Validity and Efficacy of the Elite HRV Smartphone Application during Slow-Paced Breathing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. RR Interval Collection
2.4. Respiration Belt
2.5. Breathing Conditions
2.6. Statistical Analysis
3. Results
3.1. Mean RR
3.2. SDNN
3.3. RMSSD
3.4. LF
3.5. HF
4. Discussion
4.1. Comparison of ECG- and Elite HRV-Derived HRV
4.2. Comparison of SPONT and PACED HRV
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Culver, M.N.; McMillan, N.K.; Cross, B.L.; Montoye, A.H.K.; Riemann, B.L.; Flatt, A.A. Self-recorded heart rate variability profiles are associated with health and lifestyle markers in young adults. Clin. Auton. Res. 2022, 32, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Pantelopoulos, A.; Emir-Farinas, H.; Natarajan, P. Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. Lancet Digit. Health 2020, 2, e650–e657. [Google Scholar] [CrossRef]
- Hayano, J.; Yamada, M.; Sakakibara, Y.; Fujinami, T.; Yokoyama, K.; Watanabe, Y.; Takata, K. Short- and long-term effects of cigarette smoking on heart rate variability. Am. J. Cardiol. 1990, 65, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Rennie, K.L.; Hemingway, H.; Kumari, M.; Brunner, E.; Malik, M.; Marmot, M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am. J. Epidemiol. 2003, 158, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Larson, M.G.; O’Donnell, C.J.; Wilson, P.F.; Tsuji, H.; Lloyd-Jones, D.M.; Levy, D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am. J. Cardiol. 2000, 86, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Dekker, J.M.; TenVoorde, B.J.; Kostense, P.J.; Heine, R.J.; Bouter, L.M.; Heethaar, R.M.; Stehouwer, C.D.A. Impaired Autonomic Function Is Associated With Increased Mortality, Especially in Subjects With Diabetes, Hypertension, or a History of Cardiovascular Disease: The Hoorn Study. Diabetes Care 2001, 24, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Liao, D.; Cai, J.; Barnes, R.W.; Tyroler, H.A.; Rautaharju, P.; Holme, I.; Heiss, G. Association of cardiac automatic function and the development of hypertension: The ARIC study. Am. J. Hypertens. 1996, 9, 1147–1156. [Google Scholar] [CrossRef]
- Schroeder, E.B.; Liao, D.; Chambless, L.E.; Prineas, R.J.; Evans, G.W.; Heiss, G. Hypertension, blood pressure, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Hypertension 2003, 42, 1106–1111. [Google Scholar] [CrossRef]
- Singh, J.P.; Larson, M.G.; Tsuji, H.; Evans, J.C.; O’Donnell, C.J.; Levy, D. Reduced heart rate variability and new-onset hypertension: Insights into pathogenesis of hypertension: The Framingham Heart Study. Hypertension 1998, 32, 293–297. [Google Scholar] [CrossRef]
- Tsuji, H.; Venditti, F.J., Jr.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994, 90, 878–883. [Google Scholar] [CrossRef]
- Hernández-Vicente, A.; Hernando, D.; Santos-Lozano, A.; Rodríguez-Romo, G.; Vicente-Rodríguez, G.; Pueyo, E.; Bailón, R.; Garatachea, N. Heart Rate Variability and Exceptional Longevity. Front. Physiol. 2020, 11, 566399. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Jurivich, D.A.; Gao, W.; Singer, D.H. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol. 2010, 105, 1181–1185. [Google Scholar] [CrossRef]
- Kay, M.W.; Jain, V.; Panjrath, G.; Mendelowitz, D. Targeting Parasympathetic Activity to Improve Autonomic Tone and Clinical Outcomes. Physiology 2022, 37, 39–45. [Google Scholar] [CrossRef]
- Frank, D.L.; Khorshid, L.; Kiffer, J.F.; Moravec, C.S.; McKee, M.G. Biofeedback in medicine: Who, when, why and how? Ment. Health Fam. Med. 2010, 7, 85–91. [Google Scholar] [PubMed]
- Lehrer, P. How does heart rate variability biofeedback work? Resonance, the baroreflex, and other mechanisms. Biofeedback 2013, 41, 26–31. [Google Scholar] [CrossRef]
- Chaitanya, S.; Datta, A.; Bhandari, B.; Sharma, V.K. Effect of Resonance Breathing on Heart Rate Variability and Cognitive Functions in Young Adults: A Randomised Controlled Study. Cureus 2022, 14, e22187. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.D.; Tzeng, Y.C.; Sin, P.Y.W.; Galletly, D.C. Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir. Physiol. Neurobiol. 2010, 174, 111–118. [Google Scholar] [CrossRef]
- Hirsch, J.A.; Bishop, B. Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. Am. J. Physiol. 1981, 241, H620–H629. [Google Scholar] [CrossRef]
- Dick, T.E.; Hsieh, Y.H.; Dhingra, R.R.; Baekey, D.M.; Galán, R.F.; Wehrwein, E.; Morris, K.F. Cardiorespiratory coupling: Common rhythms in cardiac, sympathetic, and respiratory activities. Prog. Brain Res. 2014, 209, 191–205. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R.T. The coherent heart heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Rev. A Transdiscipl. Transcult. J. New Thought Res. Prax. 2009, 5, 24. [Google Scholar]
- Shaffer, F.; Meehan, Z.M. A Practical Guide to Resonance Frequency Assessment for Heart Rate Variability Biofeedback. Front. Neurosci. 2020, 14, 570400. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Allen, M.S.; Borges, U.; Dosseville, F.; Hosang, T.J.; Iskra, M.; Mosley, E.; Salvotti, C.; Spolverato, L.; Zammit, N.; et al. Effects of voluntary slow breathing on heart rate and heart rate variability: A systematic review and a meta-analysis. Neurosci. Biobehav. Rev. 2022, 138, 104711. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.K.; Wachholtz, A. The benefit of heart rate variability biofeedback and relaxation training in reducing trait anxiety. Hanguk Simni Hakhoe Chi Kongang 2015, 20, 391–408. [Google Scholar] [CrossRef]
- Wang, C.H.; Yang, H.W.; Huang, H.L.; Hsiao, C.Y.; Jiu, B.K.; Lin, C.; Lo, M.T. Long-Term Effect of Device-Guided Slow Breathing on Blood Pressure Regulation and Chronic Inflammation in Patients with Essential Hypertension Using a Wearable ECG Device. Acta Cardiol. Sin. 2021, 37, 195–203. [Google Scholar] [CrossRef]
- Steffen, P.R.; Austin, T.; DeBarros, A.; Brown, T. The Impact of Resonance Frequency Breathing on Measures of Heart Rate Variability, Blood Pressure, and Mood. Front. Public Health 2017, 5, 222. [Google Scholar] [CrossRef]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef]
- Laborde, S.; Hosang, T.; Mosley, E.; Dosseville, F. Influence of a 30-Day Slow-Paced Breathing Intervention Compared to Social Media Use on Subjective Sleep Quality and Cardiac Vagal Activity. J. Clin. Med. 2019, 8, 193. [Google Scholar] [CrossRef]
- Flatt, A.A.; Howells, D. Effects of Long-Haul Travel and the Olympic Games on Heart-Rate Variability in Rugby Sevens Medalists. Int. J. Sports Physiol. Perform. 2022, 17, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, P.; Shrestha, L.; Mahotra, N.B. Validity of Elite-HRV Smartphone Application for Measuring Heart Rate Variability Compared to Polar V800 Heart Rate Monitor. J. Nepal. Health Res. Counc. 2021, 19, 809–813. [Google Scholar]
- Gambassi, B.B.; Neves, V.R.; Brito, E.Z.A.; da Silva Fernandes, D.S.; Sá, C.A.; da Rocha Nogueira, R.M.; de Jesus Furtado Almeida, F.; de Araújo Cavalcanti, P.A.; Gomes Gonçalves, E.S.D.C.; Neto, D.S.; et al. A validation study of a smartphone application for heart rate variability assessment in asymptomatic adults. Am. J. Cardiovasc. Dis. 2020, 10, 219–229. [Google Scholar] [PubMed]
- Himariotis, A.T.; Coffey, K.F.; Noel, S.E.; Cornell, D.J. Validity of a Smartphone Application in Calculating Measures of Heart Rate Variability. Sensors 2022, 22, 9883. [Google Scholar] [CrossRef] [PubMed]
- Moya-Ramon, M.; Mateo-March, M.; Peña-González, I.; Zabala, M.; Javaloyes, A. Validity and reliability of different smartphones applications to measure HRV during short and ultra-short measurements in elite athletes. Comput. Methods Programs Biomed. 2022, 217, 106696. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, A.S.; Jeklin, A.T.; Hives, B.A.; Meanwell, L.E.; Warburton, D.E.R. Validity of the Elite HRV Smartphone Application for Examining Heart Rate Variability in a Field-Based Setting. J. Strength Cond. Res. 2017, 31, 2296–2302. [Google Scholar] [CrossRef]
- Stone, J.D.; Ulman, H.K.; Tran, K.; Thompson, A.G.; Halter, M.D.; Ramadan, J.H.; Stephenson, M.; Finomore, V.S.; Galster, S.M.; Rezai, A.R.; et al. Assessing the Accuracy of Popular Commercial Technologies That Measure Resting Heart Rate and Heart Rate Variability. Front. Sports Act. Living 2021, 3, 585870. [Google Scholar] [CrossRef]
- Guzik, P.; Piekos, C.; Pierog, O.; Fenech, N.; Krauze, T.; Piskorski, J.; Wykretowicz, A. Classic electrocardiogram-based and mobile technology derived approaches to heart rate variability are not equivalent. Int. J. Cardiol. 2018, 258, 154–156. [Google Scholar] [CrossRef]
- Christiani, M.; Grosicki, G.J.; Flatt, A.A. Cardiac-autonomic and hemodynamic responses to a hypertonic, sugar-sweetened sports beverage in physically active men. Appl. Physiol. Nutr. Metab. 2021, 46, 1189–1195. [Google Scholar] [CrossRef]
- Laborde, S.; Allen, M.S.; Borges, U.; Iskra, M.; Zammit, N.; You, M.; Hosang, T.; Mosley, E.; Dosseville, F. Psychophysiological effects of slow-paced breathing at six cycles per minute with or without heart rate variability biofeedback. Psychophysiology 2022, 59, e13952. [Google Scholar] [CrossRef]
- Lipponen, J.A.; Tarvainen, M.P. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J. Med. Eng. Technol. 2019, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Rüdiger, H.; Ziemssen, T. Spectral Analysis of Heart Rate Variability: Time Window Matters. Front. Neurol. 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.M.; Gevirtz, R. Heart rate variability biofeedback: How and why does it work? Front. Psychol. 2014, 5, 756. [Google Scholar] [CrossRef]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef]
- Smith, R.; Chase, J.G.; Pretty, C.G.; Davidson, S.; Shaw, G.M.; Desaive, T. Preload & Frank-Starling curves, from textbook to bedside: Clinically applicable non-additionally invasive model-based estimation in pigs. Comput. Biol. Med. 2021, 135, 104627. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Brehm, M.A.; Scholtes, V.A.; Dallmeijer, A.J.; Twisk, J.W.; Harlaar, J. The importance of addressing heteroscedasticity in the reliability analysis of ratio-scaled variables: An example based on walking energy-cost measurements. Dev. Med. Child. Neurol. 2012, 54, 267–273. [Google Scholar] [CrossRef]
- Lawrence, I.; Lin, K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- Ludbrook, J. A primer for biomedical scientists on how to execute model II linear regression analysis. Clin. Exp. Pharmacol. Physiol. 2012, 39, 329–335. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Menghini, L.; Gianfranchi, E.; Cellini, N.; Patron, E.; Tagliabue, M.; Sarlo, M. Stressing the accuracy: Wrist-worn wearable sensor validation over different conditions. Psychophysiology 2019, 56, e13441. [Google Scholar] [CrossRef] [PubMed]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in Recreational Men and Women. Sensors 2022, 22, 6536. [Google Scholar] [CrossRef] [PubMed]
- Jeyhani, V.; Mantysalo, M.; Noponen, K.; Seppanen, T.; Vehkaoja, A. Effect of Different ECG Leads on Estimated R-R Intervals and Heart Rate Variability Parameters. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 3786–3790. [Google Scholar] [CrossRef] [PubMed]
- Apandi, Z.F.M.; Ikeura, R.; Hayakawa, S.; Tsutsumi, S. An Analysis of the Effects of Noisy Electrocardiogram Signal on Heartbeat Detection Performance. Bioengineering 2020, 7, 53. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R.; Allen, J.R.; Robinson, J.B.; Bragg, A.; Keith, C.M.; Fedewa, M.V.; Earley, R.L. Cardiac-Autonomic Responses to In-Season Training Among Division-1 College Football Players. J. Strength Cond. Res. 2020, 34, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Martins, T.C.; Nascimento, L.M.; Hoeller, A.A.; Walz, R.; Takase, E. Ultra-short heart rate variability recording reliability: The effect of controlled paced breathing. Ann. Noninvasive Electrocardiol. 2018, 23, e12565. [Google Scholar] [CrossRef] [PubMed]
- Saboul, D.; Pialoux, V.; Hautier, C. The impact of breathing on HRV measurements: Implications for the longitudinal follow-up of athletes. Eur. J. Sport. Sci. 2013, 13, 534–542. [Google Scholar] [CrossRef]
- Sakakibara, M.; Kaneda, M.; Oikawa, L.O. Efficacy of Paced Breathing at the Low-frequency Peak on Heart Rate Variability and Baroreflex Sensitivity. Appl. Psychophysiol. Biofeedback 2020, 45, 31–37. [Google Scholar] [CrossRef]
- Sevoz-Couche, C.; Laborde, S. Heart rate variability and slow-paced breathing:when coherence meets resonance. Neurosci. Biobehav. Rev. 2022, 135, 104576. [Google Scholar] [CrossRef]

| Participant | ECG SPONT | ECG PACED | App SPONT | Signal Quality | App PACED | Signal Quality |
|---|---|---|---|---|---|---|
| 1 | 4 $ | 4 $ | 0 | good | 0 | good |
| 2 | 0 | 2 $ | 0 | good | 0 | good |
| 3 | 0 | 0 | 0 | good | 0 | good |
| 4 | 0 | 0 | 0 | good | 0 | good |
| 5 | 0 | 0 | 0 | good | 2 | good |
| 6 | 0 | 0 | 6 | good | 46 | okay |
| 7 | 0 | 0 | 0 | good | 0 | good |
| 8 | 0 | 0 | 0 | good | 0 | good |
| 9 | 0 | 0 | 0 | good | 0 | good |
| 10 | 0 | 0 | 0 | good | 0 | good |
| 11 | 0 | 0 | 0 | good | 0 | good |
| 12 | 0 | 0 | 0 | good | 0 | good |
| 13 | 0 | 0 | 0 | good | 0 | good |
| 14 | 0 | 0 | 0 | good | 0 | good |
| 15 | 0 | 0 | 0 | good | 0 | good |
| 16 | 0 | 0 | 0 | good | 0 | good |
| 17 | 0 | 0 | 2 | good | 2 | okay |
| 18 | 0 | 0 | 0 | good | 0 | good |
| 19 | 0 | 0 | 0 | good | 0 | good |
| 20 | 2 ‡ | 4 ‡ | 2 | good | 6 | good |
| Median (IQR) | p | Median Bias (IQR) | Limits of Agreement | τ | LCC | Ordinary Least Products Regression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower 2.5th | Upper 97.5th | R2 | Slope (95% CI) | Intercept (95% CI) | ||||||||
| Mean RR (ms) | SPONT | ECG | 1040.8 (268.4) | 0.60 | −0.04 (2.3) | −19.8 | 29.1 | 0.36 | 1.0 | 0.99 | 1.0 (0.98–1.03) | −4.5 (−27.9–18.9) |
| App | 1026.2 (249.7) | |||||||||||
| PACED | ECG | 1018.3 (207.2) | 0.002 | 1.5 (5.3) | −1.6 | 114.6 | 0.05 | 1.0 | 0.94 | 1.0 (0.9–1.1) | −8.1 (−80.1–63.8) | |
| App | 1003.0 (198.3) | |||||||||||
| SDNN (ms) | SPONT | ECG | 84.2 (43.0) | 0.01 * | 1.3 (3.6) | −71.9 | 33.4 | 0.21 | 0.91 | 0.64 | 1.2 (0.9–1.4) | −15.6 (−45.9–14.8) |
| App | 84.8 (35.5) | |||||||||||
| PACED | ECG | 100.1 (50.7) | 0.52 | 0.1 (3.5) | −9.9 | 88.4 | 0.34 | 0.92 | 0.67 | 1.4 (0.9–1.9) | −37.2 (−86.1–11.7) | |
| App | 99.6 (44.7) | |||||||||||
| RMSSD (ms) | SPONT | ECG | 61.3 (47.1) | <0.001 * | 1.4 (5.5) | −1.4 | 34.6 | 0.41 | 0.98 | 0.98 | 1.2 (1.0–1.3) | −8.5 (−16.4–−0.5) |
| App | 61.1 (35.7) | |||||||||||
| PACED | ECG | 71.6 (46.9) | 0.01 * | 0.4 (5.7) | −12.3 | 128.2 | 0.47 | 0.80 | 0.17 | 1.7 (0.7–2.6) | −41.9 (−104.2–20.5) | |
| App | 76.9 (47.9) | |||||||||||
| HRV Metric | HRV Tool | Condition | Mean ± SD or Median (IQR) | p | LCC |
|---|---|---|---|---|---|
| Mean RR (ms) | ECG | SPONT | 1040.8 (268.4) | 0.04 * | 0.95 |
| PACED | 1018.3 (207.2) | ||||
| App | SPONT | 1026.2 (249.7) | 0.04 * | ||
| PACED | 1003.0 (198.3) | ||||
| SDNN (ms) | ECG | SPONT | 90.0 ± 37.3 | 0.006 * | 0.70 |
| PACED | 111.9 ± 45.4 | ||||
| App | SPONT | 90.3 ± 31.9 | 0.05 | ||
| PACED | 107.2 ± 32.6 | ||||
| RMSSD (ms) | ECG | SPONT | 61.3 (47.1) | 0.13 | 0.65 |
| PACED | 71.6 (46.9) | ||||
| App | SPONT | 61.1 (35.7) | 0.52 | ||
| PACED | 76.9 (47.9) | ||||
| LF (ms2) | ECG | SPONT | 1786.3 (2074.5) | <0.001 * | 0.75 |
| PACED | 6618.2 (5251.9) | ||||
| App | SPONT | 1634.0 (2084.7) | <0.001 * | ||
| PACED | 6356.2 (4081.8) | ||||
| HF (ms2) | ECG | SPONT | 1321.8 (1705.2) | 0.18 | 0.39 |
| PACED | 965.2 (1413.0) | ||||
| App | SPONT | 1276.5 (1618.7) | 0.06 | ||
| PACED | 1047.4 (1313.2) |
| Mean ± SD or Median (IQR) | p | Mean Bias ± SD or Median Bias (IQR) | Limits of Agreement (95% OR 2.5th and 97.5th) | τ | LCC | Ordinary Least Products Regression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | R2 | Slope (95% CI) | Intercept (95% CI) | ||||||||
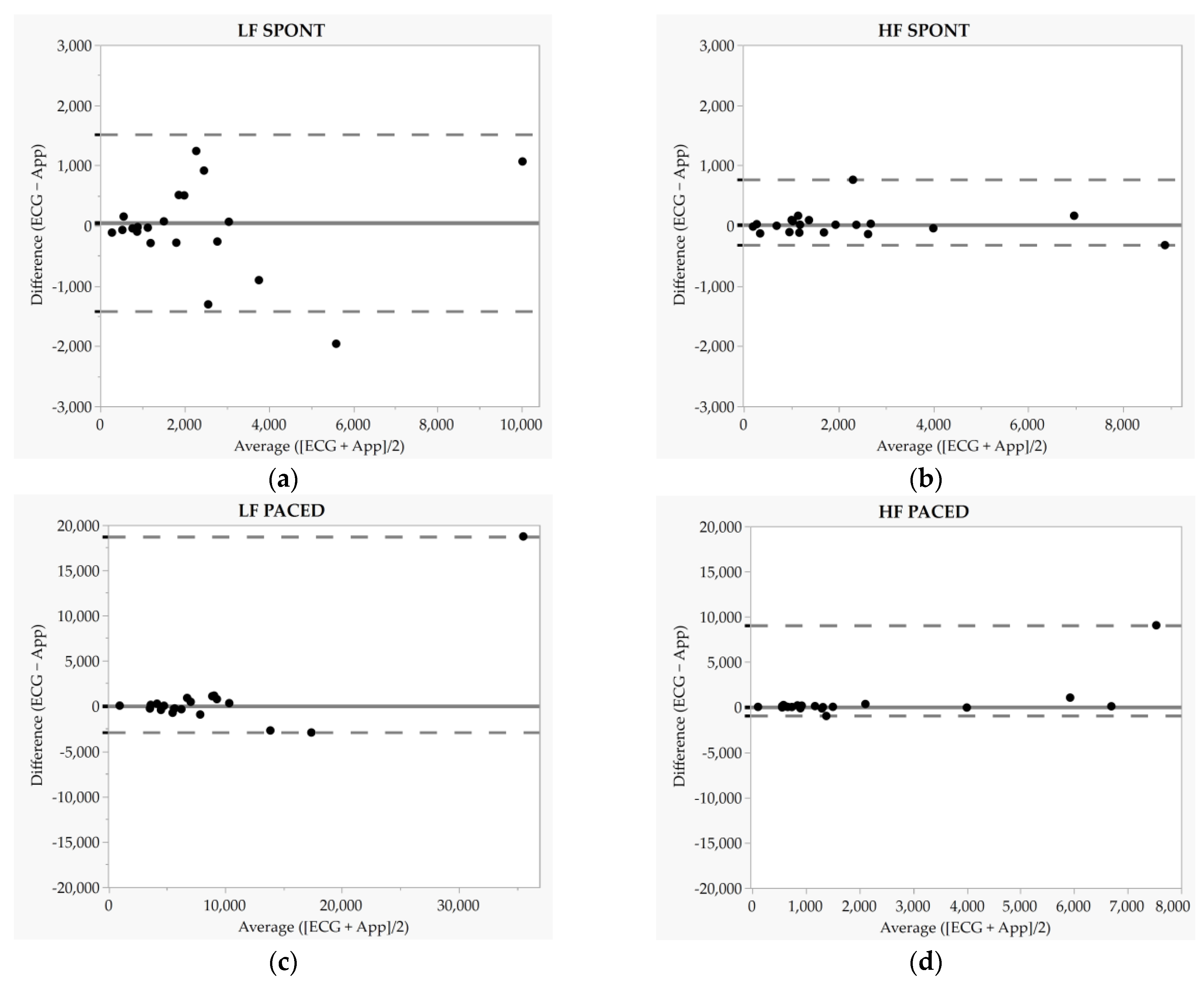
| LF (ms2) | SPONT | ECG | 2273.4 ± 2259.4 | 0.80 | 44.0 ± 748.9 | −1424.0 | 1511.9 | 0.46 | 0.95 | 0.89 | 1.0 (0.7–1.3) | −56.9 (−578.4–464.6) |
| App | 2317.4 ± 2246.9 | |||||||||||
| PACED | ECG | 6618.2 (5251.9) | 0.93 | 28.9 (1100.2) | −2901.4 | 18,752.9 | 0.67 | 0.85 | 0.63 | 1.6 (0.7–2.4) | −3869.6 (−9067.2–1327.9) | |
| App | 6356.2 (4081.8) | |||||||||||
| HF (ms2) | SPONT | ECG | 1321.8 (1705.2) | 0.96 | 14.2 (199.1) | −323.4 | 762.1 | 0.21 | 1.0 | 0.99 | 1.0 (0.9–1.1) | 52.9 (−60.4–166.2) |
| App | 1276.5 (1618.7) | |||||||||||
| PACED | ECG | 965.2 (1413.0) | 0.13 | 35.1 (208.4) | −994.8 | 9046.4 | 0.32 | 0.66 | 0.49 | 1.7 (0.0–3.4) | −707.2 (−2177.6–763.2) | |
| App | 1047.4 (1313.2) | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vondrasek, J.D.; Riemann, B.L.; Grosicki, G.J.; Flatt, A.A. Validity and Efficacy of the Elite HRV Smartphone Application during Slow-Paced Breathing. Sensors 2023, 23, 9496. https://doi.org/10.3390/s23239496
Vondrasek JD, Riemann BL, Grosicki GJ, Flatt AA. Validity and Efficacy of the Elite HRV Smartphone Application during Slow-Paced Breathing. Sensors. 2023; 23(23):9496. https://doi.org/10.3390/s23239496
Chicago/Turabian StyleVondrasek, Joseph D., Bryan L. Riemann, Gregory J. Grosicki, and Andrew A. Flatt. 2023. "Validity and Efficacy of the Elite HRV Smartphone Application during Slow-Paced Breathing" Sensors 23, no. 23: 9496. https://doi.org/10.3390/s23239496
APA StyleVondrasek, J. D., Riemann, B. L., Grosicki, G. J., & Flatt, A. A. (2023). Validity and Efficacy of the Elite HRV Smartphone Application during Slow-Paced Breathing. Sensors, 23(23), 9496. https://doi.org/10.3390/s23239496








