Failure Reason of PI Test Samples of Neural Implants
Abstract
1. Introduction
1.1. Historical Overview
1.2. Degradation of Neural Implants
1.3. This Work
2. Materials and Methods
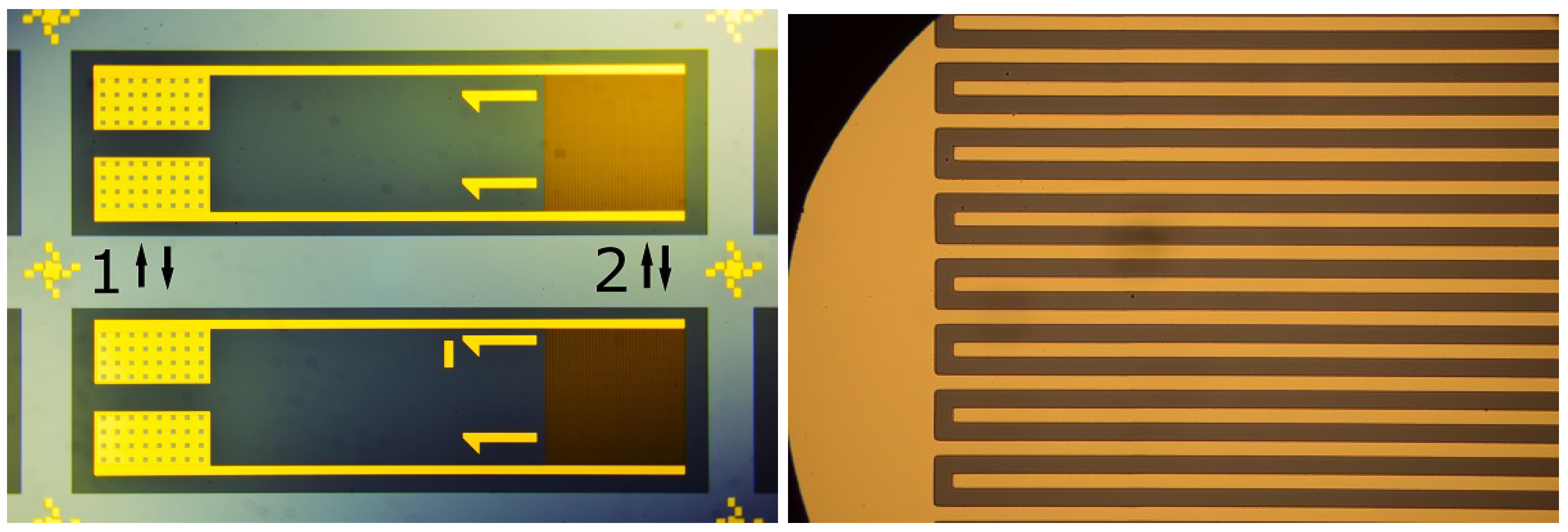
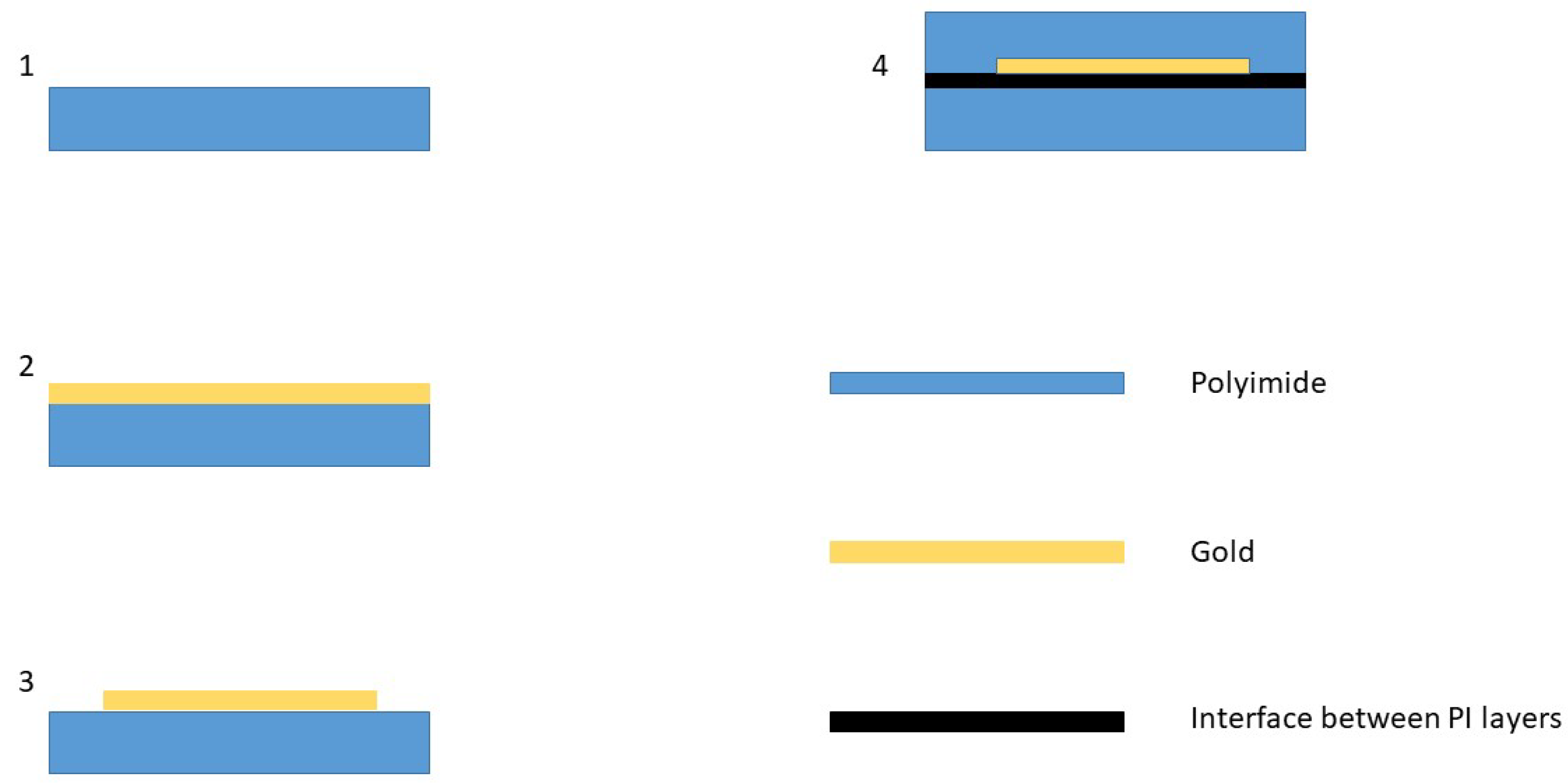
2.1. Sample Production
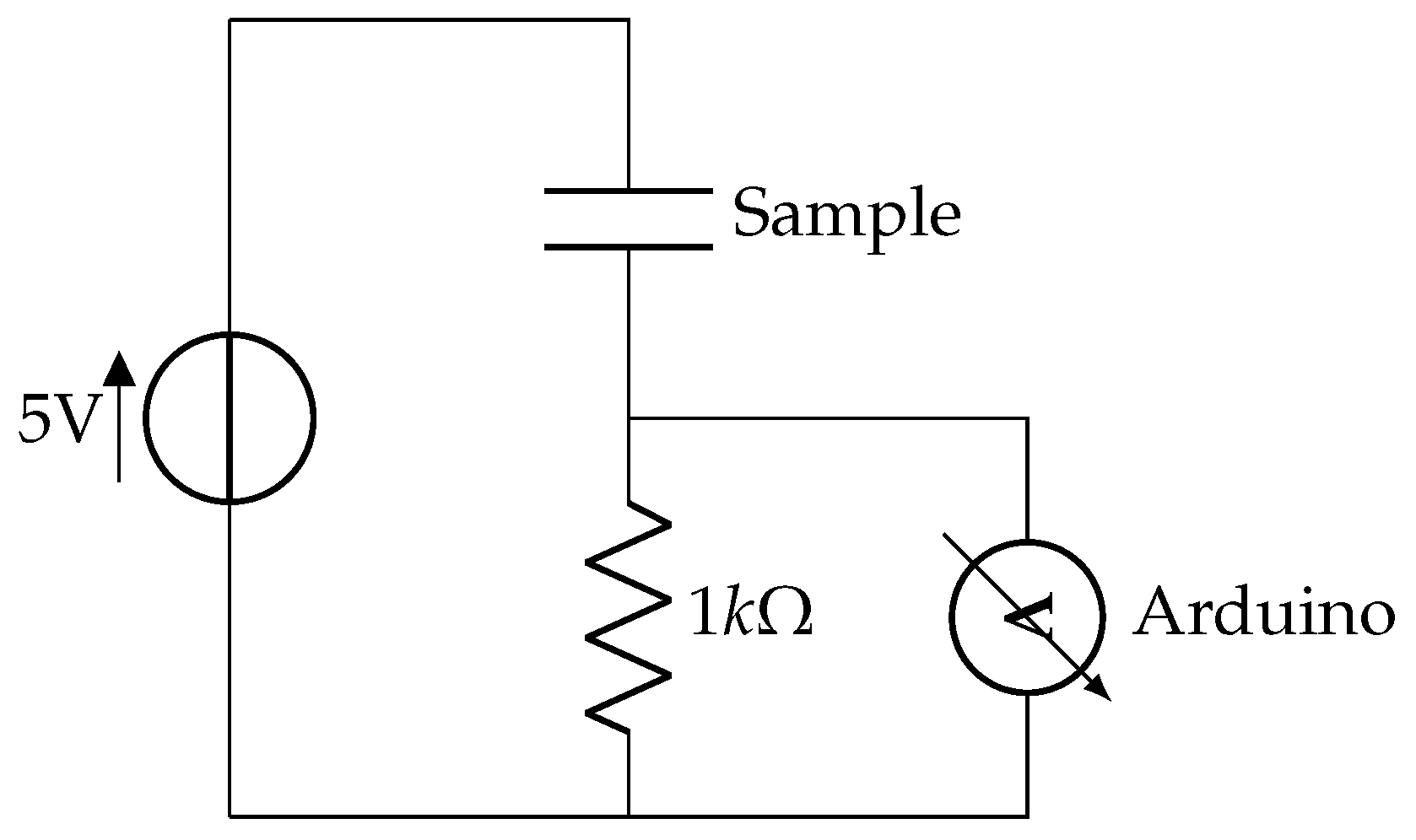
2.2. Sample Preparation and Experiment
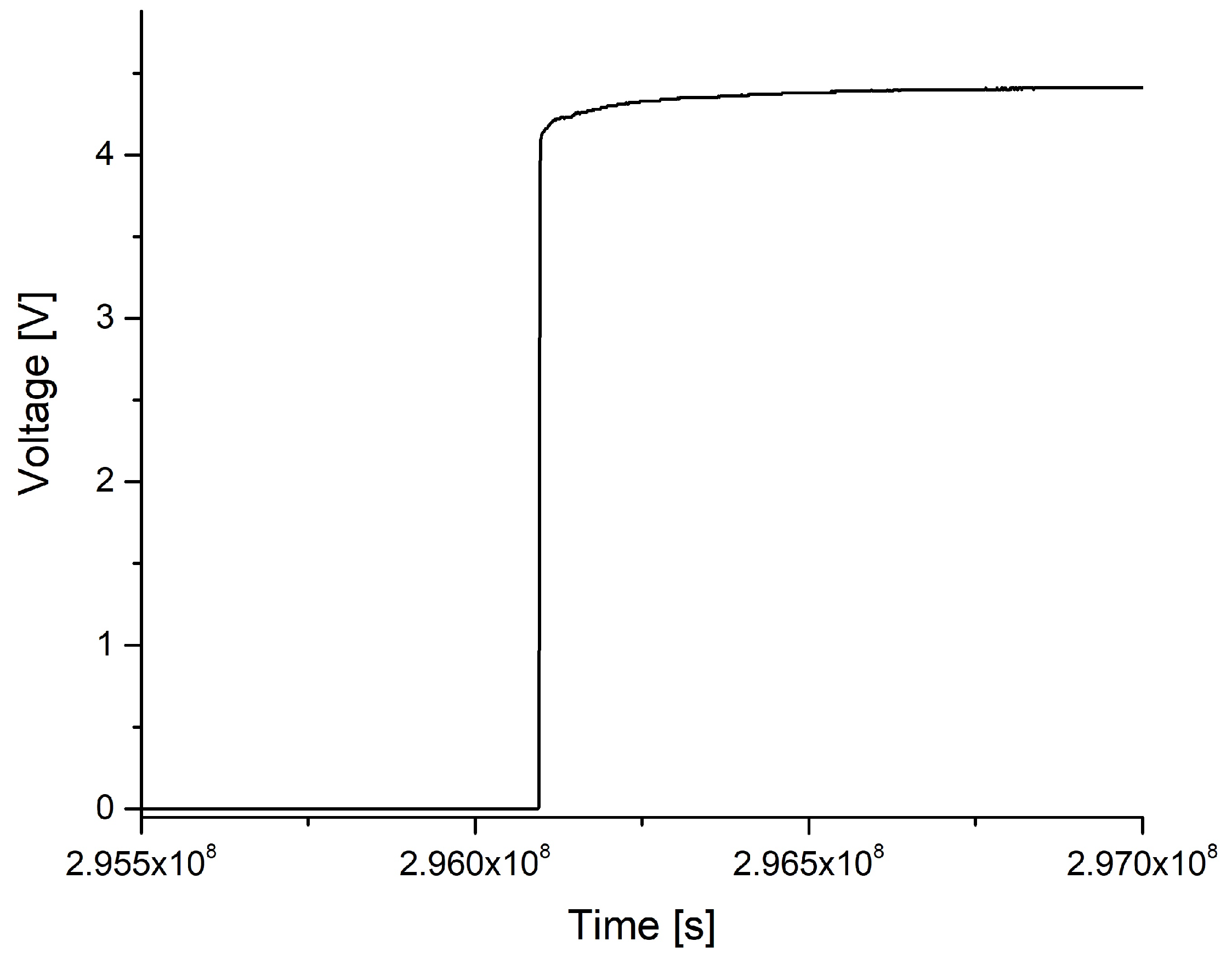
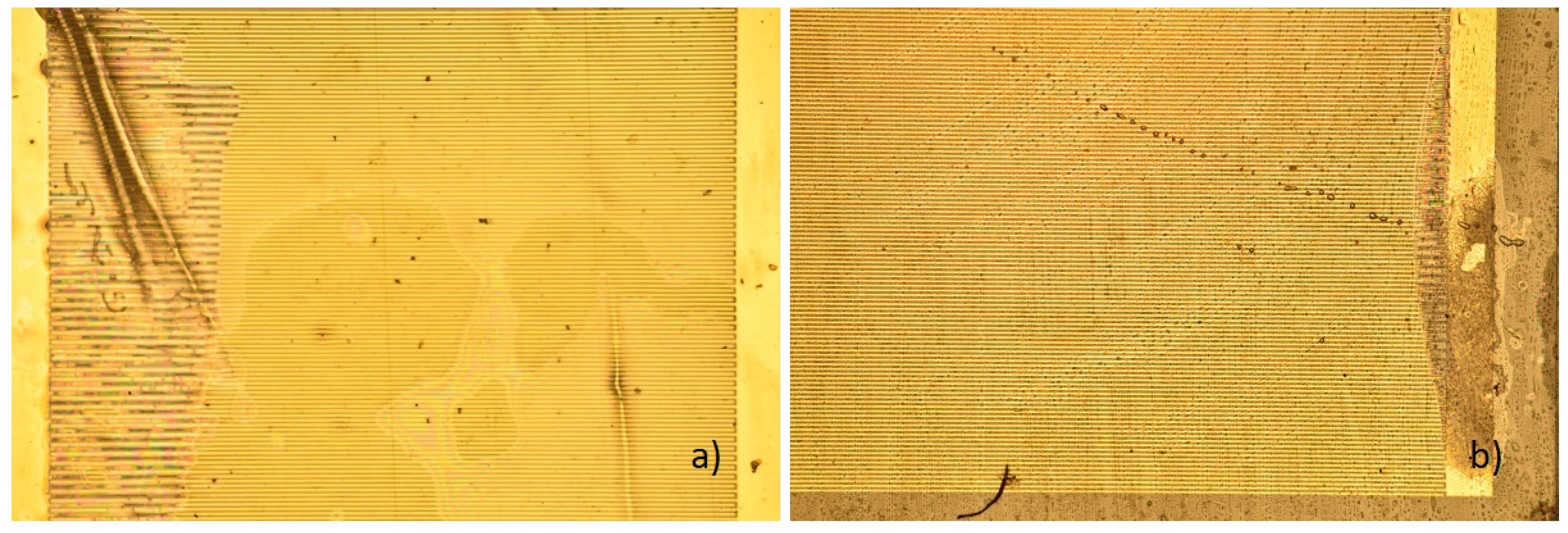
3. Results
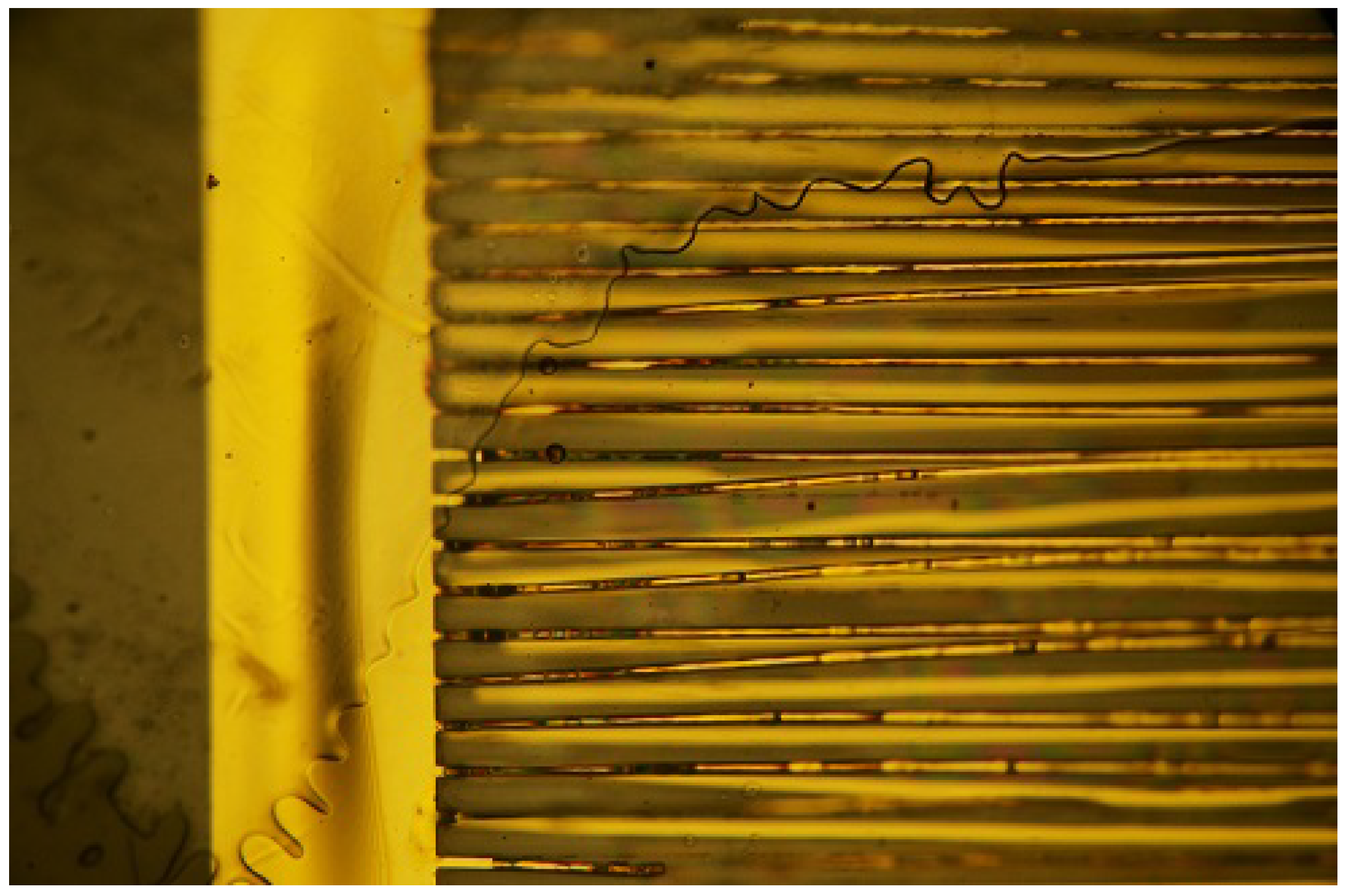
3.1. State of the Samples after the Procedure
3.2. Relation between Observed Damage and Occurring Voltage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EIS | Electrical Impedance Spectroscopy |
| ID | Interdigitated electrodes |
| PI | Polyimide |
| PPX-C | Parylene-C |
| SEM | Scanning Electron Microscope |
| SU8 | Polymer by MicroChemCorp., Newton, MA, USA |
References
- Tsang, W.M.; Je, M. Flexible electrode for implantable neural devices. In Neural Computation, Neural Devices, and Neural Prosthesis; Yang, Z., Ed.; Springer: New York, NY, USA, 2014; pp. 121–156. [Google Scholar]
- Schmidt, E.M.; Bak, M.J.; Hambrecht, F.T.; Kufta, C.V.; O’rourke, D.K.; Vallabhanath, P. Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain 1996, 119, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.D.; Weishaar, D.J. Tapered tungsten fine-wire microelectrode for chronic single unit recording. Brain Res. Bull. 1979, 4, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Davis Technologies. Available online: https://www.tdt.com (accessed on 26 July 2022).
- Wang, R.; Yu, H.; Li, Z. Microelectrode Array. In Micro Electro Mechanical Systems; Huang, Q.-A., Ed.; Springer: Singapore, 2018; pp. 1379–1411. [Google Scholar]
- Márton, G. Development and Characterization of Novel Microelectrode Arrays for Neurophysiology. Ph.D. Thesis, Semmelweis-University, Budapest, Hungary, 2016. [Google Scholar]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef]
- Schander, A.; Tolstosheeva, E.; Biefeld, V.; Kempen, L.; Stemmann, H.; Kreiter, A.; Lang, W. Design and fabrication of multi-contact flexible silicon probes for intracortical floating implantation. In Proceedings of the 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1739–1742. [Google Scholar]
- Normann, R.A.; Fernandez, E. Clinical applications of penetrating neural interfaces and Utah Electrode Array technologies. J. Neural Eng. 2016, 13, 061003. [Google Scholar] [CrossRef] [PubMed]
- Normann, R.A.; Maynard, E.M.; Rousche, P.J.; Warren, D.J. A neural interface for a cortical vision prosthesis. Vis. Res. 1999, 39, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Byers, C.E.; Loeb, C.E.; Merzenich, M.M.; Rebscher, S.J. Method for Making an Intracochlear Electrode Array. U.S. Patent 4,686,765. Available online: https://patentcenter.uspto.gov/applications/06855084 (accessed on 21 November 2022).
- Byers, C.E.; Loeb, C.E.; Merzenich, M.M.; Rebscher, S.J. Intracochlear Electrode Array. U.S. Patent 4,819,647. Available online: https://patents.google.com/patent/US4819647A/en (accessed on 21 November 2022).
- Hetke, J.F.; Najafi, K.; Wise, K. Flexible silicon interconnects for microelectromechanical systems. In Proceedings of the TRANSDUCERS’91: 1991 International Conference on Solid-State Sensors and Actuators. Digest of Technical Papers, San Francisco, CA, USA, 24–27 June 1991; pp. 764–767. [Google Scholar]
- Hetke, J.F.; Williams, J.C.; Pellinen, D.S.; Vetter, R.J.; Kipke, D.R. 3-D silicon probe array with hybrid polymer interconnect for chronic cortical recording. In Proceedings of the First International IEEE EMBS Conference on Neural Engineering, Capri, Italy, 20–22 March 2003; pp. 181–184. [Google Scholar]
- Hoffer, J.A.; Loeb, G.E.; Pratt, C.A. Single unit conduction velocities from averaged nerve cuff electrode records in freely moving cats. J. Neurosci. Methods 1981, 4, 211–225. [Google Scholar] [CrossRef]
- Loeb, G.E.; Peck, R.A. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J. Neurosci. Methods 1996, 64, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rodri, F.J.; Ceballos, D.; Schu, M.; Valero, A.; Valderrama, E.; Stieglitz, T.; Navarro, X. Polyimide cuff electrodes for peripheral nerve stimulation. J. Neurosci. Methods 2000, 98, 105–118. [Google Scholar]
- Bosse, B.; Damle, S.; Akinin, A.; Jing, Y.; Bartsch, D.-U.; Cheng, L.; Oesch, N.; Lo, Y.-H.; Cauwenberghs, G.; Freeman, W.R. In vivo photovoltaic performance of a silicon nanowire photodiode–based retinal prosthesis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5885–5892. [Google Scholar] [CrossRef]
- Luo, Y.H.-L.; Da Cruz, L. The Argus ® II retinal prosthesis system. Prog. Retin. Eye Res. 2016, 50, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Pellinen, D.; Pivin, D.; Rousche, P.; Kipke, D. Development of the thin-film longitudinal intra-fascicular electrode. In Proceedings of the 5th Annual Conference of the International Functional Electrical Stimulation Society, Aalborg, Denmark, 18–24 June 2000; pp. 279–281. [Google Scholar]
- Kipke, D.R.; Pellinen, D.S.; Vetter, R.J. Advanced neural implants using thin-film polymers. In Proceedings of the 2002 IEEE International Symposium on Circuits and Systems. Proceedings (Cat. No. 02CH37353), Phoenix-Scottsdale, AZ, USA, 26–29 May 2002; Volume 4, p. IV. [Google Scholar]
- Nikles, S.A.; Pellinen, D.S.; Kitagawa, J.; Bradley, R.M.; Kipke, D.R.; Najafi, K. Long term in vitro monitoring of polyimide microprobe electrical properties. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003; Volume 4, pp. 3340–3343. [Google Scholar]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, T.; Schuetter, M.; Koch, K.P. Implantable biomedical microsystems for neural prostheses. IEEE Eng. Med. Biol. Mag. 2005, 24, 58–65. [Google Scholar] [CrossRef]
- NeuroOne Technology. Available online: https://n1mtc.com/technology.html (accessed on 14 November 2022).
- Cortec News im Februar 2015. Available online: https://www.cortec-neuro.com/cortec-news-im-februar-2015/ (accessed on 11 November 2022).
- Fekete, Z.; Pongrácz, A. Multifunctional soft implants to monitor and control neural activity in the central and peripheral nervous system: A review. Sens. Actuators Chem. 2017, 243, 1214–1223. [Google Scholar] [CrossRef]
- Kozai, T.D.; Catt, K.; Li, X.; Gugel, Z.V.; Olafsson, V.T.; Vazquez, A.L.; Cui, X.T. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 2015, 37, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Afanasenkau, D.; Kalinina, D.; Lyakhovetskii, V.; Tondera, C.; Gorsky, O.; Moosavi, S.; Pavlova, N.; Merkulyeva, N.; Kalueff, A.V.; Minev, I.R.; et al. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 2020, 4, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.C.; Goncalves, S.B.; Pinho, F.; Silva, A.F.; Dias, N.S.; Correia, J.H. Flexible three-dimensional microelectrode array for neural applications. Sens. Actuators Phys. 2014, 217, 21–28. [Google Scholar] [CrossRef]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef]
- Hong, G.; Yang, X.; Zhou, T.; Lieber, C.M. Mesh electronics: A new paradigm for tissue-like brain probes. Curr. Opin. Neurobiol. 2018, 50, 33–41. [Google Scholar] [CrossRef]
- Altuna, A.; de la Prida, L.M.; Bellistri, E.; Gabriel, G.; Guimerá, A.; Berganzo, J.; Villa, R.; Fernández, L.J. SU-8 based microprobes with integrated planar electrodes for enhanced neural depth recording. Biosens. Bioelectron. 2012, 37, 1–5. [Google Scholar] [CrossRef]
- Kim, E.G.; John, J.K.; Tu, H.; Zheng, Q.; Loeb, J.; Zhang, J.; Xu, Y. A hybrid silicon–parylene neural probe with locally flexible regions. Sens. Actuators Chem. 2014, 195, 416–422. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.; Jeong, J.; Gwon, T.M.; Lee, S.; Kim, S.J. Novel four-sided neural probe fabricated by a thermal lamination process of polymer films. J. Neurosci. Methods 2017, 278, 25–35. [Google Scholar] [CrossRef]
- Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]
- Ejserholm, F. Development of a Polymer Based Neural Probe. Ph.D. Thesis, University Lund, Lund, Sweden, 18 March 2016. [Google Scholar]
- Torres-Martinez, N.; Ratel, D.; Cretallaz, C.; Gaude, C.; Maubert, S.; Divoux, J.-L.; Henry, C.; Guiraud, D.; Sauter-Starace, F. Reliability of parylene-based multi-electrode arrays chronically implanted in adult rat brains, and evidence of electrical stimulation on contact impedance. J. Neural Eng. 2019, 16, 066047. [Google Scholar] [CrossRef] [PubMed]
- Hukins, D.W.L.; Mahomed, A.; Kukureka, S.N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.; Street, M.G.; Sharma, R.; Takmakov, P.; Baker, B.; Rieth, L. Characterization of Parylene-C degradation mechanisms: In vitro reactive accelerated aging model compared to multiyear in vivo implantation. Biomaterials 2020, 232, 119731. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Xue, Q.-S.; Dieme, R.; Sankar, V.; Mayrand, R.C.; Nishida, T.; Streit, W.J.; Sanchez, J.C. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Rieth, L.; Williams, L.; Negi, S.; Bhandari, R.; Caldwell, R.; Sharma, R.; Tathireddy, P.; Solzbacher, F. Long-term reliability of Al2O3 and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J. Neural Eng. 2014, 11, 026016. [Google Scholar] [CrossRef] [PubMed]
- Rubehn, B.; Stieglitz, T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials 2010, 31, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.; Fellows, M.R.; Vargas-Irwin, C.; Nakata, G.K.; Donoghue, J.P. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Tintelott, M.; Schander, A.; Lang, W. Understanding Electrical Failure of Polyimide-Based Flexible Neural Implants: The Role of Thin Film Adhesion. Polymers 2022, 14, 3702. [Google Scholar] [CrossRef] [PubMed]
- Prodanov, D.; Delbeke, J. Mechanical and biological interactions of implants with the brain and their impact on implant design. Front. Neurosci. 2016, 10, 11. [Google Scholar] [CrossRef]
- Loeb, G.E.; Walker, A.E.; Uematsu, S.; Konigsmark, B.W. Histological reaction to various conductive and dielectric films chronically implanted in the subdural space. J. Biomed. Mater. Res. 1977, 11, 195–210. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Ultraflexible neural electrodes for long-lasting intracortical recording. IScience 2020, 23, 101387. [Google Scholar] [CrossRef]
- Karumbaiah, L.; Saxena, T.; Carlson, D.; Patil, K.; Patkar, R.; Gaupp, E.A.; Betancur, M.; Stanley, G.B.; Carin, L.; Bellamkonda, R.V. Relationship between intracortical electrode design and chronic recording function. Biomaterials 2013, 34, 8061–8074. [Google Scholar] [CrossRef] [PubMed]
- Stiller, A.M.; Black, B.J.; Kung, C.; Ashok, A.; Cogan, S.F.; Varner, V.D.; Pancrazio, J.J. A meta-analysis of intracortical device stiffness and its correlation with histological outcomes. Micromachines 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Zeniieh, D.; Bajwa, A.; Ledernez, L.; Urban, G. Effect of Plasma Treatments and Plasma-P olymerized Films on the Adhesion of Parylene-C to Substrates. Plasma Process. Polym. 2013, 10, 1081–1089. [Google Scholar] [CrossRef]
- Forssell, M. Long-Term Insulation of Active Electronics Embedded in Compliant Neural Probes. Ph.D. Thesis, Carnegie Mellon University, Pittsburgh, PA, USA, May 2019. [Google Scholar]
- Schander, A.; Gancz, J.M.; Tintelott, M.; Lang, W. Towards long-term stable polyimide-based flexible electrical insulation for chronically implanted neural electrodes. Micromachines 2021, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Takmakov, P.; Ruda, K.; Phillips, K.S.; Isayeva, I.S.; Krauthamer, V.; Welle, C.G. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species. J. Neural Eng. 2015, 12, 026003. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guljakow, J.; Lang, W. Failure Reason of PI Test Samples of Neural Implants. Sensors 2023, 23, 1340. https://doi.org/10.3390/s23031340
Guljakow J, Lang W. Failure Reason of PI Test Samples of Neural Implants. Sensors. 2023; 23(3):1340. https://doi.org/10.3390/s23031340
Chicago/Turabian StyleGuljakow, Jürgen, and Walter Lang. 2023. "Failure Reason of PI Test Samples of Neural Implants" Sensors 23, no. 3: 1340. https://doi.org/10.3390/s23031340
APA StyleGuljakow, J., & Lang, W. (2023). Failure Reason of PI Test Samples of Neural Implants. Sensors, 23(3), 1340. https://doi.org/10.3390/s23031340




