|
| Triceps surae NMES studies (n = 19) | ||||||
| Collins, 2002 [5] | Cohort Extra force | 21/7 | - | - | To investigate the optimal stimulus properties for evoking plateau-like phenomena to motoneurons | Experimental proof of contractions generated by central contribution with activation of plateau-like potentials. |
| Dean, 2007 [8] | Cohort Mechanisms | 8/2 | 21–43 | - | The effect of NMES parameters (frequency, duration, and intensity) on the central contribution to torque using several stimulation patterns | Torque was generated via stimulation of motor axons at low-frequency (≤20 Hz) NMES and via stimulation of sensory axons at high-frequency (≥80 Hz), low intensity, and wide-pulse duration NMES. |
| Dean, 2008 [37] | Cohort Extra force | 10/1 | 18–42 | - | Methods to activate the antagonist muscles, with practical implications for functional electrical stimulation. | Plantar flexor extra torque can be diminished by volitional or electrical activation of the antagonist muscles |
| Lagerquist, 2009 [41] | Cohort Extra force | 13/0 | 28–53 | - | To enhance the central contribution to the evoked contractions by using stimulation frequencies of 20 Hz and 100 Hz. | During NMES evoked torque increases possibly due to the contribution of central neural pathways, since blocking the antidromic volley the same NMES protocol demonstrates a decrease in evoked torque. |
| Neyroud, 2014 [42] | Cohort Fatigue | 14/3 | 27.0 ± 4.0 | - | Investigation whether central recruitment occurs during WPHF contractions and comparison of the extent and origin of muscle fatigue | WPHF fatigue protocol induced fatigue at faster rate than a fatigue protocol with short pulse duration and low frequency. |
| Papaiordanidou, 2014 [43] | Cohort Fatigue | 10/0 | 32.0 ± 3.8 | - | To understand the nature of fatigue induced by two NMES protocols, matched for the number of delivered pulses, and to determine whether the stimulation pattern induces different neuromuscular adaptations. | Variable frequency fatigue protocol (with doublets at 100 Hz) demonstrated greater torque decrease than constant frequency, whereas both protocols had similar effects on all examined muscular, spinal and supraspinal mechanisms. |
| Papaiordanidou, 2014 [44] | Cohort Fatigue | 8/- | 27.8 ± 7.1 | - | Examination of fatigue development during NMES protocols with different stimulation frequencies and pulse widths. | Evoked torque decreased at similar extent relative to the initial value, independent of the pulse duration. |
| Regina Dias Da Silva, 2015 [45] | Cohort Extra force | 13/4 | 30.0 ± 7.0 | - | Examination whether the twitch potentiation would be greater following conventional than WPHF or voluntary contractions of the plantar flexor muscles | There is no different in twitch torque potentiation when using WPHF or short-pulse low-frequency NMES. |
| Wegrzyk, 2015 [34] | Cohort Extra force | 42/22 | 28.0 ± 6.0 | - | Evaluation of twitch potentiation, H-reflex, and M-wave to understand the neuromuscular mechanisms of extra forces in response to WPHF. | Individuals that exhibit extra force demonstrate depressed H-reflex after NMES, which suggests the contribution of central mechanisms. |
| Wegrzyk, 2015 [47] | Cohort analytic Extra force | 18/5 | 29.0 ± 7.0 | - | Comparison of metabolic demand during WPHF NMES and voluntary contractions, using 31P-magnetic resonance spectroscopy. | Extra force induced by WPHF is less demanding metabolically than short-pulse low-frequency NMES, and possibly exhibits a muscle activation pattern similar to voluntary contractions. |
| Cheng, 2017 [36] | Cohort Mechanisms | 11/5 | 30.0 ± 7.0 | - | Investigation of the mechanisms underlying the low-frequency force potentiation following high-frequency stimulation. | High-frequency wide-pulse NMES results in a potentiation in torque produced by a subsequent low-frequency NMES train possibly due to increased sensitivity of myofibrillar Ca2+. |
| Wegrzyk (2017) [15] | Cohort Mechanisms | 18/6 | 26.0 ± 5.0 | 2/0(11.1%/0%) | Investigation of the cerebral activation pattern during WPHF NMES protocols, as compared to voluntary contractions, matched for the same initial isometric force. | NMES-induced isometric contractions resulted in brain activation patterns including sensorimotor areas and subcortical structures, similar to the activation patterns of voluntary movements. |
| Grosprêtre, 2017 [39] | Cohort Fatigue | 10/3 | 24.6 ± 4.2 | - | Acute fatigue effects of submaximal NMES delivered at different frequencies during NMES-evoked and maximal voluntary contractions. | For the same exerted torque, high frequency/low intensity fatigue protocols induce greater fatigue, whereas low or high frequency stimulation induce alterations at muscular or neural (spinal and supraspinal) level, respectively. |
| Grosprêtre, 2018 [40] | Cohort Mechanisms | 10/3 | 24.6 ± 4.2 | - | To understand the origins of spinal excitability modulation after NMES. | Spinal excitability decreased and presynaptic inhibition increased after NMES, especially at high-frequency mode. |
| Mani, 2018 [32] | RCT Training | 30/17 | 73.5 ± 4.8 | 2/0 (6.6%/0%) | To compare the effect of a 6-week NMES protocol with long and short pulse duration on the mobility of older adults | Wide- and short-pulse NMES training resulted in gains in functional mobility tests (walking speed, chair rise etc.). |
| Neyroud, 2019 [16] | RCT Training | 10/4 | 24.0 ± 1.0 | - | Evaluation of neuromuscular adaptations after 3 weeks of WPHF NMES | Evoked force time integral was increased after 3 weeks of WPHF NMES, with no changes in plantar flexor neuromuscular properties. |
| Bouguetoch, 2021 [29] | RCT Training | 10/3 | 24.0 ± 5.8 | - | Investigation of strength gains as well as the muscular and neural plasticity after training that combines motor imagery with NMES. | MVC peak twitch torque and M-wave amplitude increased after NMES training, whereas no changes in muscle architecture or other neural variables (H-reflex, V-wave) were observed. |
| Donnelly, 2021 [31] | RCT Extra force | 23/3 | 26.7 ± 2 | - | Evaluation of torque evoked by WPHF using transcutaneous electrical nerve stimulation (TENS) or transcutaneous spinal direct current stimulation (tsDCS). | Extra force produced by WPHF stimulation was diminished after a bout of TENS but was not affected after a bout of tsDCS. |
| Espeit, 2021 [33] | RCT Extra force | 30/5 | 26.6 ± 6 | - | Evaluation of the extra force magnitude with high and low frequency, wide pulse stimulation. | Low and high frequency wide pulse NMES has similar number of responders for extra force, with higher extra force for high frequency. |
| Tibial nerve NMES studies (n = 10) | ||||||
| Klakowicy, 2006 [20] | Cohort Extra force | 11/- | 21–42 | 2/1 (18.1%/9.1%) | Examination of whether the extra torque generated by 100 Hz stimulation involves the activation of spinal motoneurons and whether H-reflexes can recover during tetanic stimulation of soleus afferents | Production of extra force was accompanied by an increase in the H-reflex amplitude with no change in the M-wave amplitude. |
| Lagerquist, 2010 [11] | Cohort Mechanisms | 18/2 | 19–43 | 4/4 (22.2%/22.2%) | The effect of pulse width on M-wave, H-reflex, and torque during 2 s of 100 Hz stimulation | Wide-pulse NMES generated greater force via sensory pathways. |
| Clair, 2011 [35] | Cohort analytic Mechanisms | 11/3 | 20–46 | - | Investigation of H-reflex recovery after 10-s trains of stimulation at 5–20 Hz, during functional tasks and low-level muscle contractions. | Increasing the stimulation frequency of the afferent pathway and the intensity of background voluntary activation can recover the H-reflex amplitude, when it has been reduced due to post-activation depression. |
| Lagerquist, 2012 [12] | Cohort Mechanisms | 10/3 | 22–44 | - | Evaluation of the enhanced spinal and corticospinal excitability of the soleus muscle following voluntary plantar-flexions, NMES of the TN and combination of the two. | Spinal excitability increased after a contraction induced by NMES combined with voluntary activation, whereas there was no effect on corticospinal excitability. |
| Dean, 2014 [38] | Cohort Mechanisms | 9/2 | 22–44 | 2/- (22.2%/-) | Characterization of the recruitment and ongoing discharge of human motoneurons when they receive trains of afferent impulses over a range of physiologically relevant frequencies | Repetitive stimulation of the TN, with intensity that does not elicit H-reflex or M-wave with a single pulse recruited motor units, and this was retained even after cessation of the stimulation. |
| Doix, 2014 [21] | Cohort Fatigue | 9/0 | 26.6 ± 2.0 | 1/1 (11.1%/11.1%) | Investigation of muscle fatigue of the TS after NMES protocols with constant or increasing current intensity and comparison with fatigue induced by voluntary contractions. | MVC decreased, and neural properties changed to a similar extent independent of the variable or constant stimulation intensity fatigue protocol. |
| Martin, 2016 [26] | Cohort Fatigue | 15/3 | 28.0 ± 8.0 | 4/4 (26.4%/26.7%) | Examination of whether the WPHF paradigm applied over the nerve trunk limits muscle fatigue by favoring motor unit’s recruitment through afferent pathways, in contrast to the motor unit activation through efferent pathways associated with a conventional NMES protocol | WPHF induced greater H-reflex increase and greater M-wave decrease compared to short pulse low frequency stimulation, despite no differences in force reduction. |
| Vitry, 2019 [13] | Cohort Extra force | 12/2 | 27.1 ± 8.7 | - | Examination of the conditions to invoke extra torque by modulating the frequency and intensity of stimulation. | Spinal excitability contribution to extra forces is frequency dependent, whereas supraspinal mechanisms do not seem to be affected by the frequency of NMES. |
| Vitry, 2019 [46] | Cohort Fatigue | 9/2 | 23.2 ± 6.6 | - | The effect of stimulation frequency on neuromuscular fatigue using stimulation parameters favoring an indirect motor unit recruitment through the afferent pathway. | Low and high frequency fatigue protocols induced similar level of decrease in twitch torque and level of voluntary activation. |
| Vitry, 2019 [30] | RCT Training | 10/3 | 22.7 ± 6.4 | - | The effect of chronic application of NMES training modalities using wide pulse duration, low stimulation intensity, and nerve stimulation to maximize the central contribution to the evoked torque | Similar strength gains independent of the stimulation frequency NMES training, whereas low frequency NMES resulted in supraspinal adaptations, and high frequency NMES resulted in supraspinal and spinal adaptations. |
| Triceps surae and tibial nerve NMES studies (n = 3) | ||||||
| Baldwin, 2006 [18] | Cohort Extra force | 15/2 | 20–41 | 1/1 (6.7%/6.7%) | The optimal technique to evoke sustained contractions enhanced through spinal pathways during NMES | Larger extra forces are produced with muscle stimulation in comparison to nerve stimulation, whereas stimulating the muscle or nerve may involve different neural pathways. |
| Bergquist, 2011 [17] | Cohort Mechanisms | 14/2 | 20–48 | 4/1 (28.5%/7.1%) | To compare the contributions of central and peripheral pathways to the motor unit recruitment for contractions of similar amplitude generated by NMES applied over TN or TS. | NMES over the tibial nerve produced responses with greater involvement of the sensory pathway than stimulation over the triceps surae. |
| Neyroud, 2018 [7] | Cohort Extra force | 10/5 | 28.0 ± 4.0 | - | Evaluation of the reliability of force production induced by WPHF NMES delivered over the TN or the plantar flexor muscle. | Nerve and muscle NMES resulted in similar magnitude of extra forces. |
| First Author, Year (Reference Number) | NMES Protocols (n) | Neuromuscular Electrical Stimulation Parameters (NMES) | |||
|---|---|---|---|---|---|
| Pulse Width (ms) | Frequency (Hz) × Duration (s) × Count of Bursts within Cycle | Duty Cycle on/off × Count of Cycles | Intensity | ||
| Extra force (n = 12) | |||||
| Collins, 2002 [5] | TS: 4 | 1 | C: (100, 200) × 7” B: 100 × 2” × 1 P: 100 × 6” | 7”/0” × 1 6”/0” × 1 | 5% MVC 5% MVC 5% MVC |
| Baldwin, 2006 [18] | TS: 2 TN: 2 | 1 | B: 100 × 2” | 7”/3” × 5 | 2, 4% MVC |
| Klakowicy, 2006 [20] | TN: 1 | 1 | B: 100 × 2” | 7”/3” × 5 | 0.3–4% Mmax |
| Dean, 2008 [37] | TS: 1 | 1 | B: 100 × 2” × 4 | 3% MVC | |
| Lagerquist, 2009 [41] | TS: 3 | 1 | C: 100 × 30” B: 100 × 2” × 4 C: 100 × 30” | 1”/1” × 15 | 13.0 ± 2.7 mA 7.5% MVC |
| Regina Dias Da Silva, 2015 [45] | TS: 1 | 1 | C: 100 × 10” | 16.5 ± 10.3 mA 10% MVC | |
| Wegrzyk, 2015 [34] | TS: 1 | 1 | C: 100 × 20” | 20”/90” × 5 | 12.9–15.4 mA ~5% MVC |
| Wegrzyk, 2015 [47] | TS: 1 | 1 | C: 100 | 20”/20” × 20 | 15–18 mA 8.5–11% MVC |
| Neyroud, 2018 [7] | TS: 1 TN: 1 | 1 | C: 100 | 20”/40” × 10 | 2.3–4.6 mA 5% MVC |
| Vitry, 2019 [13] | TN: 15 | 1 | C: (20, 50, 100) × 20” | 1.1, 1.2, 1.3, 1.4, 1.5 MT | |
| Donnelly, 2021 [31] | TS: 1 | 1 | C: 100 | 20”/40” × 1 or 3 | 5% MVC |
| Espeit, 2021 [33] | TS: 2 | 1 | C: 20 Hz C: 100 Hz | 20”/90” × 3 20”/90” × 3 | 9 ± 3 mA 8 ± 3 mA 10% MVC |
| Mechanisms (n = 9) | |||||
| Dean, 2007 [8] | TS: 8 | 1 | C: 50 × 20” C: 100 × 20” B: 80 × 2” × 4 B: 100 × 2” × 4 | 1, 3% MVC 1, 3% MVC 3% MVC 1, 3, 5% MVC | |
| Lagerquist, 2010 [11] | TN: 6 | 0.2, 0.5, 1 | B: 100 × 2” | 7”/n.r. × 4 | 5–15 mA1–2, 5% Mmax |
| Bergquist, 2011 [17] | TS: 2 | 1 | B: 100 × 2” | 8”/45” × 5 | 28.3 ± 1.9 mA 10% MVC 34.2 ± 2.7 mA 20–40% MVC |
| TN: 4 | 1 | C: 20 × 8” B: 100 × 2” | 8”/45” × 5 | 7.8 ± 0.9 mA 10% MVC 8.4 ± 0.8 mA 20–40% MVC | |
| Clair, 2011 [35] | TN: 6 | 1 | C: (1,5,10,20) × 10” | 10”/30” × 3 | 1,5,10,20% Mmax |
| Lagerquist, 2012 [12] | TN: 1 | 1 | C: 100 × 40′ | 5”/5” × 240 | 2–3% MVC |
| Dean, 2014 [38] | TN: 2 | 1 | C: (80, 100) × 30” | 2.5–10 mA ≤1 MT | |
| Wegrzyk, 2017 [15] | TS: 2 | 0.051 | C: 25 C: 100 | 20”/20” × 20 20”/20” × 20 | 135 ± 31 mA 25.0 ± 11.0 mA 10% MVC |
| Cheng, 2017 [36] | TS: 1 | B: 100 × 2” × 1 | 6”/0” × 1 | 17.0 ± 11.0 mA 5–10% MVC | |
| Grosprêtre, 2018 [40] | TS: 1 | 1 | C: 100 | 6”/6” × 40 | 12.8 ± 4.3 mA 20% MVC |
| Fatigue (n = 7) | |||||
| Doix, 2014 [21] | TN: 2 | 1 | C: 50 × 6” C: 50 × 6” | 6”/6” × 2 6”/6” × 2 | 20.6 ± 1.7 mA variable20% MVC |
| Neyroud, 2014 [42] | TS: 1 | 1 | C: 100 | 20”/40” × 20 | 18.0 ± 8.0 mA 10% MVC |
| Papaiordanidou, 2014 [43] | TS: 1 | 0.5 | C: 30 B: 100 doublets | 0.167”/0.5” × 450 0.146”/0.5” × 450 | 30% MVC |
| Papaiordanidou, 2014 [44] | TS: 2 | 0.5, 1 | C: 100 | 4”/6” × 60 | 30% MVC |
| Martin, 2016 [26] | TN: 1 | 1 | C: 80 | 6”/6” × 40 | 20% MVC |
| Grosprêtre, 2017 [39] | TS: 1 | 1 | C: 100 | 6”/6” × 40 | 12.9 ± 1.4 mA 20% MVC |
| Vitry, 2019 [46] | TN: 2 | 1 | C: 20 C: 100 | 20”/20” × 25 20”/20” × 25 | 11.3 ± 5.6 mA 11.9 ± 6.0 mA 10% MVC |
| Training (n = 4) | |||||
| Mani, 2018 [32] | TS: 2 | 0.261 | C: 50C: 100 | 4”/12” × 75 4”/12” × 75 | 33 ± 10 mA 18.5 ± 8 mA until tolerance level |
| Neyroud, 2019 [16] | TS: 1 | 1 | C: 100 | 20”/40” × 10 | 13.3 ± 9.1 mA 5% MVC |
| Vitry, 2019 [30] | TN: 2 | 1 | C: 20 C: 100 | 20”/20” × 25 20”/20” × 25 | 12.2 ± 6.4 mA 11.2 ± 4.7 mA 10% MVC |
| Bouguetoch, 2021 [29] | TN: 1 | 0.5 | C: 80 | 6”/6” × 40 | 10–45 mA 20% MVC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papavasileiou, A.; Xenofondos, A.; Baudry, S.; Lapole, T.; Amiridis, I.G.; Metaxiotis, D.; Tsatalas, T.; Patikas, D.A. Protocols Targeting Afferent Pathways via Neuromuscular Electrical Stimulation for the Plantar Flexors: A Systematic Review. Sensors 2023, 23, 2347. https://doi.org/10.3390/s23042347
Papavasileiou A, Xenofondos A, Baudry S, Lapole T, Amiridis IG, Metaxiotis D, Tsatalas T, Patikas DA. Protocols Targeting Afferent Pathways via Neuromuscular Electrical Stimulation for the Plantar Flexors: A Systematic Review. Sensors. 2023; 23(4):2347. https://doi.org/10.3390/s23042347
Chicago/Turabian StylePapavasileiou, Anastasia, Anthi Xenofondos, Stéphane Baudry, Thomas Lapole, Ioannis G. Amiridis, Dimitrios Metaxiotis, Themistoklis Tsatalas, and Dimitrios A. Patikas. 2023. "Protocols Targeting Afferent Pathways via Neuromuscular Electrical Stimulation for the Plantar Flexors: A Systematic Review" Sensors 23, no. 4: 2347. https://doi.org/10.3390/s23042347
APA StylePapavasileiou, A., Xenofondos, A., Baudry, S., Lapole, T., Amiridis, I. G., Metaxiotis, D., Tsatalas, T., & Patikas, D. A. (2023). Protocols Targeting Afferent Pathways via Neuromuscular Electrical Stimulation for the Plantar Flexors: A Systematic Review. Sensors, 23(4), 2347. https://doi.org/10.3390/s23042347









