Advancements in Home-Based Devices for Detecting Obstructive Sleep Apnea: A Comprehensive Study
Abstract
:1. Introduction
2. Methodology
- -
- In the last ten years, what commercial devices have had the most significant impact on detecting OSA in the market?
- -
- In the last ten years, what methods have been used to detect OSA using a low quantity of signals?
- -
- In the last ten years, which devices have been developed for signal monitoring to diagnose OSA?
- -
- Based on the gathered information, what is the most effective signal and method for diagnosing OSA?
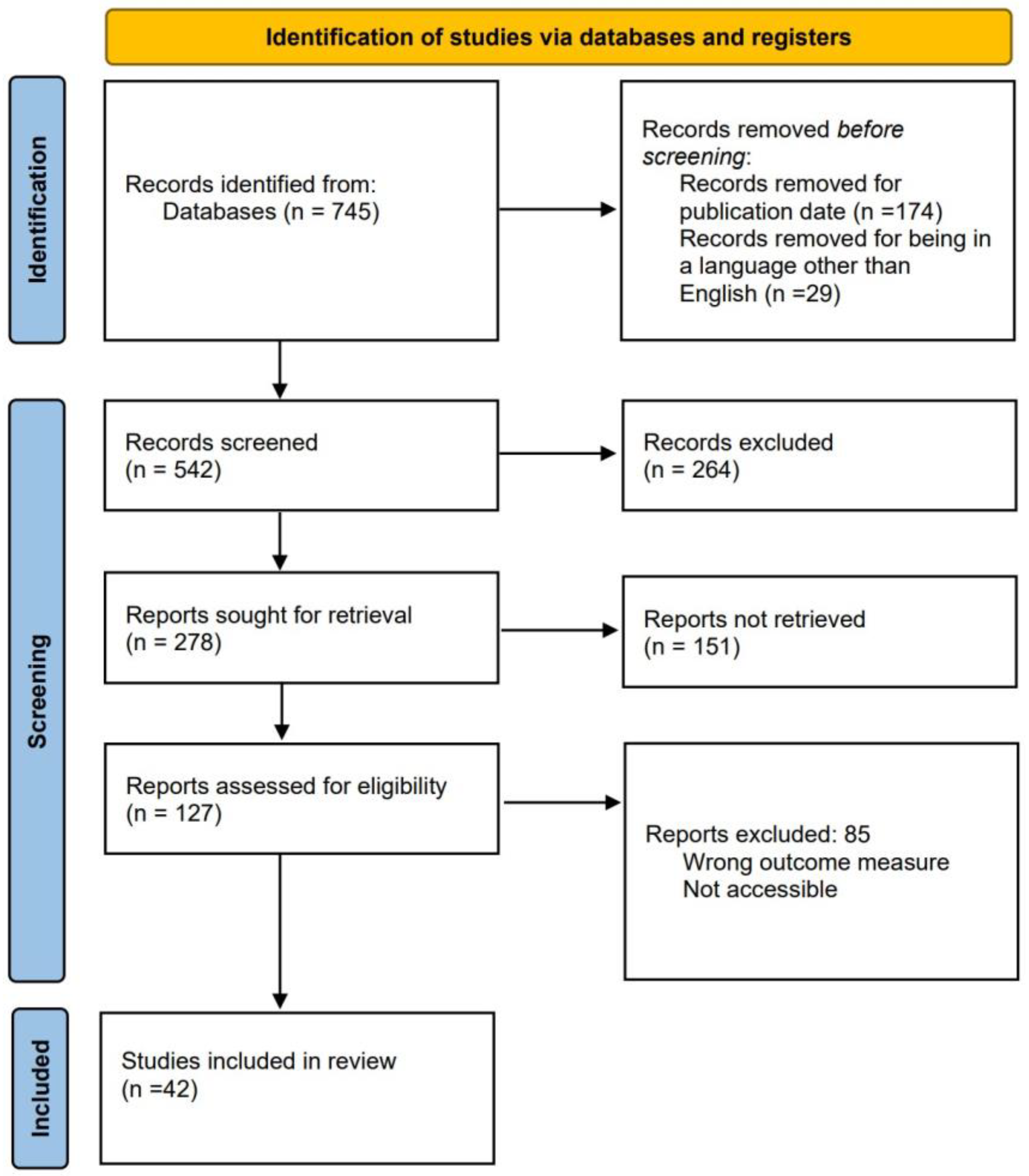
Literature Review Process
3. Modules and Technology Available for OSA Detection
3.1. Commercial Devices for OSA Diagnosis
3.2. Hardware and Software Systems for OSA Diagnosis Available in Scientific Research
3.3. Applicable Regulations for Signal Monitoring Modules
3.4. Applicable Regulations for Medical Materials
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Apnea Hypopnea Index (AHI). Available online: https://www.webmd.com/sleep-disorders/sleep-apnea/sleep-apnea-ahi-numbers (accessed on 7 September 2022).
- Ronquidos y Apnea, Trastornos del Sueño Más Comunes en México. 2020. Available online: https://www.dgcs.unam.mx/boletin/bdboletin/2020_226.html (accessed on 30 September 2023).
- de Salud, S. En México, Cuatro por Ciento de Hombres y dos por Ciento de Mujeres Sufren Apnea del Sueño.gob.mx. 2016. Available online: http://www.gob.mx/salud/articulos/en-mexico-cuatro-por-ciento-de-hombres-y-dos-por-ciento-de-mujeres-sufren-apnea-del-sueno (accessed on 30 September 2023).
- Rundo, J.V.; Downey, R. Polysomnography. Handb. Clin. Neurol. 2019, 160, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Learn about Polysomnography. Available online: https://www.chegg.com/learn/medicine-and-health/medical-terminology/polysomnography (accessed on 8 September 2022).
- Kapoor, M.; Greenough, G. Home sleep tests for obstructive sleep apnea (OSA). J. Am. Board Fam. Med. 2015, 28, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Zúñiga, S.; Gaona-Pineda, E.B.; Cuevas-Nasu, L.; Torre-Bouscoulet, L.; Reyes-Zúñiga, M.; Shamah-Levy, T.; Perez-Padilla, R. Prevalencia de síntomas de sueño y riesgo de apnea obstructiva del sueño en México. In Salud Pública de México; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2018; Volume 60, pp. 347–355. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Obstructive Sleep Apnea and Home Sleep Monitoring: Overview of Obstructive Sleep Apnea, Efficacy of Home Sleep Tests, Advantages of HSTsPublication: Medscape–eMedicine. Available online: https://emedicine.medscape.com/article/1518830-overviewa7 (accessed on 12 September 2022).
- Gao, X.; Li, Y.; Xu, W.; Han, D. Diagnostic accuracy of level IV portable sleep monitors versus polysomnography for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. 2021, 87, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Chesson, A.L., Jr.; Berry, R.B.; Pack, A.; American Academy of Sleep Medicine; American Thoracic Society; American College of Chest Physicians. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep 2003, 26, 907–913. [Google Scholar] [CrossRef]
- Mendonça, F.; Mostafa, S.S.; Ravelo-García, A.G.; Morgado-Dias, F.; Penzel, T. Devices for home detection of obstructive sleep apnea: A review. Sleep Med. Rev. 2018, 41, 149–160. [Google Scholar] [CrossRef]
- Philips-Alice PDx. Available online: https://www.philips.co.in/healthcare/product/HC1043844/alice-pdx-portable-sleep-diagnostic-system (accessed on 19 September 2022).
- Nilius, G.; Domanski, U.; Schroeder, M.; Franke, K.-J.; Hogrebe, A.; Margarit, L.; Stoica, M.; d’Ortho, M.-P. A randomized controlled trial to validate the Alice PDX ambulatory device. Nat. Sci. Sleep 2017, 9, 171–180. [Google Scholar] [CrossRef]
- Tedjasukmana, R.; Purba, J.S.; Wanandi, S.I.; Suyatna, F.D. Neuroglobin correlates with cryptochrome-1 in obstructive sleep apnea with primary aldosteronism. PLoS ONE 2018, 13, e0204390. [Google Scholar] [CrossRef]
- ResMed. ApneaLink Air. Available online: https://www.resmed.lat/healthcare-professional/products/diagnostics/apnealink-air (accessed on 19 September 2022).
- Stehling, F.; Keull, J.; Olivier, M.; Große-Onnebrink, J.; Mellies, U.; Stuck, B.A. Validation of the screening tool ApneaLink® in comparison to polysomnography for the diagnosis of sleep-disordered breathing in children and adolescents. Sleep Med. 2017, 37, 13–18. [Google Scholar] [CrossRef]
- Muñoz-Pindado, C.; Muñoz-Herrera, E.; Arribas-Peña, V.; Roura-Poch, P.; Ruiz-Mori, F.; Sánchez-Belmonte, S.; Mateu-Carralero, B.; Callís-Privat, M.; Darnés-Surroca, A.; Casademunt-Codina, I.; et al. Implementación del método simplificado Apnealink™Air® por médicos de atención primaria para el diagnóstico del síndrome de apnea hipopnea durante el sueño. Med. Fam. SEMERGEN 2022, 48, 3–13. [Google Scholar] [CrossRef]
- WatchPAT Home Sleep Test (HST) Sleep Apnea Device|Itamar Medical Ltd. Available online: https://www.itamar-medical.com/ (accessed on 24 September 2022).
- Jen, R.; Orr, J.E.; Li, Y.; DeYoung, P.; Smales, E.; Malhotra, A.; Owens, R.L. Accuracy of WatchPAT for the Diagnosis of Obstructive Sleep Apnea in Patients with Chronic Obstructive Pulmonary Disease. COPD 2020, 17, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Alma, M.A.; Nijenhuis-Huls, R.; de Jong, Z.; Ulgiati, A.M.; de Vries, A.; Dekker, A.D. Detecting sleep apnea in adults with Down syndrome using WatchPAT: A feasibility study. Res. Dev. Disabil. 2022, 129, 104302. [Google Scholar] [CrossRef] [PubMed]
- Embletta® MPR Sleep System. Available online: https://natus.com/neuro/embletta-mpr-sleep-system/ (accessed on 27 September 2022).
- Ng, S.S.S.; Chan, T.-O.; To, K.-W.; Ngai, J.; Tung, A.; Ko, F.W.S.; Hui, D.S.C. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnea syndrome (OSAS). Respirology 2010, 15, 336–342. [Google Scholar] [CrossRef]
- Jonassen, T.M.; Bjorvatn, B.; Saxvig, I.W.; Eagan, T.M.; Lehmann, S. Clinical information predicting severe obstructive sleep apnea: A cross-sectional study of patients waiting for sleep diagnostics. Respir. Med. 2022, 197, 106860. [Google Scholar] [CrossRef] [PubMed]
- ARES™ Home Sleep Test–SleepMed|Better Sleep. Better Health. Available online: https://www.watermarkmedical.com/ares-hst-solution/device-features (accessed on 29 September 2022).
- Ayappa, I.; Norman, R.G.; Seelall, V.; Rapoport, D.M. Validation of a Self-Applied Unattended to Monitor for Sleep Disordered Breathing. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2008, 4, 26–37. [Google Scholar] [CrossRef]
- Sunderram, J.; Weintraub, M.; Black, K.; Alimokhtari, S.; Twumasi, A.; Sanders, H.; Udasin, I.; Harrison, D.; Chitkara, N.; de la Hoz, R.E.; et al. Chronic Rhinosinusitis Is an Independent Risk Factor for OSA in World Trade Center Responders. Chest 2019, 155, 375–383. [Google Scholar] [CrossRef]
- Yagi, H.; Nakata, S.; Tsuge, H.; Yasuma, F.; Noda, A.; Morinaga, M.; Tagaya, M.; Nakashima, T. Significance of a screening device (Apnomonitor5) for sleep apnea syndrome. Auris Nasus Larynx 2009, 36, 176–180. [Google Scholar] [CrossRef]
- Kakutani-Hatayama, M.; Kadoya, M.; Morimoto, A.; Miyoshi, A.; Kosaka-Hamamoto, K.; Kusunoki, Y.; Shoji, T.; Koyama, H. Associations of sleep quality, sleep apnea and autonomic function with insulin secretion and sensitivity: HSCAA study. Metab. Open 2020, 6, 100033. [Google Scholar] [CrossRef]
- Goodrich, S.; Orr, W.C. An investigation of the validity of the Lifeshirt in comparison to standard polysomnography in the detection of obstructive sleep apnea. Sleep Med. 2009, 10, 118–122. [Google Scholar] [CrossRef]
- Jayasekera, S.; Hensel, E.; Robinson, R. Feasibility Assessment of Wearable Respiratory Monitors for Ambulatory Inhalation Topography. Int. J. Environ. Res. Public Health 2021, 18, 2990. [Google Scholar] [CrossRef]
- Somnocheck Micro Cardio Sleep Diagnostic Device. Available online: https://www.medi-shop.gr/en/sleep-diagnostic-systems/weinmann-somnocheck-micro-cardio#:~:text=Weinmann%20SOMNOcheck%20micro%20CARDIO%20analysis,Stokes%20breathing%20can%20be%20detected (accessed on 30 September 2022).
- Bilgin, C.; Erkorkmaz, U.; Uçar, M.K.; Akın, N.; Nalbant, A.; Annakkaya, A.N. Use of a portable monitoring device (Somnocheck Micro) for the investigation and diagnosis of obstructive sleep apnea in comparison with polysomnography. Pak. J. Med. Sci. 2016, 32, 471–475. [Google Scholar] [CrossRef]
- Pataka, A.; Kalamaras, G.; Vlachogianni, E.; Argyropoulou, P. Combination of oximetry and sleep questionnaires as screening tools for CPAP initiation in patients with obstructive sleep apnea. Pulmonology 2019, 25, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Braebon-Medibyte Features. Available online: https://www2.braebon.com/products/medibyte (accessed on 30 September 2022).
- Driver, H.S.; Pereira, E.J.; Bjerring, K.; Toop, F.; Stewart, S.C.; Munt, P.W.; Fitzpatrick, M.F. Validation of the MediByte® type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can. Respir. J. J. Can. Thorac. Soc. 2011, 18, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.I.; Patwari, P.P.; Adavadkar, P.A.; Arantes, H.; Park, C.; Carley, D.W. Validation of the MediByte Portable Monitor for the Diagnosis of Sleep Apnea in Pediatric Patients. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2019, 15, 733–742. [Google Scholar] [CrossRef] [PubMed]
- ResMed. ApneaLink Plus. Available online: https://support.resmed.com/en-in/diagnostics/apnealink-plus/ (accessed on 5 October 2022).
- Cho, J.H.; Kim, H.J. Validation of ApneaLink™ Plus for the diagnosis of sleep apnea. Sleep Breath. 2017, 21, 799–807. [Google Scholar] [CrossRef]
- Lisabeth, L.D.; Scheer, R.V.; Li, C.; Case, E.; Chervin, R.D.; Zahuranec, D.B.; Morgenstern, L.B.; Garcia, N.M.; Tower, S.; Brown, D.L. Intracerebral hemorrhage and sleep-disordered breathing. Sleep Med. 2018, 46, 114–116. [Google Scholar] [CrossRef]
- Nox T3-Polisomnógrafo con EEG by Nox Medical|MedicalExpo. Available online: https://noxmedical.com/ (accessed on 10 October 2022).
- Xu, L.; Han, F.; Keenan, B.T.; Kneeland-Szanto, E.; Yan, H.; Dong, X.; Chang, Y.; Zhao, L.; Zhang, X.; Li, J.; et al. Validation of the Nox-T3 Portable Monitor for Diagnosis of Obstructive Sleep Apnea in Chinese Adults. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 675–683. [Google Scholar] [CrossRef]
- Valério, M.P.; Pereira, S.; Moita, J.; Teixeira, F.; Travassos, C.; Coutinho, A.S.; Rodrigues, D.M. Is the Nox-T3 device scoring algorithm accurate enough for the diagnosis of obstructive sleep apnea. Adv. Respir. Med. 2021, 89, 262–267. [Google Scholar] [CrossRef]
- Penzel, T.; Schöbel, C.; Fietze, I. New technology to assess sleep apnea: Wearables, smartphones, and accessories. F1000Research 2018, 7, 413. [Google Scholar] [CrossRef]
- Abdel-Basset, M.; Ding, W.; Abdel-Fatah, L. The fusion of Internet of Intelligent Things (IoIT) in remote diagnosis of obstructive Sleep Apnea: A survey and a new model. Inf. Fusion 2020, 61, 84–100. [Google Scholar] [CrossRef]
- Bianchi, M.T. Sleep devices: Wearables and nearables, informational and interventional, consumer and clinical. Metab. Clin. Exp. 2018, 84, 99–108. [Google Scholar] [CrossRef]
- Camcı, B.; Ersoy, C.; Kaynak, H. Abnormal respiratory event detection in sleep: A pre-screening system with smart wearables. J. Biomed. Inform. 2019, 95, 103218. [Google Scholar] [CrossRef]
- Gu, W.; Leung, L.; Kwok, K.C.; Wu, I.-C.; Folz, R.J.; Chiang, A.A. Belun Ring Platform: A novel home sleep apnea testing system for assessment of obstructive sleep apnea. Sleep Med. 2020, 16, 1611–1617. [Google Scholar] [CrossRef]
- Baptista, P.M.; Martin, F.; Ross, H.; Reina, C.O.; Plaza, G.; Casale, M. A systematic review of smartphone applications and devices for obstructive sleep apnea. Braz. J. Otorhinolaryngol. 2022, 88, S188–S197. [Google Scholar] [CrossRef]
- Pinheiro, G.D.L.; Cruz, A.F.; Domingues, D.M.; Genta, P.R.; Drager, L.F.; Strollo, P.J.; Lorenzi-Filho, G. Validation of an Overnight Wireless High-Resolution Oximeter plus Cloud-Based Algorithm for the Diagnosis of Obstructive Sleep Apnea. Clinics 2020, 75, e2414. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Diagnostic value of smartphone in obstructive sleep apnea syndrome: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268585. [Google Scholar] [CrossRef]
- Shelgikar, A.V.; Anderson, P.F.; Stephens, M.R. Sleep Tracking, Wearable Technology, and Opportunities for Research and Clinical Care. Chest 2016, 150, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Abad, J.; Muñoz-Ferrer, A.; Cervantes, M.; Esquinas, C.; Marin, A.; Martínez, C.; Morera, J.; Ruiz, J. Automatic Video Analysis for Obstructive Sleep Apnea Diagnosis. Sleep 2016, 39, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, A.; Giannouli, E.; Moussavi, Z. Sleep apnea monitoring and diagnosis based on pulse oximetry and tracheal sound signals. Med. Biol. Eng. Comput. 2010, 48, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tang, X.; Ai, H.; Li, Y.; Xu, W.; Wang, X.; Han, D. Obstructive Sleep Apnea Detection Based on Sleep Sounds via Deep Learning. Nat. Sci. Sleep 2022, 14, 2033–2045. [Google Scholar] [CrossRef]
- Kang, S.; Kim, D.-K.; Lee, Y.; Lim, Y.-H.; Park, H.-K.; Cho, S.H.; Cho, S.H. Non-contact diagnosis of obstructive sleep apnea using impulse-radio ultra-wideband radar. Sci. Rep. 2020, 10, 5261. [Google Scholar] [CrossRef]
- Manoni, A.; Loreti, F.; Radicioni, V.; Pellegrino, D.; Della Torre, L.; Gumiero, A.; Halicki, D.; Palange, P.; Irrera, F. A New Wearable System for Home Sleep Apnea Testing, Screening, and Classification. Sensors 2020, 20, 7014. [Google Scholar] [CrossRef]
- Sadek, I.; Heng, T.T.S.; Seet, E.; Abdulrazak, B. A New Approach for Detecting Sleep Apnea Using a Contactless Bed Sensor: Comparison Study. J. Med. Internet Res. 2020, 22, e18297. [Google Scholar] [CrossRef]
- Gaiduk, M.; Orcioni, S.; Conti, M.; Seepold, R.; Penzel, T.; Madrid, N.M.; Ortega, J.A. Embedded system for non-obtrusive sleep apnea detection. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2776–2779. [Google Scholar] [CrossRef]
- Mendonça, F.; Mostafa, S.S.; Morgado-Dias, F.; Ravelo-García, A.G. An Oximetry Based Wireless Device for Sleep Apnea Detection. Sensors 2020, 20, 888. [Google Scholar] [CrossRef]
- Sabil, A.; Glos, M.; Günther, A.; Schöbel, C.; Veauthier, C.; Fietze, I.; Penzel, T. Comparison of Apnea Detection Using Oronasal Thermal Airflow Sensor, Nasal Pressure Transducer, Respiratory Inductance Plethysmography and Tracheal Sound Sensor. J. Clin. Sleep Med. 2019, 15, 285–292. [Google Scholar] [CrossRef]
- Surrel, G.; Aminifar, A.; Rincon, F.; Murali, S.; Atienza, D. Online Obstructive Sleep Apnea Detection on Medical Wearable Sensors. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 762–773. [Google Scholar] [CrossRef]
- Akbarian, S.; Ghahjaverestan, N.M.; Yadollahi, A.; Taati, B. Non-contact Sleep Monitoring With Infrared Video Data to Estimate Sleep Apnea Severity and Distinguish Between Positional and Non-positional Sleep Apnea: Model Development and Experimental Validation. J. Med. Internet Res. 2021, 23, e26524. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, A.; Arons, E.; Radwan, H.; Silverberg, D.S. Positional vs non-positional obstructive sleep apnea patients: Anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest 1997, 112, 629–639. [Google Scholar] [CrossRef]
- Lin, X.; Cheng, H.; Lu, Y.; Luo, H.; Li, H.; Qian, Y.; Zhou, L.; Zhang, L.; Wang, M. Contactless sleep apnea detection in snoring signals using hybrid deep neural networks targeted for embedded hardware platform with real-time applications. Biomed. Signal Process. Control 2022, 77, 103765. [Google Scholar] [CrossRef]
- Filter Bank: What Is It? (DCT, Polyphase and More). Available online: https://www.electrical4u.com/filter-bank/ (accessed on 10 December 2022).
- Chen, X.; Chen, Y.; Ma, W.; Fan, X.; Li, Y. Toward sleep apnea detection with lightweight multi-scaled fusion network. Knowl.-Based Syst. 2022, 247, 108783. [Google Scholar] [CrossRef]
- Zarei, A.; Beheshti, H.; Asl, B.M. Detection of sleep apnea using deep neural networks and single-lead ECG signals. Biomed. Signal Process. Control 2022, 71, 103125. [Google Scholar] [CrossRef]
- Erdenebayar, U.; Kim, Y.J.; Park, J.-U.; Joo, E.Y.; Lee, K.-J. Deep learning approaches for automatic detection of sleep apnea events from an electrocardiogram. Comput. Methods Programs Biomed. 2019, 180, 105001. [Google Scholar] [CrossRef]
- Tuncer, S.A.; Akılotu, B.; Toraman, S. A deep learning-based decision support system for diagnosis of OSAS using PTT signals. Med Hypotheses 2019, 127, 15–22. [Google Scholar] [CrossRef]
- Deviaene, M.; Testelmans, D.; Buyse, B.; Borzee, P.; Van Huffel, S.; Varon, C. Automatic Screening of Sleep Apnea Patients Based on the SpO2 Signal. IEEE J. Biomed. Health Inform. 2019, 23, 607–617. [Google Scholar] [CrossRef]
- Sklearn.ensemble.RandomForestClassifier. Available online: https://scikit-learn/stable/modules/generated/sklearn.ensemble.RandomForestClassifier.html (accessed on 15 December 2022).
- Wang, T.; Lu, C.; Shen, G. Detection of Sleep Apnea from SingleLead ECG Signal Using a Time Window Artificial Neural Network. BioMed Res. Int. 2019, 2019, e9768072. [Google Scholar] [CrossRef]
- Penzel, T.; Moody, G.B.; Mark, R.G.; Goldberger, A.L.; Peter, J.H. Apnea-ECG Database. Comput. Cardiol. 2000, 27, 255–258. [Google Scholar] [CrossRef]
- Choi, S.H.; Yoon, H.; Kim, H.S.; Kim, H.B.; Bin Kwon, H.; Oh, S.M.; Lee, Y.J.; Park, K.S. Real-time apnea-hypopnea event detection during sleep by convolutional neural networks. Comput. Biol. Med. 2018, 100, 123–131. [Google Scholar] [CrossRef]
- Jung, D.W.; Hwang, S.H.; Cho, J.G.; Choi, B.H.; Baek, H.J.; Lee, Y.J.; Jeong, D.-U.; Park, K.S. Real-Time Automatic Apneic Event Detection Using Nocturnal Pulse Oximetry. IEEE Trans. Biomed. Eng. 2018, 65, 706–712. [Google Scholar] [CrossRef]
- Martinot, J.-B.; Le-Dong, N.-N.; Cuthbert, V.; Denison, S.; Gozal, D.; Lavigne, G.; Pépin, J.-L. Artificial Intelligence Analysis of Mandibular Movements Enables Accurate Detection of Phasic Sleep Bruxism in OSA Patients: A Pilot Study. Nat. Sci. Sleep 2021, 13, 1449–1459. [Google Scholar] [CrossRef]
- Teng, F.; Wang, D.; Yuan, Y.; Zhang, H.; Singh, A.K.; Lv, Z. Multimedia Monitoring System of Obstructive Sleep Apnea via a Deep Active Learning Model. IEEE Multimed. 2022, 29, 48–56. [Google Scholar] [CrossRef]
- Cheng, L.; Luo, S.; Li, B.; Liu, R.; Zhang, Y.; Zhang, H. Multiple-instance learning for EEG-based OSA event detection. Biomed. Signal Process. Control 2023, 80, 104358. [Google Scholar] [CrossRef]
- Cheng, L.; Luo, S.; Yu, X.; Ghayvat, H.; Zhang, H.; Zhang, Y. EEG-CLNet: Collaborative Learning for Simultaneous Measurement of Sleep Stages and OSA Events Based on Single EEG Signal. IEEE Trans. Instrum. Meas. 2023, 72, 2503910. [Google Scholar] [CrossRef]
- ISO 80601-2-61:2017(en); Medical Electrical Equipment—Part 2-61: Particular Requirements for Basic Safety and Essential Performance of Pulse Oximeter Equipment. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/67963.html (accessed on 23 December 2022).
- Panamerican Health Organization. Technical and Regulatory Aspects of the Use of Pulse Oximeters in Monitoring COVID-19 Patients. 7 August 2020. Available online: https://iris.paho.org/handle/10665.2/52589 (accessed on 24 December 2022).
- ISO 23747:2015; Anaesthetic and Respiratory Equipment—Peak Expiratory Flow Meters for the Assessment of Pulmonary Function in Spontaneously Breathing Humans. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/64926.html (accessed on 10 January 2023).
- ISO 4135:2022(en); Anaesthetic and Respiratory Equipment-Vocabulary. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/obp/ui#iso:std:iso:4135:ed-4:v1:en (accessed on 12 January 2023).
- ISO 13485:2016; Anaesthetic and Respiratory Equipment. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/59752.html (accessed on 13 January 2023).
- Compliance Center|ActiGraph. Available online: https://actigraphcorp.com/compliance/ (accessed on 14 January 2023).
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 14 February 2023).
- Pan, L.; Meng, F.; Zhang, L.; Shen, H.; Kong, D.; Wang, W.; Kang, J. Global research trends of obstructive sleep apnea from 2011 to 2020: A 10- year bibliometric analysis. Ann. Palliat. Med. 2022, 11, 1671–1686. [Google Scholar] [CrossRef] [PubMed]
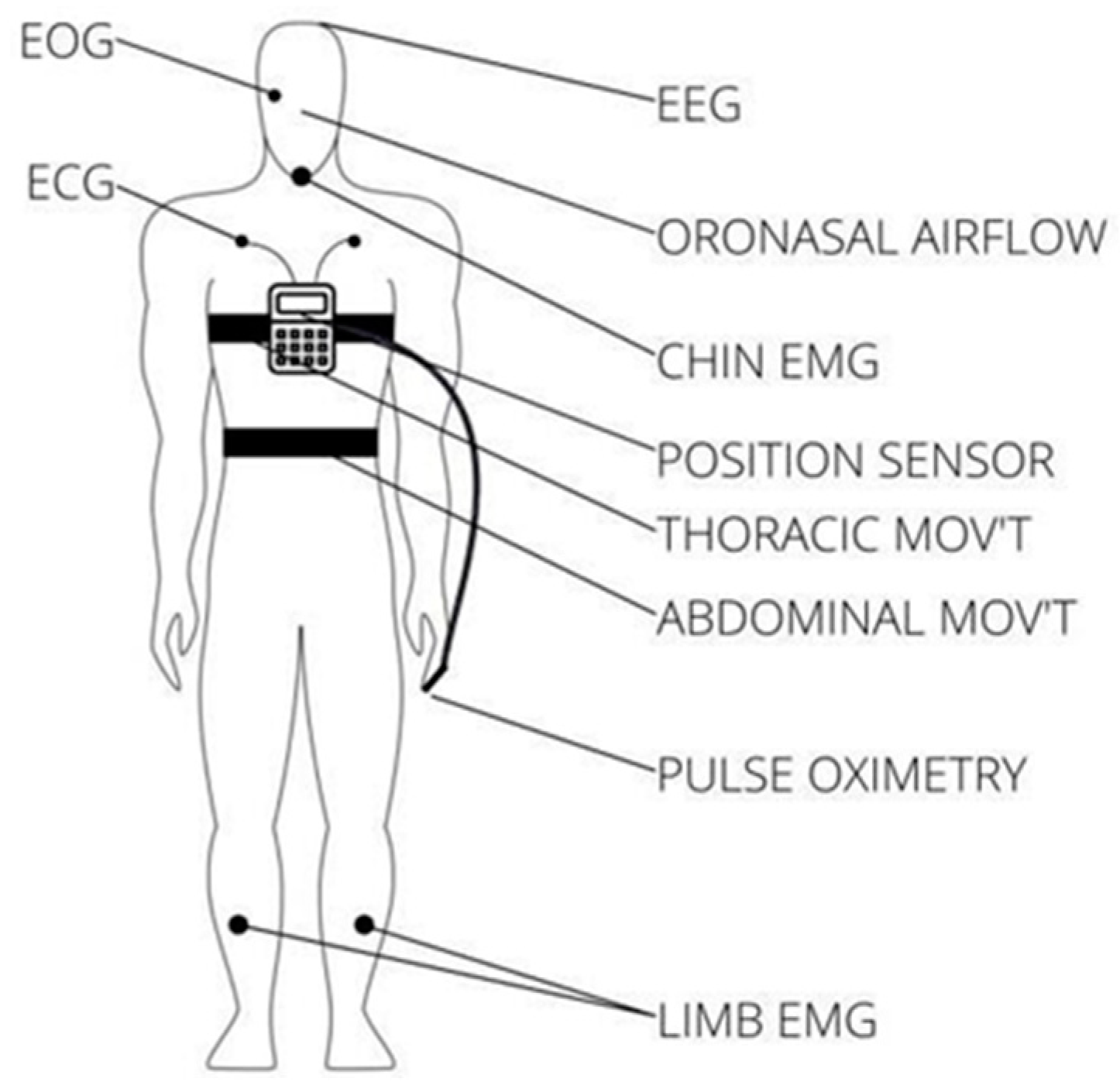
| Device | Measurements | Category | Validation | Application |
|---|---|---|---|---|
| Alice PDx [14] | Oxygen, SpO2, Heart Rate, Snoring, Respiratory Flow | III | Eighty-five patients with suspected OSA were studied. The Alice PDX was used in diagnostic agreement with PSG in 96.4% of the studies [15]. | The study was conducted involving subjects from Indonesia. Its main objective is to prove that there is a correlation between primary aldosteronism and OSA [16]. |
| Apnea Link Air [17] | Oxygen, SpO2, Heart Rate, Snoring, Respiratory Flow | III | Sixty children and adolescents with suspected OSA were studied. When the AHI threshold was adjusted to 1 h, the sensitivity and specificity were 94% and 29% [18]. | Comparison between the interpretation of Apnea link Air by primary care physicians (PCPs) and the one achieved through respiratory polygraphy (RP) at the Hospital Sleep Unit (HSU) [19]. |
| WatchPat One [20] | PAT signal, Heart Rate, Oximetry, Actigraphy, Body, Position, Snoring, Chest Motion | II | A total of thirty-six subjects exhibiting suspected OSA underwent an examination. The obtained sensitivity of WatchPAT One at an AHI cut-off of ≥5 was 95.8% [21]. | To detect OSA in patients with Down syndrome using the WatchPat One. OSA was seen in 95% of participants [22]. |
| Embletta [23] | Abdominal Strain, Chest Strain, Nasal Pressure, Nasal Flow, Snore, SpO2, Heart Rate, Position, Microphone | II | Eighty subjects exhibiting suspected OSA underwent examination. The sensitivity at an AHI > or =5 h was 0.924 [24]. | The Embletta device was used in a study to predict severe OSA in patients awaiting sleep studies [25]. |
| ARES Unicode [26] | Actigraphy, Pulse, Oximetry, Position, Effort, Nasal Pressure, Audio | II | The study was conducted involving eighty subjects with suspected OSA and twenty-two volunteers. The sensitivity and specificity of ARES Unicode were 85% and 91%, respectively [27]. | A study to examine the relationship between chronic rhinosinusitis and the prevalence and severity of OSA [28]. |
| Apnomonitor 5 | Oximetry, Position, Respiratory, Audio, Effort | III | Twenty-two adults with suspected OSA underwent an examination. The sensitivity of the Apnomonitor 5 was 95% against PSG [29]. | The study aimed to identify the relationship between quantitative measures of sleep quality, sleep apnea, autonomic function, and insulin secretion and sensitivity [30]. |
| Lifeshirt | EOG, Pulse, Oximetry, Respiratory Flow | III | Fifty subjects with suspected OSA underwent examination. The sensitivity of the Lifeshirt ranged from 0.85 to 1, depending on the AHI. Specificity ranged from 0.67 to 1.00 [31]. | The application aimed to examine the possibility of modifying Wireless Respiratory Monitors (WRMs), like the Lifeshirt, to measure inhalation patterns [32]. |
| SOMNOcheck micro [33] | Pulse, Oximetry, Position, Nasal pressure, Audio | III | The study involved one hundred five subjects with suspected OSA. There were no differences between the AHI of the device and PSG [34]. | The study aimed to assess the effectiveness of various sleep questionnaires. Subsequently, PSG and other evaluation methods were conducted, and the questionnaires were administered again after treatment [35]. |
| Medibyte [36] | ECG, Oximetry, Effort, Nasal Pressure | III | Seventy-three subjects with suspected OSA were involved in the study. The sensitivity and specificity of the screener were 80% and 97%, respectively [37]. | The study aimed to validate the Medibyte device by comparing it with PSG in pediatric patients who wore both setups simultaneously [38]. |
| ApneaLink Plus [39] | Respiratory effort, Pulse, Oxygen saturation, Nasal Flow | III | The study involved one hundred fifty subjects with suspected OSA. The specificity of the device was 93% [40]. | A study aimed to characterize the objective measures of sleep-disordered breathing (SDB) in patients with post-intracerebral hemorrhage. The Apnea Link Plus was used to screen SDB [41]. |
| NOX T3 [42] | Oximeter, Thorax and Abdomen Respiratory Inductance, Plethysmography (RIP), Effort, Flow, Snore Microphone | III | The study was conducted on eighty adults with suspected OSA. The NOX T3 has a sensitivity of 85% and a specificity of 89% [43]. | A study aimed to evaluate the accuracy of the NOX T3 method in diagnosing OSA through random tests on patients [44]. |
| Article | Number of Sensors | Type of Sensors | Performance |
|---|---|---|---|
| [58] | 2 | Photoplethysmography and accelerometer | Sensitivity and precision of 90% |
| [59] | 1 | MFOS | Accuracy of 49.96%, sensitivity of 57.07%, specificity of 45.26% |
| [60] | 1 | Force-sensitive resistor | Accuracy rate of 91% |
| [61] | 1 | SPO2 sensor | Accuracy of 88%, sensitivity of 80%, specificity of 91% |
| [62] | 1 | Tracheal sound sensor | Sensitivity of 96.06% and specificity of 76.07% |
| [63] | 1 | ECG sensor | Accuracy of 88.2% |
| Article | Input | Method | Accuracy |
|---|---|---|---|
| [64] | Infrared video | Deep learning | 83% |
| [66] | Snoring signals | Deep neural network | 74.27% |
| [68] | ECG signal | Multiscaled neural network | 90.64% |
| [69] | ECG signal | Deep neural network | 97.21% |
| [70] | ECG signal | Deep learning | 99% |
| [71] | Pulse transition time | Deep learning | 92.64% |
| [72] | SPO2 signal | Random forest classifier | 82.8% |
| [74] | ECG signal | Artificial neural network | 87.3% |
| [76] | Nasal pressure signal | Convolutional neural network | 96.6% |
| [77] | Pulse oximetry | Regression modeling | 96.7% |
| [78] | Mandibular jaw movements | Artificial intelligence | 86.6% |
| [79] | ECG signal | Deep learning | 92.15% |
| [80] | EEG signal | EEG-MIL | 69.3–73.4% |
| [81] | EEG signal | EEG-CLNet | 1–5% better than baseline |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa, M.A.; Ponce, P.; Molina, A.; Borja, V.; Torres, M.G.; Rojas, M. Advancements in Home-Based Devices for Detecting Obstructive Sleep Apnea: A Comprehensive Study. Sensors 2023, 23, 9512. https://doi.org/10.3390/s23239512
Espinosa MA, Ponce P, Molina A, Borja V, Torres MG, Rojas M. Advancements in Home-Based Devices for Detecting Obstructive Sleep Apnea: A Comprehensive Study. Sensors. 2023; 23(23):9512. https://doi.org/10.3390/s23239512
Chicago/Turabian StyleEspinosa, Miguel A., Pedro Ponce, Arturo Molina, Vicente Borja, Martha G. Torres, and Mario Rojas. 2023. "Advancements in Home-Based Devices for Detecting Obstructive Sleep Apnea: A Comprehensive Study" Sensors 23, no. 23: 9512. https://doi.org/10.3390/s23239512
APA StyleEspinosa, M. A., Ponce, P., Molina, A., Borja, V., Torres, M. G., & Rojas, M. (2023). Advancements in Home-Based Devices for Detecting Obstructive Sleep Apnea: A Comprehensive Study. Sensors, 23(23), 9512. https://doi.org/10.3390/s23239512








