Electrochemical Sensing of Lead in Drinking Water Using Copper Foil Bonded with Polymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sensor Fabrication
2.3. Electrode Characterization
2.4. Voltammetry Experiments
3. Results and Discussion
3.1. Directly Bonded Cu/LCP for Sensing Electrodes
3.2. Cu-Based Sensing Electrodes
3.3. Optimization of Sensing Parameters
3.4. Voltammetric Measurements and Sensor Calibration
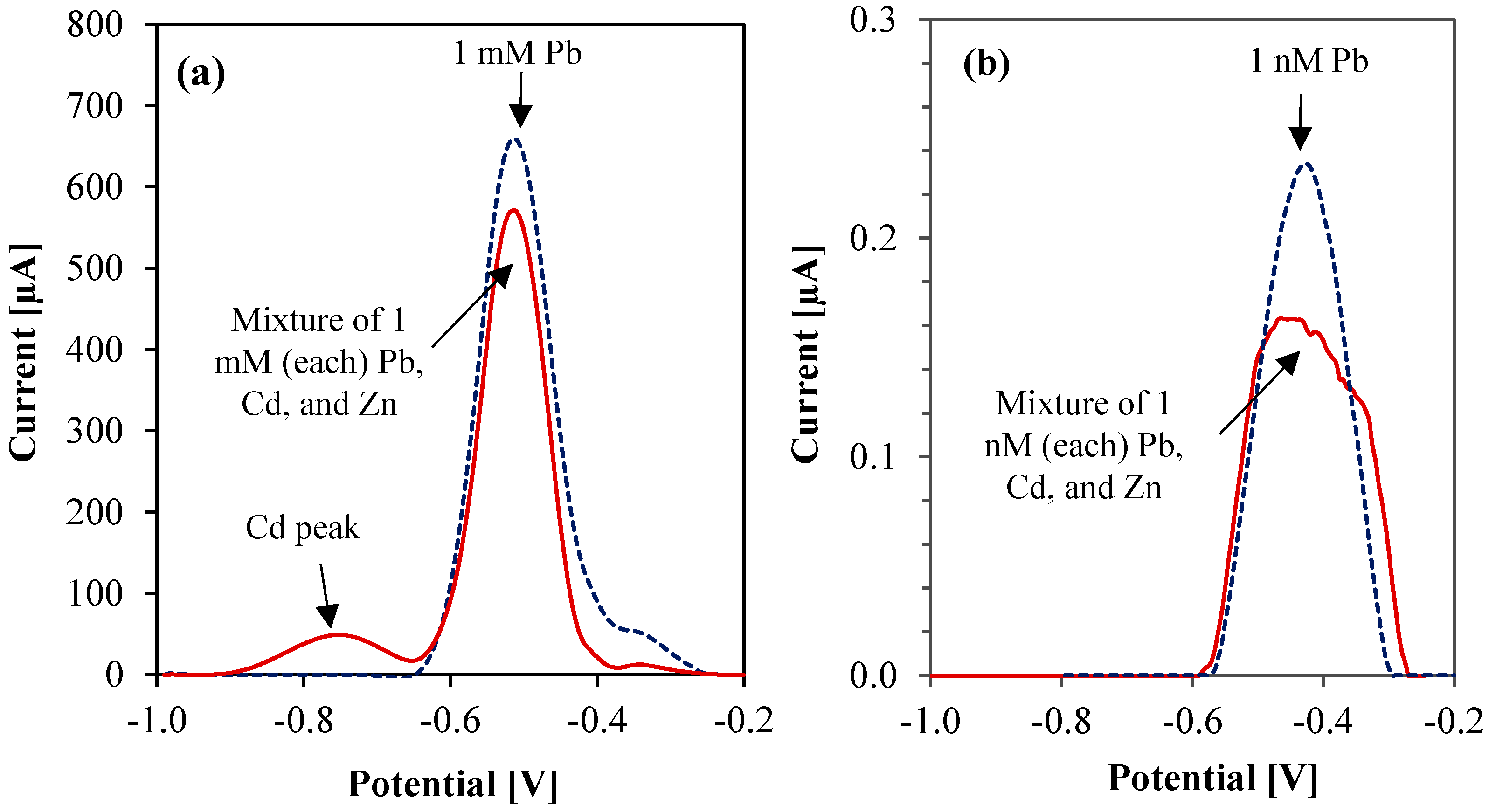
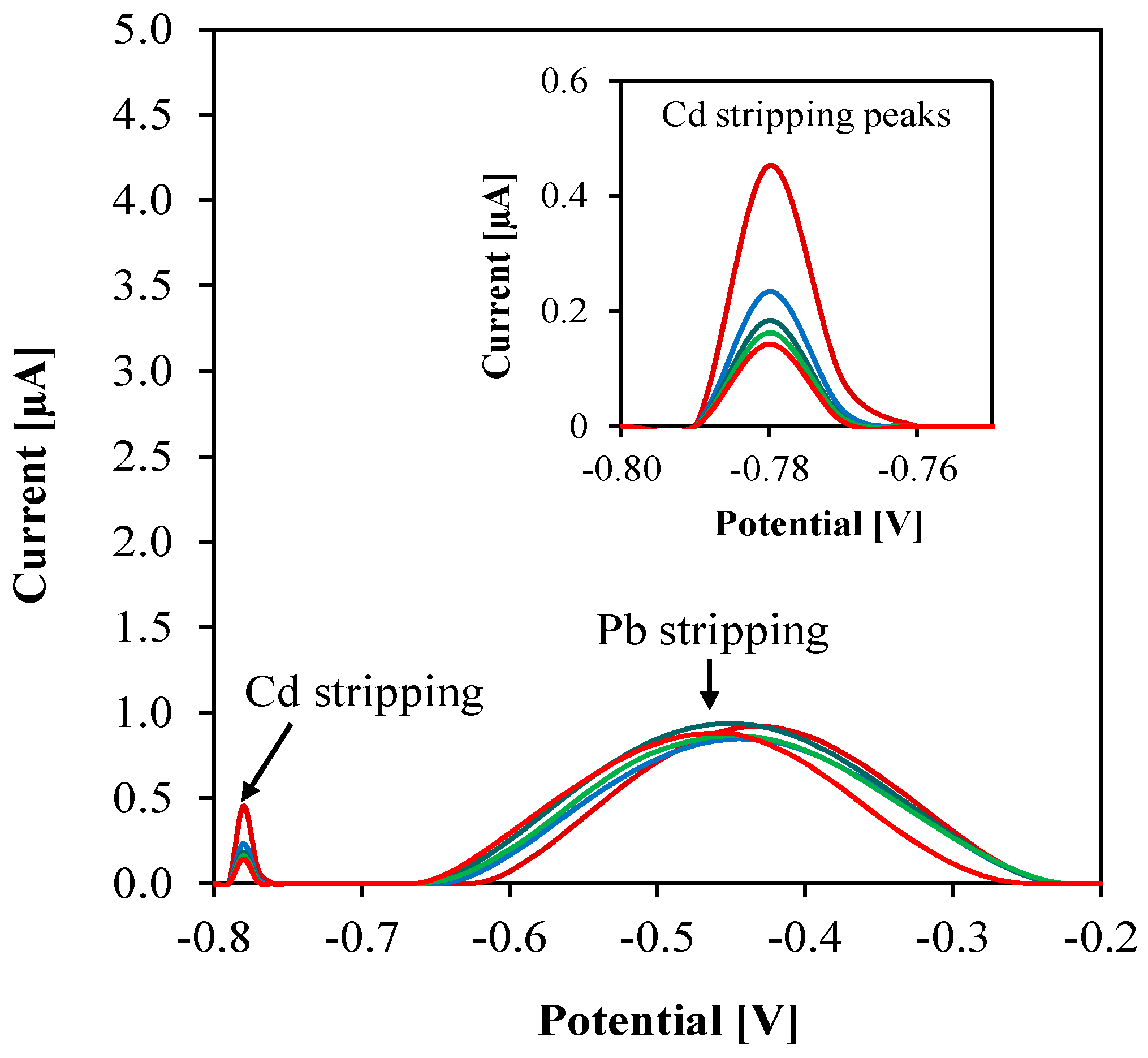
3.5. Interference Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xuan, X.; Hossain, M.; Park, J.Y. A fully integrated and miniaturized heavy-metal-detection sensor based on micro-patterned reduced graphene oxide. Sci. Rep. 2016, 6, 33125. [Google Scholar] [CrossRef] [PubMed]
- Redhwan, T.Z.; Alam, A.U.; Haddara, Y.M.; Howlader, M.M. Copper and liquid crystal polymer bonding towards lead sensing. Jpn. J. Appl. Phys. 2018, 57, 02BB03. [Google Scholar] [CrossRef]
- Canfield, R.L.; Henderson Jr, C.R.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Dietrich, K.; Auinger, P.; Cox, C. Cognitive deficits associated with blood lead concentrations< 10 microg/dL in US children and adolescents. Public Health Rep. 2000, 115, 521. [Google Scholar] [CrossRef]
- WHO. Childhood Lead Poisoning. Available online: https://www.who.int/publications/i/item/childhood-lead-poisoning (accessed on 21 March 2018).
- Goyer, R.A. Lead toxicity: Current concerns. Environ. Health Perspect. 1993, 100, 177–187. [Google Scholar] [CrossRef]
- Federal-Provincial-Territorial Committee on Drinking Water (CDW). Consulation on Lead in Drinking Water. Available online: https://www.canada.ca/en/health-canada/programs/consultation-lead-drinking-water.html (accessed on 21 March 2018).
- Canada, H. Guidelines for Canadian Drinking Water Quality—Summary Tables. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/guidelines-canadian-drinking-water-quality-summary-table.html (accessed on 19 January 2023).
- Conti, M.E. The content of heavy metals in food packaging paper boards: An atomic absorption spectroscopy investigation. Food Res. Int. 1997, 30, 343–348. [Google Scholar] [CrossRef]
- Aydin, F.A.; Soylak, M. Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium (IV), titanium (IV), iron (III), lead (II) and chromium (III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin. J. Hazard. Mater. 2010, 173, 669–674. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. TrAC Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Thiruppathi, A.R.; Sidhureddy, B.; Keeler, W.; Chen, A. Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 2017, 76, 42–46. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Zuo, Y.; Zhang, Y.; Wang, W. A new electrochemical sensor based on carboimidazole grafted reduced graphene oxide for simultaneous detection of Hg2+ and Pb2+. J. Electroanal. Chem. 2016, 782, 250–255. [Google Scholar] [CrossRef]
- Gunasingham, H.; Dalangin, R.R. Anodic stripping voltammetry of lead using a copper-mercury film electrode. Anal. Chim. Acta 1991, 246, 309–313. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Ogorevc, B. Bismuth-coated screen-printed electrodes for stripping voltammetric measurements of trace lead. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 13–16. [Google Scholar] [CrossRef]
- Lee, G.J.; Kim, C.K.; Lee, M.K.; Rhee, C.K. Simultaneous voltammetric determination of Zn, Cd and Pb at bismuth nanopowder electrodes with various particle size distributions. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 530–535. [Google Scholar] [CrossRef]
- Hwang, G.-H.; Han, W.-K.; Park, J.-S.; Kang, S.-G. An electrochemical sensor based on the reduction of screen-printed bismuth oxide for the determination of trace lead and cadmium. Sens. Actuators B Chem. 2008, 135, 309–316. [Google Scholar] [CrossRef]
- Ouyang, R.; Zhu, Z.; Tatum, C.E.; Chambers, J.Q.; Xue, Z.-L. Simultaneous stripping detection of Zn (II), Cd (II) and Pb (II) using a bimetallic Hg–Bi/single-walled carbon nanotubes composite electrode. J. Electroanal. Chem. 2011, 656, 78–84. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.; Hu, N.-X.; Deen, M.J. Electrochemical sensing of lead in drinking water using β-cyclodextrin-modified MWCNTs. Sensors Actuators B Chem. 2019, 296, 126632. [Google Scholar] [CrossRef]
- Toghill, K.E.; Xiao, L.; Wildgoose, G.G.; Compton, R.G. Electroanalytical determination of cadmium (II) and lead (II) using an antimony nanoparticle modified boron-doped diamond electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1113–1118. [Google Scholar] [CrossRef]
- Brand, M.; Eshkenazi, I.; Kirowa-Eisner, E. The silver electrode in square-wave anodic stripping voltammetry. Determination of Pb2+ without removal of oxygen. Anal. Chem. 1997, 69, 4660–4664. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Veerapandian, M.; Krishnan, U.M.; Rayappan, J.B.B. Simultaneous electrochemical detection of Cd (II), Pb (II), As (III) and Hg (II) ions using ruthenium (II)-textured graphene oxide nanocomposite. Talanta 2017, 162, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Sui, X.; Pu, H.; Bottum, K.J.; Jin, B.; Chang, J.; Zhou, G.; Lu, G.; Chen, J. Sensitive field-effect transistor sensors with atomically thin black phosphorus nanosheets. Nanoscale 2020, 12, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Li, Z.; Burns, M.A. A drinking water sensor for lead and other heavy metals. Anal. Chem. 2017, 89, 8748–8756. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, P.; Dai, H.; Chen, L.; Ma, H.; Lin, M.; Shen, D. An electrochemical sensor based on Co 3 O 4 nanosheets for lead ions determination. RSC Adv. 2017, 7, 39611–39616. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Voltammetric determination of lead (II) and cadmium (II) using a bismuth film electrode modified with mesoporous silica nanoparticles. Electrochim. Acta 2014, 132, 223–229. [Google Scholar] [CrossRef]
- Avuthu, S.G.R.; Narakathu, B.B.; Eshkeiti, A.; Emamian, S.; Bazuin, B.J.; Joyce, M.; Atashbar, M.Z. Detection of heavy metals using fully printed three electrode electrochemical sensor. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; pp. 669–672. [Google Scholar] [CrossRef]
- Kang, W.; Pei, X.; Rusinek, C.A.; Bange, A.; Haynes, E.N.; Heineman, W.R.; Papautsky, I. Determination of lead with a copper-based electrochemical sensor. Anal. Chem. 2017, 89, 3345–3352. [Google Scholar] [CrossRef]
- Howlader, M.M.; Alam, A.U.; Sharma, R.P.; Deen, M.J. Materials analyses and electrochemical impedance of implantable metal electrodes. PCCP 2015, 17, 10135–10145. [Google Scholar] [CrossRef]
- Wei, H.; Sun, J.-J.; Xie, Y.; Lin, C.-G.; Wang, Y.-M.; Yin, W.-H.; Chen, G.-N. Enhanced electrochemical performance at screen-printed carbon electrodes by a new pretreating procedure. Anal. Chim. Acta 2007, 588, 297–303. [Google Scholar] [CrossRef]
- Heidelbach, F.; Wenk, H.-R.; Chen, S.; Pospiech, J.; Wright, S. Orientation and misorientation characteristics of annealed, rolled and recrystallized copper. Mater. Sci. Eng. A 1996, 215, 39–49. [Google Scholar] [CrossRef]
- Merchant, H.; Liu, W.; Giannuzzi, L.; Morris, J. Grain structure of thin electrodeposited and rolled copper foils. Mater. Charact. 2004, 53, 335–360. [Google Scholar] [CrossRef]
- Brist, G.; Hall, S.; Clouser, S.; Liang, T. Non-classical conductor losses due to copper foil roughness and treatment. In Proceedings of the 2005 IPC Electronic Circuits World Convention, Anaheim, CA, USA, 22–24 February 2005. [Google Scholar]
- Coonrod, J. Different copper foils for different reasons. The PCB Magazine, April 2012; pp. 60–64. [Google Scholar]
- Howlader, M.; Doyle, T.; Mohtashami, S.; Kish, J. Charge transfer and stability of implantable electrodes on flexible substrate. Sens. Actuators B Chem. 2013, 178, 132–139. [Google Scholar] [CrossRef]
- Howlader, M.; Selvaganapathy, P.; Deen, M.J.; Suga, T. Nanobonding technology toward electronic, fluidic, and photonic systems integration. IEEE J. Sel. Top. Quantum Electron. 2010, 17, 689–703. [Google Scholar] [CrossRef]
- Redhwan, T.Z.; Alam, A.U.; Catalano, M.; Wang, L.; Kim, M.J.; Haddara, Y.M.; Howlader, M.M. Direct bonding of copper and liquid crystal polymer. Mater. Lett. 2018, 212, 214–217. [Google Scholar] [CrossRef]
- Howlader, M.; Suga, T.; Takahashi, A.; Saijo, K.; Ozawa, S.; Nanbu, K. Surface activated bonding of LCP/Cu for electronic packaging. J. Mater. Sci. 2005, 40, 3177–3184. [Google Scholar] [CrossRef]
- Howlader, M.; Iwashita, M.; Nanbu, K.; Saijo, K.; Suga, T. Enhanced Cu/LCP adhesion by pre-sputter cleaning prior to Cu deposition. IEEE Trans. Adv. Packag. 2005, 28, 495–502. [Google Scholar] [CrossRef]
- Li, X.; Wu, N.; Rojanasakul, Y.; Liu, Y. Selective stamp bonding of PDMS microfluidic devices to polymer substrates for biological applications. Sens. Actuators A Phys. 2013, 193, 186–192. [Google Scholar] [CrossRef]
- Howlader, M.M.; Kagami, G.; Lee, S.H.; Wang, J.G.; Kim, M.J.; Yamauchi, A. Sequential plasma-activated bonding mechanism of silicon/silicon wafers. J. Microelectromechanical Syst. 2010, 19, 840–848. [Google Scholar] [CrossRef]
- Kibria, M.; Zhang, F.; Lee, T.; Kim, M.; Howlader, M. Comprehensive investigation of sequential plasma activated Si/Si bonded interfaces for nano-integration on the wafer scale. Nanotechnology 2010, 21, 134011. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Howlader, M.M.; Deen, M.J. Direct bonding of liquid crystal polymer to glass. RSC Adv. 2016, 6, 107200–107207. [Google Scholar] [CrossRef]
- Moriceau, H.; Fournel, F.; Rayssac, O.; Cartier, A.; Morales, C.; Pocas, S.; Zussy, M.; Jalaguier, E.; Biasse, B.; Bataillou, B. Overview on some recent advances in wafer bonding technologies. In Semiconductor Wafer Bonding Science: Technology and Applications VI, Electrochemical Society Proceedings; Electrochemical Society: Pennington, NJ, USA, 2001; pp. 1–16. [Google Scholar]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated reference electrodes and their biosensing applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef]
- Harvey, D. Analytical Chemistry 2.0—an open-access digital textbook. Anal. Bioanal. Chem. 2011, 399, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Bui, M.-P.N.; Li, C.A.; Han, K.N.; Pham, X.-H.; Seong, G.H. Electrochemical determination of cadmium and lead on pristine single-walled carbon nanotube electrodes. Anal. Sci. 2012, 28, 699–704. [Google Scholar] [CrossRef]
- Laschi, S.; Palchetti, I.; Mascini, M. Gold-based screen-printed sensor for detection of trace lead. Sens. Actuators B Chem. 2006, 114, 460–465. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Zhai, Y.; Wang, E. High-sensitivity determination of lead and cadmium based on the Nafion-graphene composite film. Anal. Chim. Acta 2009, 649, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.; Meng, Y.; Zhang, P.; Su, Z.; Liu, Y.; Huang, Y.; Zhou, Y.; Xie, Q.; Yao, S. Sensitive square wave anodic stripping voltammetric determination of Cd2+ and Pb2+ ions at Bi/Nafion/overoxidized 2-mercaptoethanesulfonate-tethered polypyrrole/glassy carbon electrode. Sens. Actuators B Chem. 2014, 191, 94–101. [Google Scholar] [CrossRef]
- Hocevar, S.B.; Wang, J.; Deo, R.P.; Ogorevc, B. Potentiometric stripping analysis at bismuth-film electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2002, 14, 112–115. [Google Scholar] [CrossRef]
- Rajamani, A.; Ragula, U.B.R.; Kothurkar, N.; Rangarajan, M. Nano-and micro-hexagons of bismuth on polycrystalline copper: Electrodeposition and heavy metal sensing. CrystEngComm 2014, 16, 2032–2038. [Google Scholar] [CrossRef]
- Arantes, T.M.; Sardinha, A.; Baldan, M.R.; Cristovan, F.H.; Ferreira, N.G. Lead detection using micro/nanocrystalline boron-doped diamond by square-wave anodic stripping voltammetry. Talanta 2014, 128, 132–140. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zhang, Z.; Liu, G. Electrochemical determination of lead and cadmium in rice by a disposable bismuth/electrochemically reduced graphene/ionic liquid composite modified screen-printed electrode. Sens. Actuators B Chem. 2014, 199, 7–14. [Google Scholar] [CrossRef]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, V.; Bartoš, M.; Vytřas, K.; Kuhn, A. Porous bismuth film electrodes for signal increase in anodic stripping voltammetry. Electroanalysis 2010, 22, 1524–1530. [Google Scholar] [CrossRef]
- Nestorović, S.; Milićević, B.; Marković, D. Anneal hardening effect in sintered copper alloys. Sci. Sinter. 2002, 34, 169–174. [Google Scholar] [CrossRef]
- Pei, X.; Kang, W.; Yue, W.; Bange, A.; Heineman, W.R.; Papautsky, I. Disposable copper-based electrochemical sensor for anodic stripping voltammetry. Anal. Chem. 2014, 86, 4893–4900. [Google Scholar] [CrossRef] [PubMed]
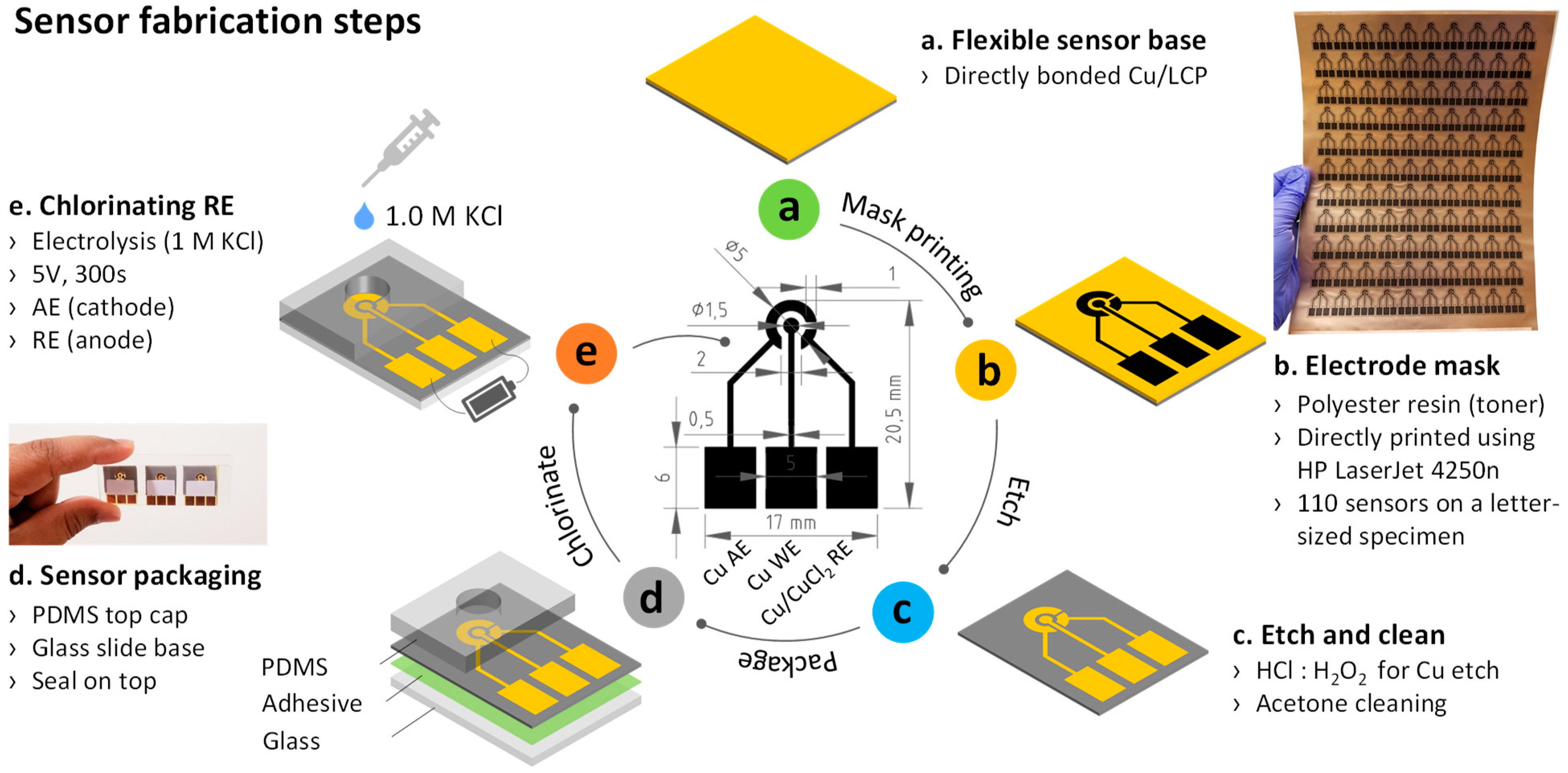

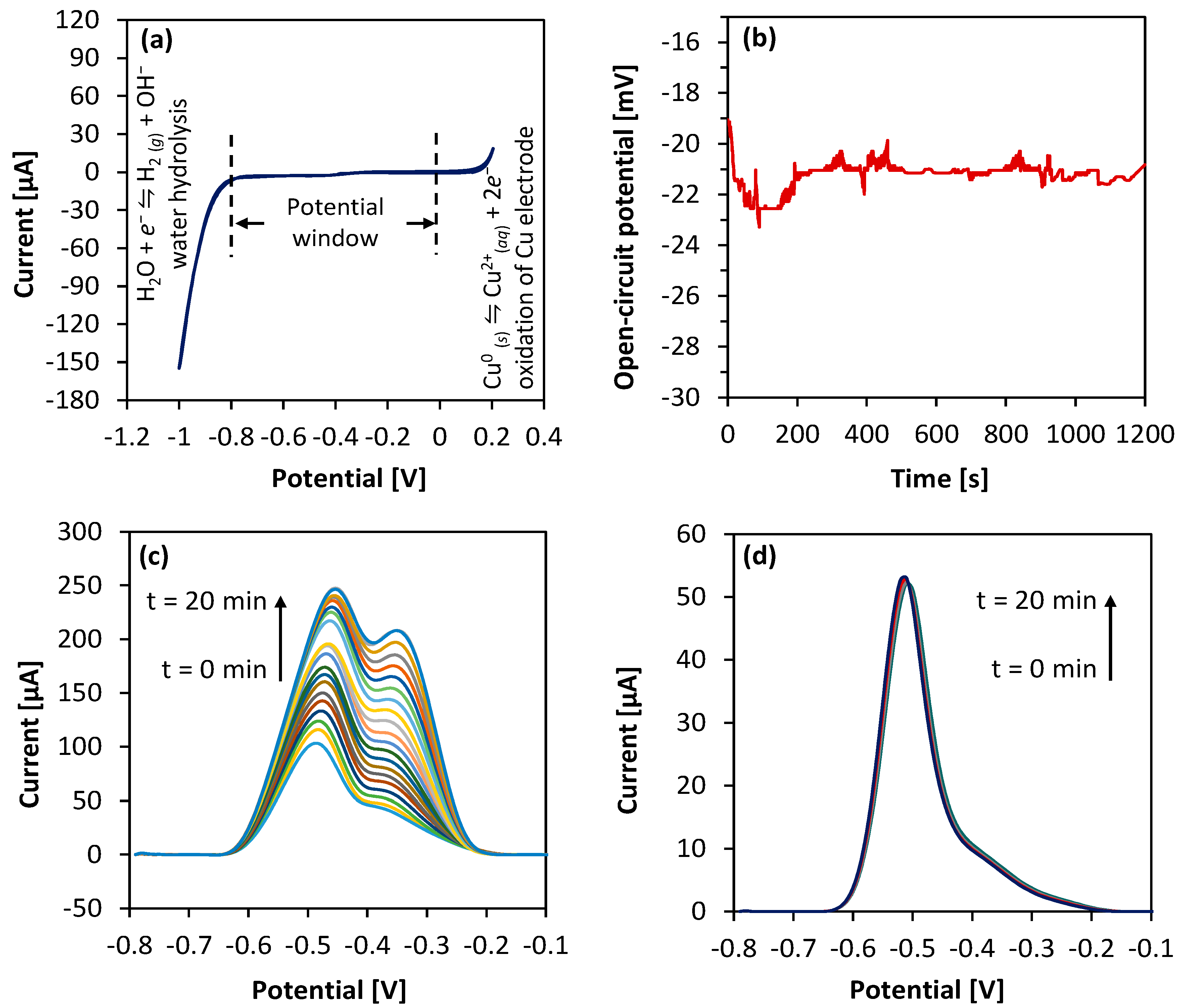

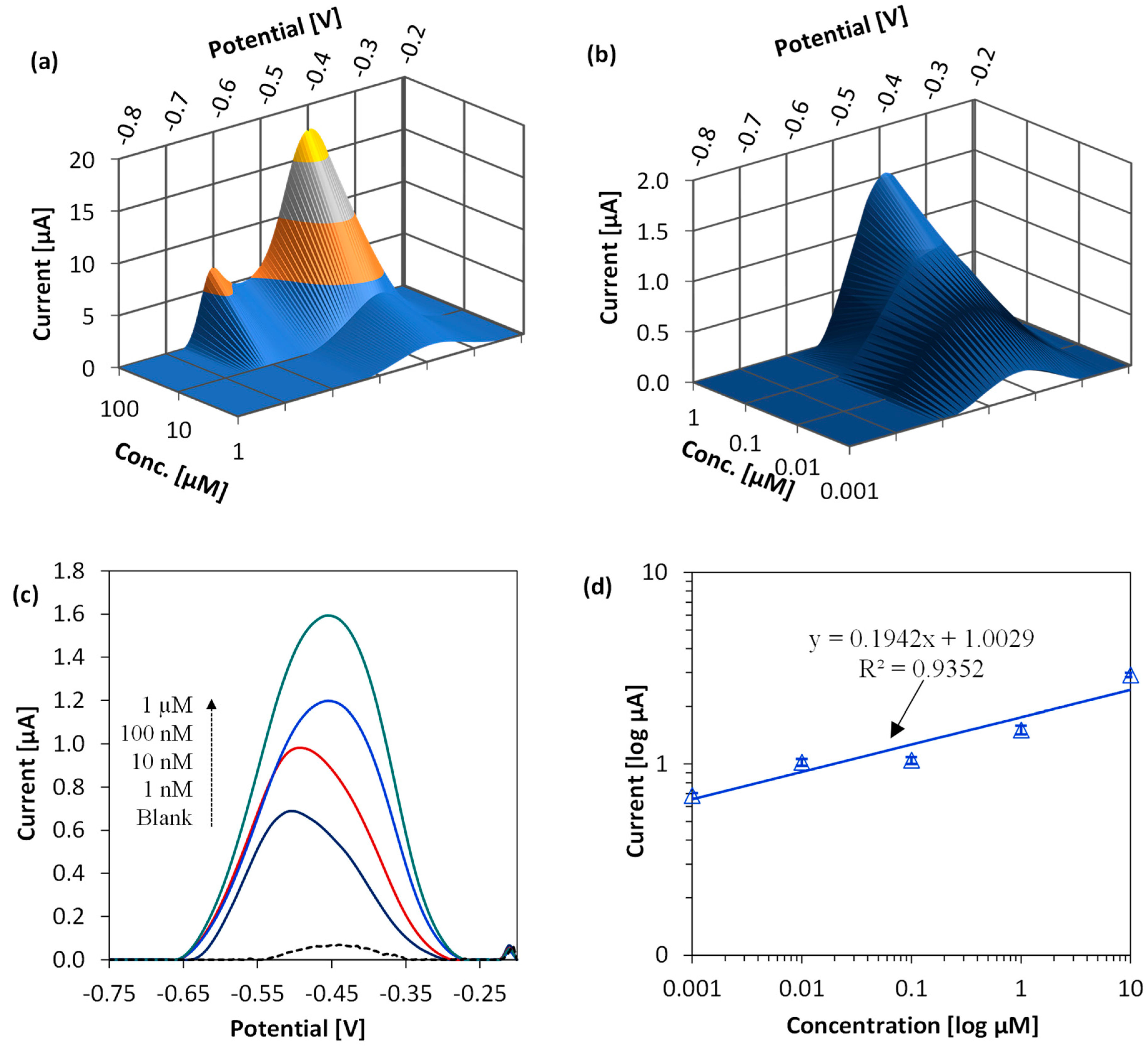
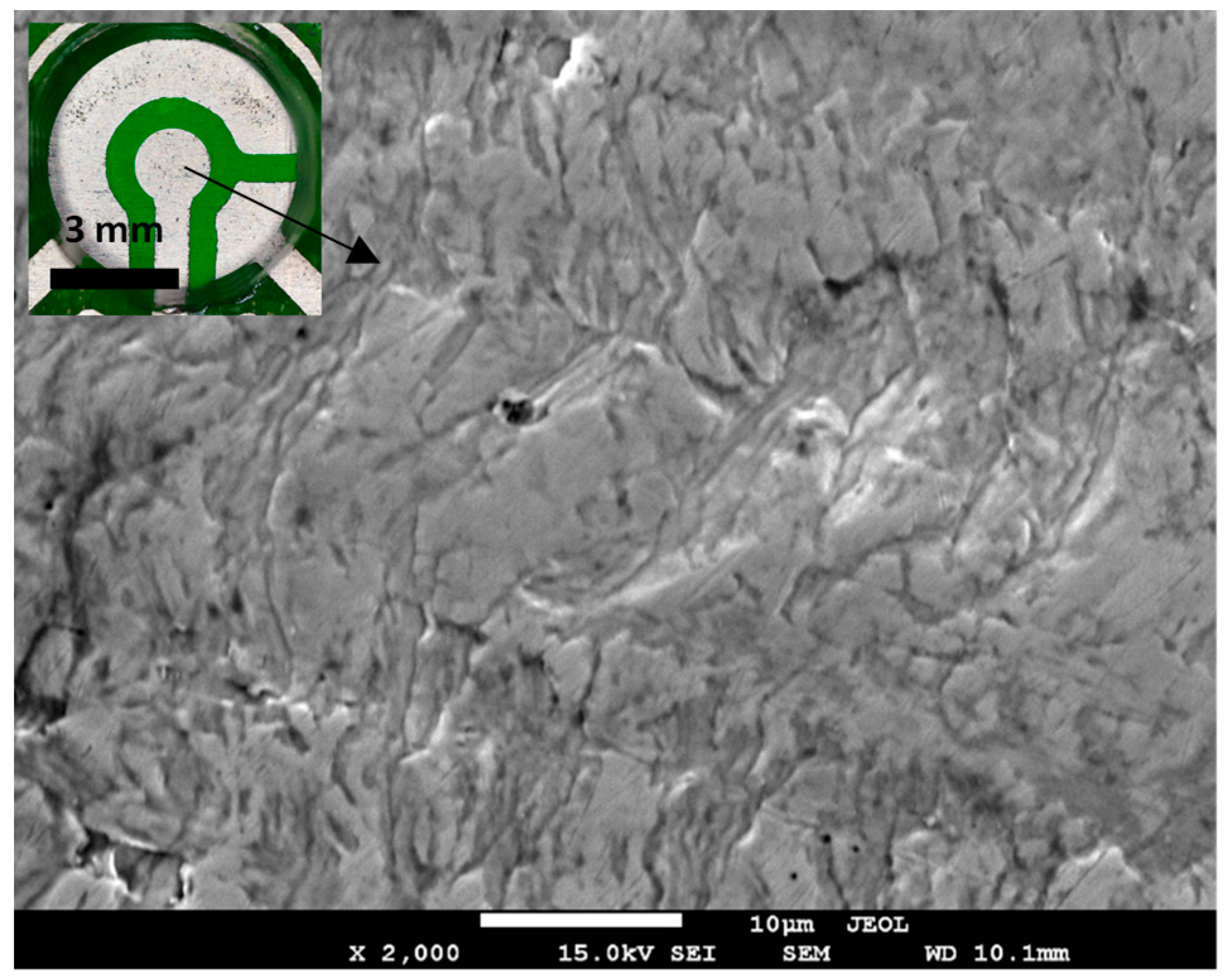
| Process Parameter | Value |
|---|---|
| RIE power (W) | 100 |
| RIE time (s) | 240 |
| O2 flow (sccm) | 98 |
| Vacuum Pressure (Pa) | 100 |
| Bonding temperature (°C) | 230 for 1 h |
| Bond-head pressure (MPa) | 0.3 |
| Process Parameter | Value |
|---|---|
| Deposition potential (V) | −0.6 |
| Deposition time (s) | 10 |
| Pulse frequency (Hz) | 98 |
| Amplitude (mV) | 100 |
| Increment (mV) | 10 |
| Electrode Material | Electrode Fabrication | LOD (µg/L) | Deposition Time (s) | Reference | ||
|---|---|---|---|---|---|---|
| WE | CE | RE | ||||
| Rolled-annealed Cu foil | Cu | Cu/CuCl2 | LaserJet printing of electrode mask on rolled-annealed Cu/polymer. Integrated metal foil-based microelectrodes bonded to a polymer substrate. RE fabricated by anodic chlorination of Cu using embedded electrodes. | 0.2 | 10 | This work. |
| MWCNTs with β-CD | Pt | Ag/AgCl | MWCNTs with β-CD drop casted on screen-printed carbon electrode. | 0.9 | 600 | Our previous work [21] |
| Bi-TRGO/Au | Au | Ag/AgCl | Photolithographic processing of fully-integrated microelectrodes on thermally-reduced graphene oxide on Si substrate followed by electrodeposition of Bismuth. | 0.4 | 150 | [1] |
| Cu thin-film | Cu | Cu/CuCl2 | Fully-integrated thin-film electrodes formed by e-beam evaporation of Cu on adhesive Ti layer, followed by photolithography and Ti/Cu etch. | 4.4 | 300 | [30] |
| Bi-C | Pt | Ag/AgCl | Screen printing & electrodeposition of bismuth- coated carbon electrode. | 0.3 | 120 | [17] |
| Bi-nanopowder/Nafion | Pt | SCE | Dispersion of gas-condensed bismuth nanopowder on carbon with Nafion. | 0.17–1.97 | 180 | [18] |
| Bi | Pt | SCE | Screen printing and electrochemical reduction of Bi2O3. | 2.3 | 300 | [19] |
| Hg-Bi/SWCNT on GCE | Pt | Ag/AgCl | Single-walled carbon nanotube functionalization of glassy-carbon electrode by liquid drop followed by ex-situ chemical deposition of Hg and Bi metals. | 1.2 × 10−3 | 300 | [20] |
| Sb-boron doped diamond | C | SCE | Electrochemical modification of antimony nanoparticles on boron-doped diamond electrode. | 18.5 | 120 | [22] |
| SWCNT | Pt | SCE | Vacuum filtering of single-walled carbon nanotube on an anodic membrane followed by photolithographic processing. | 0.8 | 150 | [50] |
| Au | C | Ag | Screen printing of Au and Ag. | 0.5 | 120 | [51] |
| Bi-Nafion-graphene on GCE | Pt | Ag/AgCl | Glassy-carbon electrode with composite paste made by dispersion of graphene in Nafion solution and in-situ plating of Bi film. | 0.5 | 120 | [52] |
| Bi/Nafion/poly-pyrrole on GCE | Pt | SCE | Pyrrole polymerization followed by thiolene overoxidation & Nafion coat on glassy-carbon electrode. | 0.05 | 300 | [53] |
| Bi/GCE | Pt | Ag/AgCl | In-situ deposition of bismuth on glassy-carbon electrode. | 0.8 | 120 | [54] |
| Bi nano-hexagons on Cu | Pt | Ag/AgCl | Hexagon-shaped bismuth nano- and micro-architectures electrodeposition onto polycrystalline Cu film. | 0.05 | 600 | [55] |
| Boron doped diamond | Pt | Ag/AgCl | Synthesis of diamond films in a hot filament chemical vapor deposition reactor. | 1 | 180 | [56] |
| Bi/graphene-ionic composite | Pt | Ag/AgCl | Electrochemical reduction of graphene oxide on an ionic liquid followed by in-situ bismuth deposition to form a composite paste for electrode. | 0.1 | 120 | [57] |
| Bi-CNT | Pt | SCE | In-situ bismuth plating on screen-printed carbon nanotube electrode. | 1.3 | 300 | [58] |
| Porous Bi | Pt | Ag/AgCl | Electrochemical deposition of bismuth into a polystyrene-based nano-spherical porous template. | 1.3 | 90 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redhwan, T.Z.; Ali, Y.; Howlader, M.M.R.; Haddara, Y.M. Electrochemical Sensing of Lead in Drinking Water Using Copper Foil Bonded with Polymer. Sensors 2023, 23, 1424. https://doi.org/10.3390/s23031424
Redhwan TZ, Ali Y, Howlader MMR, Haddara YM. Electrochemical Sensing of Lead in Drinking Water Using Copper Foil Bonded with Polymer. Sensors. 2023; 23(3):1424. https://doi.org/10.3390/s23031424
Chicago/Turabian StyleRedhwan, Taufique Z., Younus Ali, Matiar M. R. Howlader, and Yaser M. Haddara. 2023. "Electrochemical Sensing of Lead in Drinking Water Using Copper Foil Bonded with Polymer" Sensors 23, no. 3: 1424. https://doi.org/10.3390/s23031424
APA StyleRedhwan, T. Z., Ali, Y., Howlader, M. M. R., & Haddara, Y. M. (2023). Electrochemical Sensing of Lead in Drinking Water Using Copper Foil Bonded with Polymer. Sensors, 23(3), 1424. https://doi.org/10.3390/s23031424






