Convergent Validity between Electromyographic Muscle Activity, Ultrasound Muscle Thickness and Dynamometric Force Measurement for Assessing Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
2.4. Fatigue Measurement Instruments
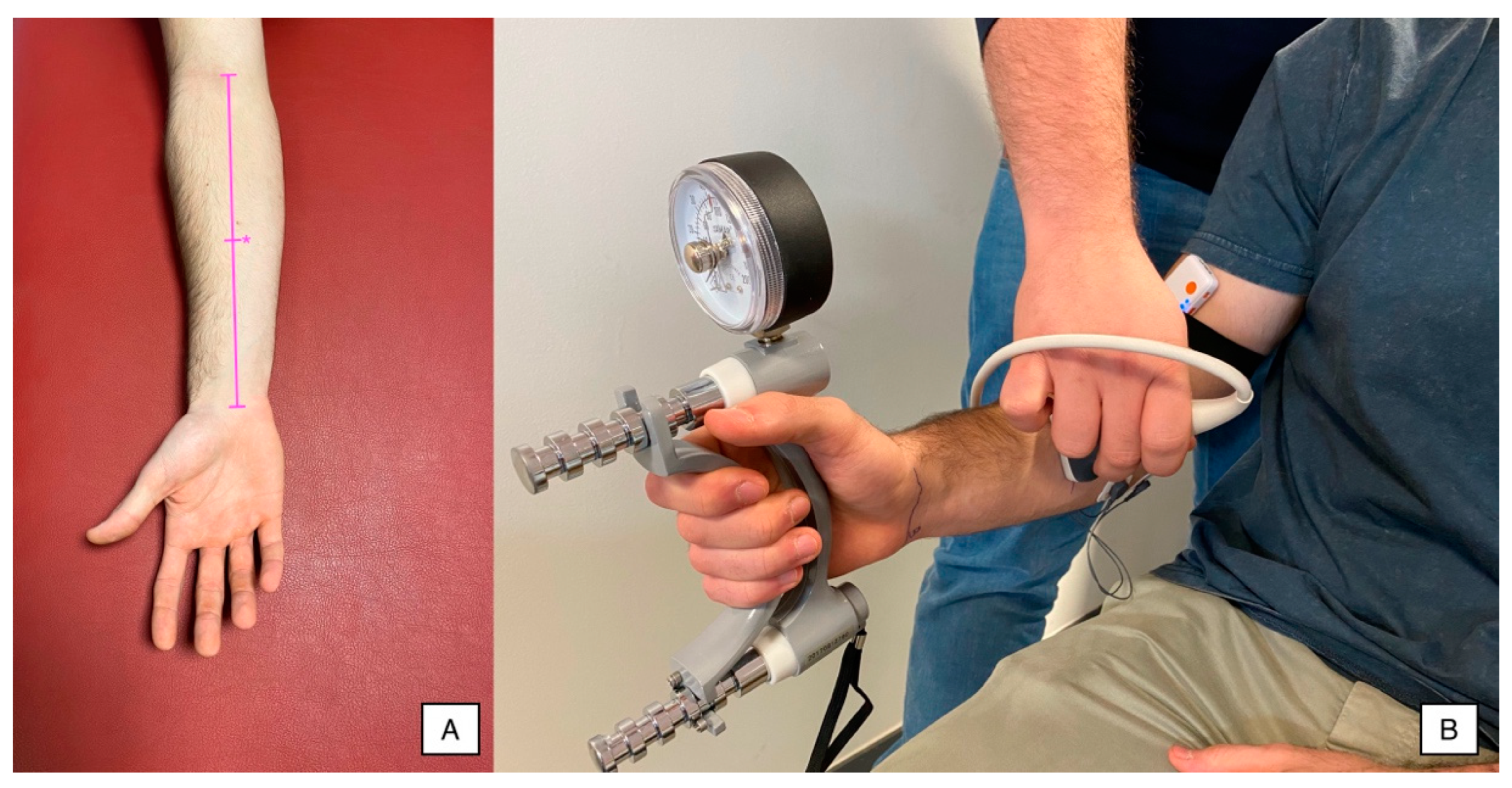
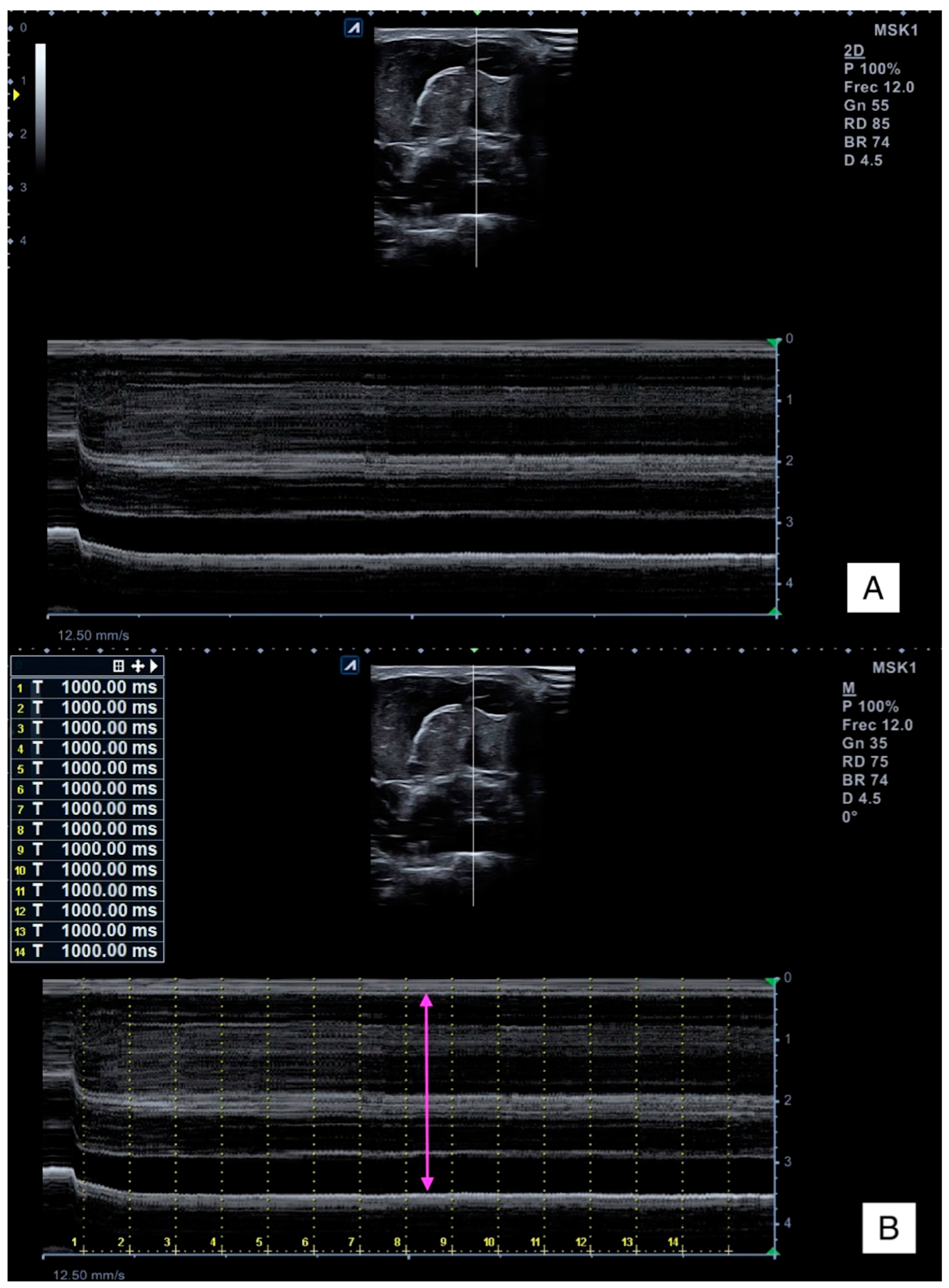
2.4.1. Ultrasound Imaging
2.4.2. Hand Grip Force
2.4.3. Surface Electromyography
2.5. Outcomes
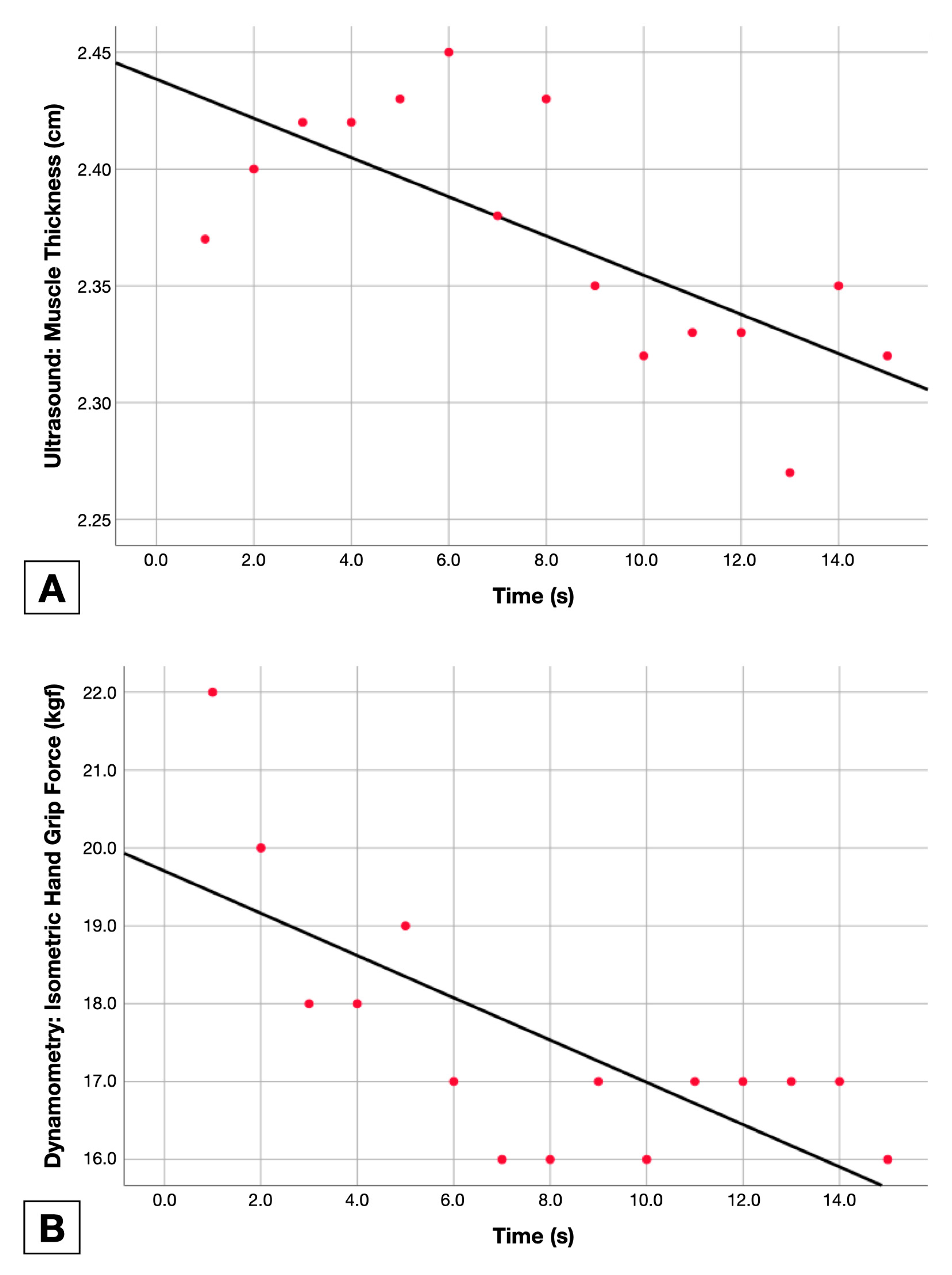
2.5.1. Isometric Force Holding
2.5.2. Muscle Thickness Change
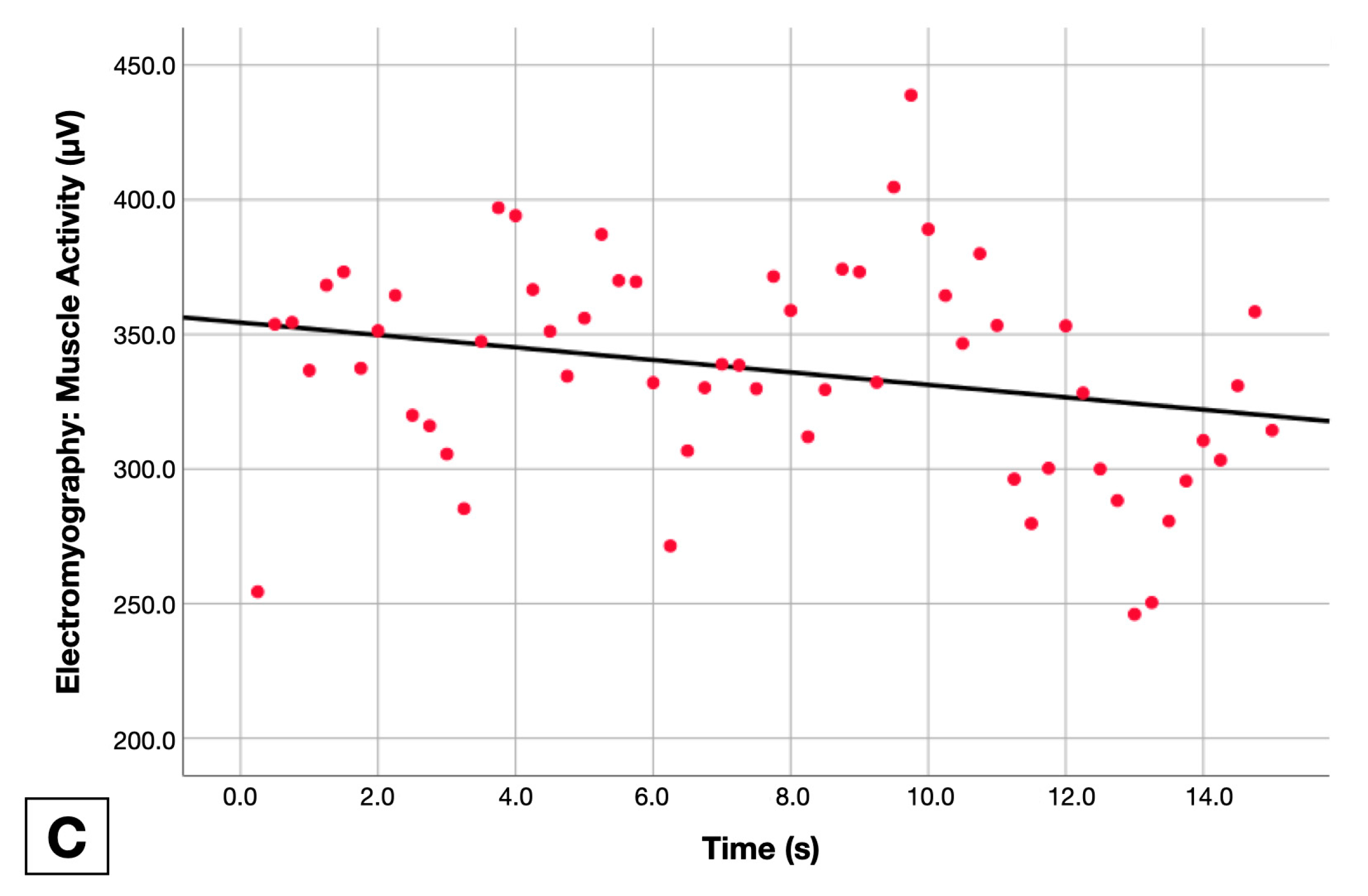
2.5.3. Muscle Activity Change
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, A.J.; Duxson, M.J.; Butler, J.E.; Hodges, P.W.; Taylor, J.L.; Gandevia, S.C. Muscle fiber and motor unit behavior in the longest human skeletal muscle. J. Neurosci. 2005, 25, 8528–8533. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Exeter, D.; Connell, D.A. Skeletal muscle: Functional anatomy and pathophysiology. Semin. Musculoskelet. Radiol. 2010, 14, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Schomacher, J.; Falla, D. Function and structure of the deep cervical extensor muscles in patients with neck pain. Man. Ther. 2013, 18, 360–366. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Constantin, D. Molecular Mechanisms of Muscle Fatigue. Int. J. Mol. Sci. 2021, 22, 11587. [Google Scholar] [CrossRef]
- Vøllestad, N.K. Measurement of human muscle fatigue. J. Neurosci. Methods 1997, 74, 219–227. [Google Scholar] [CrossRef]
- Kossoff, G.; Garrett, W.J.; Carpenter, D.A.; Jellins, J.; Dadd, M.J. Principles and classification of soft tissues by grey scale echography. Ultrasound Med. Biol. 1976, 2, 89–111. [Google Scholar] [CrossRef]
- Varol, U.; Navarro-Santana, M.J.; Gómez-Sánchez, S.; Plaza-Manzano, G.; Sánchez-Jiménez, E.; Valera-Calero, J.A. Inter-Examiner Disagreement for Assessing Cervical Multifidus Ultrasound Metrics Is Associated with Body Composition Features. Sensors 2023, 23, 1213. [Google Scholar] [CrossRef]
- So, S.; Patel, R.M.; Orebaugh, S.L. Ultrasound imaging in medical student education: Impact on learning anatomy and physical diagnosis. Anat. Sci. Educ. 2017, 10, 176–189. [Google Scholar] [CrossRef]
- Varol, U.; Sánchez-Jiménez, E.; Leloup, E.A.A.; Navarro-Santana, M.J.; Fernández-de-las-Peñas, C.; Sánchez-Jorge, S.; Valera-Calero, J.A. Correlation between Body Composition and Inter-Examiner Errors for Assessing Lumbar Multifidus Muscle Size, Shape and Quality Metrics with Ultrasound Imaging. Bioengineering 2023, 10, 133. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Ellis, R.; Hodges, P.W.; OSullivan, C.; Hides, J.; Fernandez-Carnero, S.; Arias-Buria, J.L.; Teyhen, D.S.; Stokes, M.J. Imaging with ultrasound in physical therapy: What is the PT’s scope of practice? A competency-based educational model and training recommendations. Br. J. Sports Med. 2019, 53, 1447–1453. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-de-las-Peñas, C.; Fernández-Rodríguez, T.; Arias-Buría, J.L.; Varol, U.; Gallego-Sendarrubias, G.M. Influence of Examiners’ Experience and Region of Interest Location on Semi-quantitative Elastography Validity and Reliability. Appl. Sci. 2021, 11, 9247. [Google Scholar] [CrossRef]
- Shriki, J. Ultrasound physics. Crit. Care Clin. 2014, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Felici, F.; Del Vecchio, A. Surface Electromyography: What Limits Its Use in Exercise and Sport Physiology? Front. Neurol. 2020, 11, 578504. [Google Scholar] [CrossRef]
- Neblett, R. Surface Electromyographic (SEMG) Biofeedback for Chronic Low Back Pain. Healthcare 2016, 4, 27. [Google Scholar] [CrossRef]
- Santana-Mora, U.; López-Ratón, M.; Mora, M.J.; Cadarso-Suárez, C.; López-Cedrún, J.; Santana-Penín, U. Surface raw electromyography has a moderate discriminatory capacity for differentiating between healthy individuals and those with TMD: A diagnostic study. J. Electromyogr. Kinesiol. 2014, 24, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.V., Jr. Electromyography; uses and limitations. Calif. Med. 1958, 89, 250–252. [Google Scholar]
- Cram, J.R.; Kasman, G.S.; Holtz, J. Introduction to Surface Electromyography; Aspen Publishers: Boston, MA, USA, 1998. [Google Scholar]
- Cigarán-Méndez, M.; Úbeda-D’Ocasar, E.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Gallego-Sendarrubias, G.M.; Valera-Calero, J.A. The hand grip force test as a measure of physical function in women with fibromyalgia. Sci. Rep. 2022, 12, 3414. [Google Scholar] [CrossRef]
- Trinidad-Fernández, M.; González-Molina, F.; Moya-Esteban, A.; Roldán-Jiménez, C.; González-Sánchez, M.; Cuesta-Vargas, A.I. Muscle activity and architecture as a predictor of hand-grip strength. Physiol. Meas. 2020, 41, 075008. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr. EQUATOR reporting guidelines should also be used by clinicians. J. Clin. Epidemiol. 2020, 117, 149–150. [Google Scholar] [CrossRef]
- Beneciuk, J.M.; Bishop, M.D.; George, S.Z. Clinical prediction rules for physical therapy interventions: A systematic review. Phys. Ther. 2009, 89, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Edmond, T.; Laps, A.; Case, A.L.; O’Hara, N.; Abzug, J.M. Normal Ranges of Upper Extremity Length, Circumference, and Rate of Growth in the Pediatric Population. Hand 2020, 15, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.F.; McDonald, C.; Chenier, T.C. Measurement of grip strength: Validity and reliability of the sphygmomanometer and jamar grip dynamometer. J. Orthop. Sports Phys. Ther. 1992, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.K.; Lowe, B.D. Optimal cylindrical handle diameter for grip force tasks. Int. J. Ind. Ergon. 2005, 35, 495–507. [Google Scholar] [CrossRef]
- Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles. Available online: http://www.seniam.org (accessed on 24 January 2023).
- Stegeman, D.; Hermens, H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede Roessingh Res. Dev. 2007, 10, 8–12. [Google Scholar]
- Hashemi Oskouei, A.; Paulin, M.G.; Carman, A.B. Intra-session and inter-day reliability of forearm surface EMG during varying hand grip forces. J. Electromyogr. Kinesiol. 2013, 23, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Sorbie, G.G.; Hunter, H.H.; Grace, F.M.; Gu, Y.; Baker, J.S.; Ugbolue, U.C. An electromyographic study of the effect of hand grip sizes on forearm muscle activity and golf performance. Res. Sports Med. 2016, 24, 222–233. [Google Scholar] [CrossRef]
- Blackwell, J.R.; Kornatz, K.W.; Heath, E.M. Effect of grip span on maximal grip force and fatigue of flexor digitorum superficialis. Appl. Ergon. 1999, 30, 401–405. [Google Scholar] [CrossRef]
- Goislard de Monsabert, B.; Rossi, J.; Berton, E.; Vigouroux, L. Quantification of hand and forearm muscle forces during a maximal power grip task. Med. Sci. Sports Exerc. 2012, 44, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Robertson, L.D.; Mullinax, C.M.; Brodowicz, G.R.; Swafford, A.R. Muscular fatigue patterning in power grip assessment. J. Occup. Rehabil. 1996, 6, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.J. Factors which contribute to fatigue associated with rheumatoid arthritis. J. Adv. Nurs. 1991, 16, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Dawson, L.; Kleppinger, A.; Iannuzzi-Sucich, M.; Judge, J.O. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M436–M440. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Hardy, J.; Broadley, K.; Tookman, A.J.; Kurowska, A.; A’Hern, R. Fatigue in advanced cancer: A prospective controlled cross-sectional study. Br. J. Cancer 1999, 79, 1479–1486. [Google Scholar] [CrossRef]
- Petrofsky, J.; Prowse, M.; Remigio, W.; Raju, C.; Salcedo, S.; Sirichotiratana, M.; Madani, P.; Chamala, R.R.; Puckett, E.; Wong, M.; et al. The use of an isometric handgrip test to show autonomic damage in people with diabetes. Diabetes Technol. Ther. 2009, 11, 361–368. [Google Scholar] [CrossRef]
- Kwon, J.W.; Lee, B.H.; Lee, S.B.; Sung, S.; Lee, C.U.; Yang, J.H.; Park, M.S.; Byun, J.; Lee, H.M.; Moon, S.H. Hand grip strength can predict clinical outcomes and risk of falls after decompression and instrumented posterolateral fusion for lumbar spinal stenosis. Spine J. 2020, 20, 1960–1967. [Google Scholar] [CrossRef]
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B359–B365. [Google Scholar] [CrossRef]
- Sunnerhagen, K.S.; Cider, A.; Schaufelberger, M.; Hedberg, M.; Grimby, G. Muscular performance in heart failure. J. Card Fail. 1998, 4, 97–104. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Gallego-Sendarrubias, G.M.; Cimadevilla-Fernández-Pola, E. Echo-intensity and fatty infiltration ultrasound imaging measurement of cervical multifidus and short rotators in healthy people: A reliability study. Musculoskelet. Sci. Pract. 2021, 53, 102335. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Manzano, G.; Navarro-Santana, M.J.; Valera-Calero, J.A.; Fabero-Garrido, R.; Fernández-de-Las-Peñas, C.; López-de-Uralde-Villanueva, I. Reliability of lumbar multifidus ultrasound assessment during the active straight leg raise test. Eur. J. Clin. Investig. 2022, 52, e13728. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Sharma, N.; Kim, K. Quantitative Assessment of Changes in Muscle Contractility Due to Fatigue During NMES: An Ultrasound Imaging Approach. IEEE Trans. Biomed. Eng. 2020, 67, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.C.; Hullfish, T.J.; O’Connor, K.M.; Hast, M.W.; Baxter, J.R. Ultrasound echogenicity is associated with fatigue-induced failure in a cadaveric Achilles tendon model. J. Biomech. 2020, 105, 109784. [Google Scholar] [CrossRef]
- Wallwork, T.L.; Stanton, W.R.; Freke, M.; Hides, J.A. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man. Ther. 2009, 14, 496–500. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-de-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R.; Gallego-Sendarrubias, G.M.; Arias-Buría, J.L. Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7554. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, Y.P.; Chen, X.; Huang, Q.H. Assessment of muscle fatigue using sonomyography: Muscle thickness change detected from ultrasound images. Med. Eng. Phys. 2007, 29, 472–479. [Google Scholar] [CrossRef]
- Bunce, S.M.; Hough, A.D.; Moore, A.P. Measurement of abdominal muscle thickness using M-mode ultrasound imaging during functional activities. Man. Ther. 2004, 9, 41–44. [Google Scholar] [CrossRef]
- Bobos, P.; Nazari, G.; Lu, Z.; MacDermid, J.C. Measurement Properties of the Hand Grip Strength Assessment: A Systematic Review With Meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 553–565. [Google Scholar] [CrossRef]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Falla, D.; Rainoldi, A.; Merletti, R.; Jull, G. Myoelectric manifestations of sternocleidomastoid and anterior scalene muscle fatigue in chronic neck pain patients. Clin. Neurophysiol. 2003, 114, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.E.; Cassisi, J.E.; O’Connor, P.D.; MacMillan, M. Lumbar iEMG during isotonic exercise: Chronic low back pain patients versus controls. J. Spinal Disord. 1992, 5, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Elfving, B.; Dedering, A.; Németh, G. Lumbar muscle fatigue and recovery in patients with long-term low-back trouble—Electromyography and health-related factors. Clin. Biomech. 2003, 18, 619–630. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Sample (n = 50) | Gender | |
|---|---|---|---|
| Male (n = 26) | Female (n = 24) | ||
| Age (years) | 22.3 ± 5.4 | 23.7 ± 6.7 | 20.8 ± 2.9 |
| Height (m) | 1.72 ± 0.09 | 1.78 ± 0.07 | 1.65 ± 0.06 |
| Weight (kg) | 70.8 ± 17.3 | 79.7 ± 18.3 | 61.1 ± 9.5 |
| BMI (kg/m2) | 23.5 ± 3.8 | 24.7 ± 4.2 | 22.1 ± 2.7 |
| Forearm Length (cm) | 25.6 ± 1.9 | 26.6 ± 1.6 | 24.5 ± 1.6 |
| Forearm Perimeter (cm) | 21.9 ± 2.4 | 23.4 ± 1.9 | 20.3 ± 1.9 |
| Difference | |||
| Age (years) | 2.9 (−0.1; 5.9) p = 0.057 | ||
| Height (m) | 0.12 (0.08; 0.16) p < 0.001 | ||
| Weight (kg) | 18.6 (10.1; 27.0) p < 0.001 | ||
| BMI (kg/m2) | 2.6 (0.6; 4.7) p = 0.013 | ||
| Forearm Length (cm) | 2.0 (1.1; 3.0) p < 0.001 | ||
| Forearm Perimeter (cm) | 3.1 (2.0; 4.2) p < 0.001 | ||
| Variables | Side | Dominance | ||
|---|---|---|---|---|
| Left (n = 50) | Right (n = 50) | Dominant (n = 50) | Non-Dominant (n = 50) | |
| Forearm Length (cm) | 25.6 ± 1.9 | 25.6 ± 1.9 | 25.6 ± 2.0 | 25.6 ± 1.9 |
| Forearm Perimeter (cm) | 21.6 ± 2.4 | 22.2 ± 2.4 | 22.2 ± 2.5 | 21.6 ± 2.4 |
| Difference | ||||
| Forearm Length (cm) | 0.0 (−0.7; 0.8) p = 0.927 | 0.0 (−0.7; 0.7) p = 0.967 | ||
| Forearm Perimeter (cm) | 0.7 (−0.3; 1.6) p = 0.189 | 0.6 (−0.4; 1.6) p = 0.218 | ||
| Variables | Gender | |
|---|---|---|
| Male (n = 26) | Female (n = 24) | |
| Muscle Activity Changes: Surface Electromyography | ||
| Adjusted Line Slope | −1.08 ± 3.33 | −0.70 ± 2.19 |
| Difference | −0.38 (−1.52; 0.76); p = 0.508 | |
| Variance (R2) | 0.29 ± 0.26 | 0.30 ± 0.26 |
| Difference | −0.006 (−0.11; 0.10); p = 0.910 | |
| Muscle Thickness Changes: Ultrasound Imaging | ||
| Adjusted Line Slope | −0.0043 ± 0.0110 | −0.0036 ± 0.0062 |
| Difference | −0.00074 (−0.0048; 0.00299) p = 0.693 | |
| Variance (R2) | 0.37 ± 0.30 | 0.34 ± 0.27 |
| Difference | 0.028 (−0.093; 0.149) p = 0.644 | |
| Force Holding Capacity: Hand Grip Dynamometry | ||
| Adjusted Line Slope | −1.39 ± 1.04 | −1.04 ± 0.59 |
| Difference | −0.35 (−0.69; −0.009); p = 0.022 | |
| Variance (R2) | 0.74 ± 0.21 | 0.76 ± 0.18 |
| Difference | −0.018 (−0.099; 0.063); p = 0.656 | |
| Variables | Side | |
|---|---|---|
| Left (n = 50) | Right (n = 50) | |
| Muscle Activity Changes: Surface Electromyography | ||
| Adjusted Line Slope | −0.50 ± 2.42 | −1.27 ± 3.15 |
| Difference | −0.648 (−1.78; 0.48); p = 0.258 | |
| Variance (R2) | 0.26 ± 0.25 | 0.33 ± 0.27 |
| Difference | 0.075 (−0.029; 0.181); p = 0.159 | |
| Muscle Thickness Changes: Ultrasound Imaging | ||
| Adjusted Line Slope | −0.0033 ± 0.0079 | −0.0045 ± 0.0098 |
| Difference | −0.000863 (−0.004598; 0.002872); p = 0.647 | |
| Variance (R2) | 0.38 ± 0.29 | 0.33 ± 0.29 |
| Difference | 0.273 (−0.188; 0.054); p = 0.272 | |
| Force Holding Capacity: Hand Grip Dynamometry | ||
| Adjusted Line Slope | −1.22 ± 0.80 | −1.21 ± 0.93 |
| Difference | 0.0173 (−0.3327; 0.3673); p = 0.922 | |
| Variance (R2) | 0.75 ± 0.19 | 0.76 ± 0.20 |
| Difference | −0.0078 (−0.0886; 0.0728); p = 0.847 | |
| Variables | Dominance | |
|---|---|---|
| Dominant (n = 50) | Non-Dominant (n = 50) | |
| Muscle Activity Changes: Surface Electromyography | ||
| Adjusted Line Slope | 1.21 ± 3.19 | −0.57 ± 2.38 |
| Difference | −0.769 (−1.897; 0.358); p = 0.179 | |
| Variance (R2) | 0.33 ± 0.27 | 0.26 ± 0.25 |
| Difference | 0.071 (−0.033; 0.177); p = 0.180 | |
| Muscle Thickness Changes: Ultrasound Imaging | ||
| Adjusted Line Slope | −0.0044 ± 0.0099 | −0.0035 ± 0.0079 |
| Difference | −0.0012 (−0.0049; 0.0025); p = 0.524 | |
| Variance (R2) | 0.32 ± 0.29 | 0.39 ± 0.28 |
| Difference | −0.052 (−0.174; 0.068); p = 0.329 | |
| Force Holding Capacity: Hand Grip Dynamometry | ||
| Adjusted Line Slope | −1.21 ± 0.94 | −1.22 ± 0.79 |
| Difference | 0.006 (−0.344; 0.355); p = 0.974 | |
| Variance (R2) | 0.75 ± 0.21 | 0.76 ± 0.18 |
| Difference | 0.0076 (−0.073; 0.088); p = 0.852 | |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | |||||||||
| 2. Height | n.s. | ||||||||
| 3. Weight | n.s. | 0.758 ** | |||||||
| 4. BMI | n.s. | 0.455 ** | 0.919 ** | ||||||
| 5. Forearm girth | 0.295 * | 0.577 ** | 0.663 ** | 0.600 ** | |||||
| 6. Forearm length | n.s. | 0.811 ** | 0.473 ** | n.s. | 0.462 ** | ||||
| 7. Gender | n.s. | −0.678 ** | −0.539 ** | −0.350 * | −0.619 ** | −0.546 ** | |||
| 8. EMG slope | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| 9. US slope | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.359 ** | |
| 10. Dynamometry slope | n.s. | −0.337 * | n.s. | n.s. | −0.248 * | −0.278 ** | 0.202 * | 0.305 ** | 0.227 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varol, U.; Navarro-Santana, M.J.; Valera-Calero, J.A.; Antón-Ramírez, S.; Álvaro-Martínez, J.; Díaz-Arribas, M.J.; Fernández-de-las-Peñas, C.; Plaza-Manzano, G. Convergent Validity between Electromyographic Muscle Activity, Ultrasound Muscle Thickness and Dynamometric Force Measurement for Assessing Muscle. Sensors 2023, 23, 2030. https://doi.org/10.3390/s23042030
Varol U, Navarro-Santana MJ, Valera-Calero JA, Antón-Ramírez S, Álvaro-Martínez J, Díaz-Arribas MJ, Fernández-de-las-Peñas C, Plaza-Manzano G. Convergent Validity between Electromyographic Muscle Activity, Ultrasound Muscle Thickness and Dynamometric Force Measurement for Assessing Muscle. Sensors. 2023; 23(4):2030. https://doi.org/10.3390/s23042030
Chicago/Turabian StyleVarol, Umut, Marcos J. Navarro-Santana, Juan Antonio Valera-Calero, Sergio Antón-Ramírez, Javier Álvaro-Martínez, María José Díaz-Arribas, César Fernández-de-las-Peñas, and Gustavo Plaza-Manzano. 2023. "Convergent Validity between Electromyographic Muscle Activity, Ultrasound Muscle Thickness and Dynamometric Force Measurement for Assessing Muscle" Sensors 23, no. 4: 2030. https://doi.org/10.3390/s23042030
APA StyleVarol, U., Navarro-Santana, M. J., Valera-Calero, J. A., Antón-Ramírez, S., Álvaro-Martínez, J., Díaz-Arribas, M. J., Fernández-de-las-Peñas, C., & Plaza-Manzano, G. (2023). Convergent Validity between Electromyographic Muscle Activity, Ultrasound Muscle Thickness and Dynamometric Force Measurement for Assessing Muscle. Sensors, 23(4), 2030. https://doi.org/10.3390/s23042030







