Effects of Functional Electrical Stimulation on Gait Characteristics in Healthy Individuals: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Data Extraction
2.5. Methodological Quality Assessment
3. Results
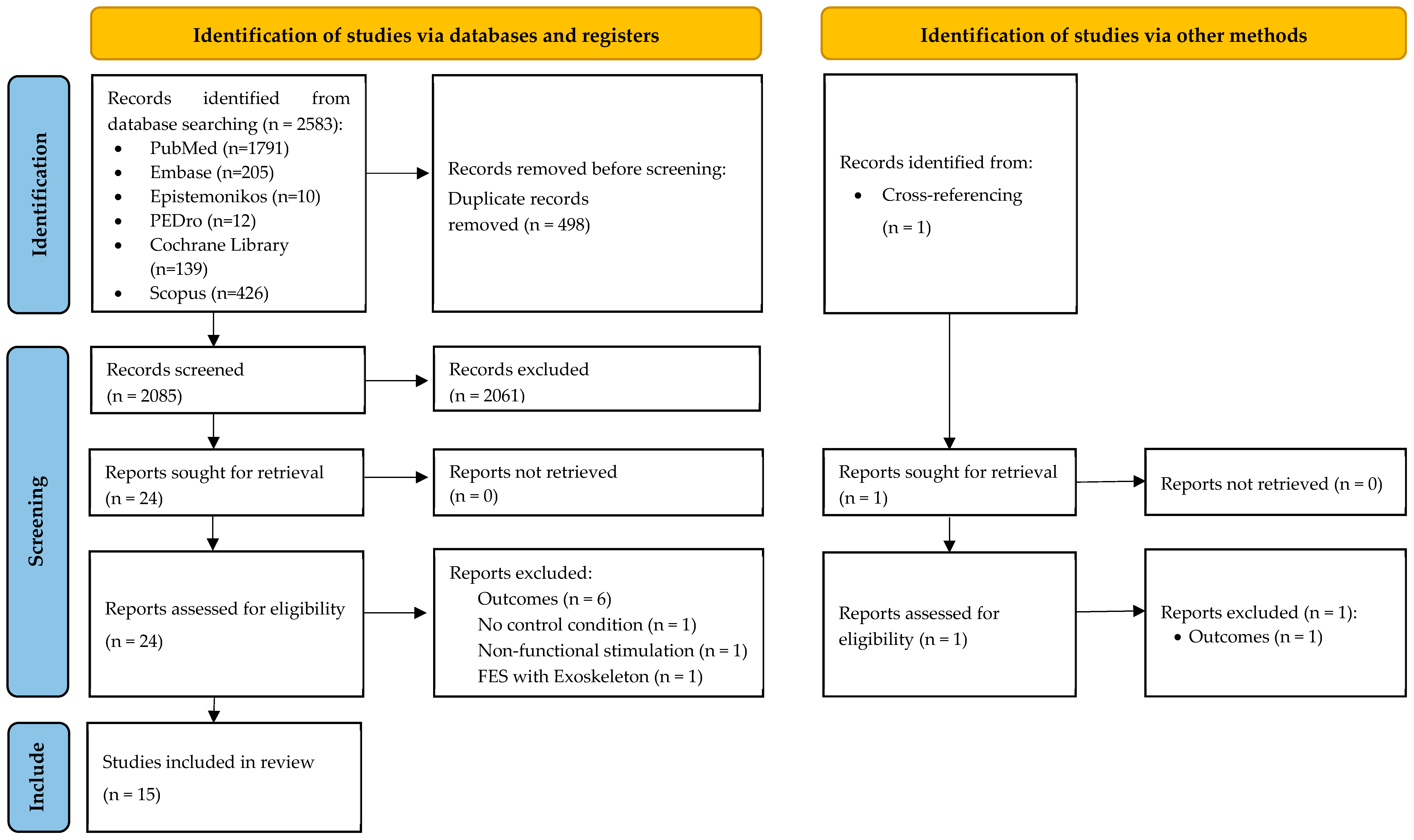
3.1. Search Results
3.2. Methodological Quality of the Included Articles
3.3. Participants
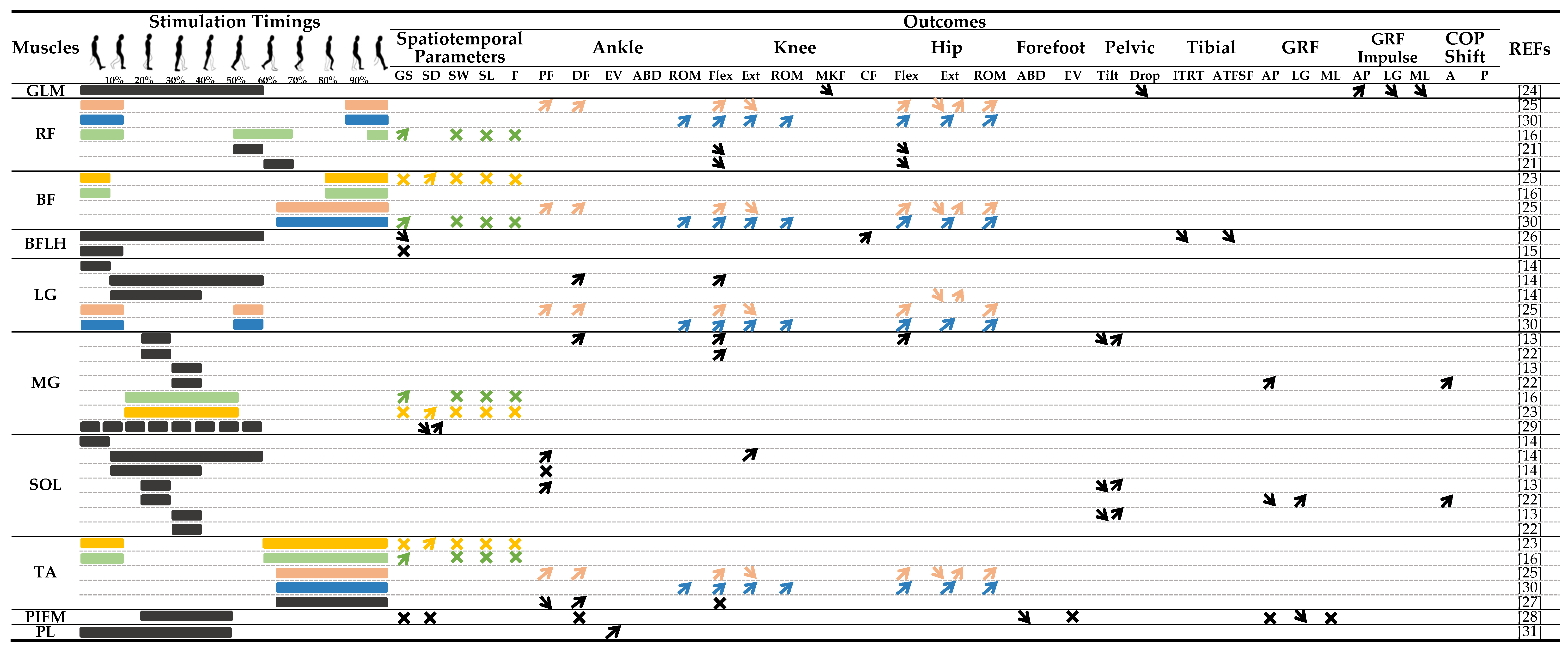
3.4. Stimulation Parameters
3.5. Spatiotemporal Parameters
3.6. Gait Kinematics
3.7. Gait Kinetics
4. Discussion
4.1. FES Parameters during Gait in Healthy Individuals
4.2. Effects of FES on Gait Parameters in Healthy Individuals
4.3. Applications, Perspectives, and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rushton, D.N. Functional Electrical Stimulation. Physiol. Meas. 1997, 18, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Chin, C.; Popovic, M.R. Functional Electrical Stimulation Therapy for Restoration of Motor Function after Spinal Cord Injury and Stroke: A Review. BioMed Eng. OnLine 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Warring, T.; Agrella, S.; Rogers, H.L.; Fox, E.J. Effects of Functional Electrical Stimulation on Gait Function and Quality of Life for People with Multiple Sclerosis Taking Dalfampridine. Int. J. MS Care 2015, 17, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.L.; Silva, M.T.; Martins, J.M.; Newman, D.J. Technical Developments of Functional Electrical Stimulation to Correct Drop Foot: Sensing, Actuation and Control Strategies. Clin. Biomech. 2015, 30, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Triolo, R.J.; Bieri, C.; Uhlir, J.; Kobetic, R.; Scheiner, A.; Marsolais, E.B. Implanted Functional Neuromuscular Stimulation Systems for Individuals with Cervical Spinal Cord Injuries: Clinical Case Reports. Arch. Phys. Med. Rehabil. 1996, 77, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Kobetic, R.; Triolo, R.J.; Marsolais, E.B. Muscle Selection and Walking Performance of Multichannel FES Systems for Ambulation in Paraplegia. IEEE Trans. Rehabil. Eng. 1997, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.R.; Curt, A.; Keller, T.; Dietz, V. Functional Electrical Stimulation for Grasping and Walking: Indications and Limitations. Spinal Cord 2001, 39, 403–412. [Google Scholar] [CrossRef]
- Liberson, W.T.; Holmquest, H.J.; Scot, D.; Dow, M. Functional Electrotherapy: Stimulation of the Peroneal Nerve Synchronized with the Swing Phase of the Gait of Hemiplegic Patients. Arch. Phys. Med. Rehabil. 1961, 42, 101–105. [Google Scholar]
- Linden, M.L.; van der Hooper, J.E.; Cowan, P.; Weller, B.B.; Mercer, T.H. Habitual Functional Electrical Stimulation Therapy Improves Gait Kinematics and Walking Performance, but Not Patient-Reported Functional Outcomes, of People with Multiple Sclerosis Who Present with Foot-Drop. PLoS ONE 2014, 9, e103368. [Google Scholar] [CrossRef]
- Allen, J.L.; Ting, L.H.; Kesar, T.M. Gait Rehabilitation Using Functional Electrical Stimulation Induces Changes in Ankle Muscle Coordination in Stroke Survivors: A Preliminary Study. Front Neurol. 2018, 9, 1127. [Google Scholar] [CrossRef]
- Kesar, T.M.; Perumal, R.; Reisman, D.S.; Jancosko, A.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Functional Electrical Stimulation of Ankle Plantarflexor and Dorsiflexor Muscles: Effects on Poststroke Gait. Stroke 2009, 40, 3821–3827. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, M.; Wagner, F.B.; Gandar, J.; Moraud, E.M.; Wenger, N.; Milekovic, T.; Shkorbatova, P.; Pavlova, N.; Musienko, P.; Bezard, E.; et al. Configuration of Electrical Spinal Cord Stimulation through Real-Time Processing of Gait Kinematics. Nat. Protoc. 2018, 13, 2031–2061. [Google Scholar] [CrossRef]
- Lenhart, R.L.; Francis, C.A.; Lenz, A.L.; Thelen, D.G. Empirical Evaluation of Gastrocnemius and Soleus Function During Walking. J. Biomech. 2014, 47, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Postans, N.; Schwartz, M.H.; Rozumalski, A.; Roberts, A. An Exploration of the Function of the Triceps Surae during Normal Gait Using Functional Electrical Stimulation. Gait Posture 2007, 26, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Azmi, N.L.; Bull, A.M.J. Validation and Use of a Musculoskeletal Gait Model to Study the Role of Functional Electrical Stimulation. IEEE Trans. Biomed. Eng. 2019, 66, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Jung, J.; Lee, D.-W.; Shin, H.C.; Lee, H.-J.; Lee, W.-H. A Wearable Electromyography-Controlled Functional Electrical Stimulation System Improves Balance, Gait Function, and Symmetry in Older Adults. Technol. Health Care 2022, 30, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; McFadyen, A.; Lord, A.C.; Hunter, R.; Paul, L.; Rafferty, D.; Bowers, R.; Mattison, P. Functional Electrical Stimulation for Foot Drop in Multiple Sclerosis: A Systematic Review and Meta-Analysis of the Effect on Gait Speed. Arch. Phys. Med. Rehabil. 2017, 98, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Khamis, S. Effects of Functional Electrical Stimulation on Gait in People with Multiple Sclerosis—A Systematic Review. Mult. Scler. Relat. Disord. 2017, 13, 4–12. [Google Scholar] [CrossRef]
- Mark, R.E.; Anne, M.M.; Catherine, S.; Robert, D.H.; Christopher, G. Maher Growth in the Physiotherapy Evidence Database (PEDro) and Use of the PEDro Scale. Br. J. Sports Med. 2013, 47, 188. [Google Scholar] [CrossRef]
- Qiu, C.G.; Chui, C.S.; Chow, S.K.H.; Cheung, W.-H.; Wong, R.M.Y. Effects of Whole-Body Vibration Therapy on Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JRM 2022, 54, jrm00266. [Google Scholar] [CrossRef]
- Hernandez, A.; Lenz, A.; Thelen, D.G. Electrical Stimulation of the Rectus Femoris During Pre-Swing Diminishes Hip and Knee Flexion During the Swing Phase of Normal Gait. IEEE Trans. Neural. Syst. Rehabil. Eng. 2010, 18, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Lenz, A.L.; Lenhart, R.L.; Thelen, D.G. The Modulation of Forward Propulsion, Vertical Support, and Center of Pressure by the Plantarflexors during Human Walking. Gait Posture 2013, 38, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Talis, V.; Ballay, Y.; Grishin, A.; Pozzo, T. Functional Electrical Stimulation Alters the Postural Component of Locomotor Activity in Healthy Humans. Front. Neurosci. 2015, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Rane, L.; Bull, A.M.J. Functional Electrical Stimulation of Gluteus Medius Reduces the Medial Joint Reaction Force of the Knee during Level Walking. Arthritis Res. Ther. 2016, 18, 255. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Porr, B.; Macleod, C.A.; Gollee, H. A Functional Electrical Stimulation System for Human Walking Inspired by Reflexive Control Principles. Proc. Inst. Mech. Eng. H 2017, 231, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.L.; Ding, Z.; Xu, R.; Bull, A.M.J. Activation of Biceps Femoris Long Head Reduces Tibiofemoral Anterior Shear Force and Tibial Internal Rotation Torque in Healthy Subjects. PLoS ONE 2018, 13, e0190672. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, L.; Song, R.; Li, L.; Wang, X.; Tong, K. Speed-Adaptive Control of Functional Electrical Stimulation for Dropfoot Correction. J. NeuroEng. Rehabil. 2018, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Kanai, S.; Hasegawa, M.; Otsuka, A.; Oki, S. The Effect of Additional Activation of the Plantar Intrinsic Foot Muscles on Foot Dynamics during Gait. Foot 2018, 34, 1–5. [Google Scholar] [CrossRef]
- Thorp, J.E.; Adamczyk, P.G. Mechanisms of Gait Phase Entrainment in Healthy Subjects during Rhythmic Electrical Stimulation of the Medial Gastrocnemius. PLoS ONE 2020, 15, e0241339. [Google Scholar] [CrossRef]
- Dong, H.; Hou, J.; Song, Z.; Xu, R.; Meng, L.; Ming, D. An Adaptive Reflexive Control Strategy for Walking Assistance System Based on Functional Electrical Stimulation. Front. Neurosci. 2022, 16, 944291. [Google Scholar] [CrossRef]
- Gottlieb, U.; Hoffman, J.R.; Springer, S. The Immediate Carryover Effects of Peroneal Functional Electrical Stimulation Differ between People with and without Chronic Ankle Instability. Sensors 2022, 22, 1622. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.L.; Davis, B.L.; O’Connor, J.C. Dynamics of Human Gait; Human Kinetics Publishers: Champaign, IL, USA, 1992; ISBN 978-0-87322-368-3. [Google Scholar]
- Downey, R.J.; Bellman, M.; Sharma, N.; Wang, Q.; Gregory, C.M.; Dixon, W.E. A Novel Modulation Strategy to Increase Stimulation Duration in Neuromuscular Electrical Stimulation: NMES Frequency and Amplitude Modulation. Muscle Nerve 2011, 44, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.J.; Clair, J.M.; Lagerquist, O.; Mang, C.S.; Okuma, Y.; Collins, D.F. Neuromuscular Electrical Stimulation: Implications of the Electrically Evoked Sensory Volley. Eur. J. Appl. Physiol. 2011, 111, 2409–2426. [Google Scholar] [CrossRef] [PubMed]
- Dreibati, B.; Lavet, C.; Pinti, A.; Poumarat, G. Influence of Electrical Stimulation Frequency on Skeletal Muscle Force and Fatigue. Ann. Phys. Rehabil. Med. 2010, 53, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Nguyen, R.; Hirabayashi, T.; Popovic, M.R.; Masani, K. Method to Reduce Muscle Fatigue During Transcutaneous Neuromuscular Electrical Stimulation in Major Knee and Ankle Muscle Groups. Neurorehabil. Neural Repair 2015, 29, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Vromans, M.; Faghri, P.D. Functional Electrical Stimulation-Induced Muscular Fatigue: Effect of Fiber Composition and Stimulation Frequency on Rate of Fatigue Development. J. Electromyogr. Kinesiol. 2018, 38, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.L.; Bowman, B.R.; McNeal, D.R. Effects of Waveform on Comfort during Neuromuscular Electrical Stimulation. Clin. Orthop. Relat. Res. 1988, 233, 75–85. [Google Scholar] [CrossRef]
- Takeda, K.; Koyama, S.; Shomoto, K.; Ushiroyama, K.; Naoi, Y.; Sakurai, H.; Kanada, Y.; Tanabe, S. Modulation of the Rate of Force Development in Hip Abductor Muscles by Neuromuscular Electrical Stimulation during Gait. Somatosens. Mot. Res. 2020, 37, 1–5. [Google Scholar] [CrossRef]
- Taylor, A.M.; Enoka, R.M. Quantification of the Factors That Influence Discharge Correlation in Model Motor Neurons. J. Neurophysiol. 2004, 91, 796–814. [Google Scholar] [CrossRef]
- Fuglevand, A.J.; Keen, D.A. Re-Evaluation of Muscle Wisdom in the Human Adductor Pollicis Using Physiological Rates of Stimulation. J. Physiol. 2003, 549, 865–875. [Google Scholar] [CrossRef]
- Grill, W.M.; Mortimer, J.T. The Effect of Stimulus Pulse Duration on Selectivity of Neural Stimulation. IEEE Trans. Biomed. Eng. 1996, 43, 161–166. [Google Scholar] [CrossRef]
- Lagerquist, O.; Collins, D.F. Influence of Stimulus Pulse Width on M-Waves, H-Reflexes, and Torque during Tetanic Low-Intensity Neuromuscular Stimulation. Muscle Nerve 2010, 42, 886–893. [Google Scholar] [CrossRef]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular Electrical Stimulation for Skeletal Muscle Function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar] [PubMed]
- Chiba, H.; Ebihara, S.; Tomita, N.; Sasaki, H.; Butler, J.P. Differential Gait Kinematics between Fallers and Non-Fallers in Community-Dwelling Elderly People. Geriatr. Gerontol. Int. 2005, 5, 127–134. [Google Scholar] [CrossRef]
- Menz, H.B.; Morris, M.E.; Lord, S.R. Foot and Ankle Risk Factors for Falls in Older People: A Prospective Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, D.C.; Lee, L.W.; Collins, J.J.; Riley, P.O.; Lipsitz, L.A. Reduced Hip Extension during Walking: Healthy Elderly and Fallers versus Young Adults. Arch. Phys. Med. Rehabil. 2001, 82, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, S.A.; Schwartz, M.H. A Baseline of Dynamic Muscle Function during Gait. Gait Posture 2006, 23, 211–221. [Google Scholar] [CrossRef]
- Neptune, R.R.; Zajac, F.E.; Kautz, S.A. Muscle Force Redistributes Segmental Power for Body Progression during Walking. Gait Posture 2004, 19, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.R. The Age-Associated Reduction in Propulsive Power Generation in Walking. Exerc. Sport Sci. Rev. 2016, 44, 129–136. [Google Scholar] [CrossRef] [PubMed]
- DeVita, P.; Hortobagyi, T. Age Causes a Redistribution of Joint Torques and Powers during Gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef]
- Begue, J.; Peyrot, N.; Dalleau, G.; Caderby, T. Effect of Increasing Speed on Whole-Body Angular Momentum during Stepping in the Elderly. J. Biomech. 2021, 122, 110436. [Google Scholar] [CrossRef]
- Robinovitch, S.N.; Feldman, F.; Yang, Y.; Schonnop, R.; Leung, P.M.; Sarraf, T.; Sims-Gould, J.; Loughin, M. Video Capture of the Circumstances of Falls in Elderly People Residing in Long-Term Care: An Observational Study. Lancet 2013, 381, 47–54. [Google Scholar] [CrossRef]
- Cicirelli, G.; Impedovo, D.; Dentamaro, V.; Marani, R.; Pirlo, G.; D’Orazio, T.R. Human Gait Analysis in Neurodegenerative Diseases: A Review. IEEE J. Biomed. Health Inform. 2022, 26, 229–242. [Google Scholar] [CrossRef]
- Mengarelli, A.; Tigrini, A.; Fioretti, S.; Verdini, F. Identification of Neurodegenerative Diseases from Gait Rhythm Through Time Domain and Time-Dependent Spectral Descriptors. IEEE J. Biomed. Health Inform. 2022, 26, 5974–5982. [Google Scholar] [CrossRef]
| First Author (Year and Reference Number) | Sex (Mean Age) of Healthy Participants | Objectives | FES Parameters (Stimulated Muscle; Wave Type; Frequency; Intensity; Stimulation Timing and Trigger Device) | Study Protocol | Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|
| Stewart et al. (2007) [14] | 5M (38 years). | To investigate the dynamic function of the calf muscles during normal gait by using FES. | Muscles: LG and SOL. Wave type: asymmetric biphasic wave. Pulse width: adapted to the subjects to make a strong muscle contraction. Frequency: 40 Hz. Intensity: below the discomfort threshold (≤70 mA). Stimulation timing: (1) initial contact to foot-flat, (2) foot-flat to toe-off, (3) heel-off to toe-off. Trigger device: foot switch. | Walking trials were performed with 3 different stimulation patterns for each muscle (LG and SOL), giving 6 stimulation conditions. A total of 6 trials were collected for the stimulated and unstimulated conditions. | Knee and ankle angles in the sagittal plane. | Stimulation of LG during stance phase increased the knee flexion angle and the ankle dorsiflexion angle, whereas stimulation of SOL increased knee extension angle and ankle plantar flexion angle. These results vary from subject to subject. |
| Hernandez et al. (2010) [21] | 7 adults (mean age: 30 years). | To evaluate the rectus femoris function during walking by synchronizing electrical stimulation to specific points of the gait cycle. | Stimulated muscle: RF of right leg. Wave type: not specified. Pulse width: 300 μs. Frequency: 33 Hz. Intensity: below the pain threshold (subjective value of 2 in a 10-point pain scale). Stimulation timing: 50% (pre-swing) or 60% (early-swing) of the gait cycle for 90 ms. Trigger device: vertical GRF. | Participants performed 90 s walking trials on a split-belt instrumented treadmill while their right RF was stimulated during the pre- or early-swing phases of randomly selected strides. | Hip and knee angles in the sagittal plane. | RF stimulation during pre-swing reduced the knee flexion angle peak in every subject and the hip flexion angle peak in 4/7 subjects. RF stimulation during early swing reduced the knee flexion angle peak in 3/7 subjects and the hip flexion angle peak in 4/7 subjects. |
| Francis et al. (2013) [22] | 20 young adults (mean age: 24 years). | To investigate the relative influence of the gastrocnemius and soleus on support, propulsion, and CoP trajectory in distinct phases of gait. | Stimulated muscles: MG and the distal–lateral quadrant of SOL. Wave type: not specified. Pulse width: 300 μs. Frequency: 33 Hz. Intensity: <50 mA. Stimulation timing: 20% (mid-stance) or 30% (terminal stance) of the gait cycle for 90 ms. Trigger device: GRF signal. | Participants performed eight 90 s walking trials at their preferred walking speed. The FES program randomly delivered stimulation to the MG or SOL at 20% or 30% of the gait cycle, with 5–10 strides between stimulation pulse trains. | GRF and CoP. | Stimulation of MG at 20% of gait cycle led to an anterior CoP shift, whereas it led to an increase in the push-off at 30% of gait cycle. Stimulation of SOL decreased the anteroposterior force at both timings, whereas it led to an anterior CoP shift and an increased vertical ground reaction force at 20% of gait cycle. |
| Lenhart et al. (2014) [13] | 20 young adults (7M and 13F, mean age: 24 years). | To evaluate the effect of electrically stimulating SOL and MG at specific portions of the stance phase of gait on lower limb kinematics. | Muscles: SOL and MG. Wave type: biphasic wave. Pulse width: 250 μs. Frequency: 40 Hz. Intensity: minimum motor threshold (value < 50 mA). Stimulation timing: 20% (mid-stance) or 30% (terminal stance) of the gait cycle for 90 ms. Trigger device: vertical GRF. | For each gait trial, muscle (MG or SOL) and stimulation timing (20% or 30% of the gait cycle) were randomized. Trials were 90 s in duration and included approximately 10 stimulations per trial. | Lower limb joint angles in the sagittal plane. | MG stimulation during mid-stance induced a greater hip and knee flexion angle 150 ms post-stimulation. Ankle dorsiflexion angle and posterior pelvic tilt were also induced at 200 ms after stimulation onset. In contrast, SOL stimulation during mid-stance induced ankle plantar flexion angle and knee extension angle. |
| Talis et al. (2015) [23] | 16 adults (13M and 3F, mean age: 35 years). | To study the effect of the FES of leg muscles on kinematics of healthy subjects during treadmill locomotion. | Stimulated muscles: BF, MG, TA, and quadriceps of both legs. Wave type: rectangular pulse. Pulse width: 0 to 250 μs. Frequency: 65 Hz. Intensity: 65 mA. Stimulation timing: timing of the activation sequence of various muscles during normal gait. Trigger device: right knee goniometer signal. | An experimental group (n = 8) and a control group (n = 8) walked for 40 min on a treadmill. After 10 min without stimulation, FES was applied for 30 min in the experimental group and finally switched off for the last 10 min. Control group walked without FES. | Spatiotemporal gait parameters, trunk oscillations and limb elevation angles in sagittal plane. | FES increased the stance duration during gait. No effect on limb elevation angles in sagittal plane, gait speed, step length, or step width and frequency. |
| Rane and Bull (2016) [24] | 15 young adults (13M and 2F, mean age: 25 years). | To study the effects of stimulating GLM on the medial knee JRF during walking. | Stimulated muscle: GLM. Wave type: asymmetrical biphasic current waveforms. Pulse width: 400 μs. Frequency: 45 Hz. Intensity: the intensity producing an abduction angle of 30–45° of the right leg while being tolerable. Stimulation timing: start before the right foot strike such that stimulation was maximal throughout the stance phase. Trigger device: not specified. | Participants performed between 10 and 15 overground walking trials at their preferred speed without and then with FES. | Medial knee JRF, GLM force, GRF, and lower limb kinematics. | Stimulating GLM during stance reduced the medial knee JRF impulse in the mid and terminal stance, increased GLM force impulse, decreased pelvic drop in the frontal plane toward the swing leg, decreased both the mediolateral and vertical GRF impulses, and increased the anteroposterior GRF impulse during stance phase compared to normal gait. |
| Meng et al. (2017) [25] | 5M and 2F young adults (29 years). | To test a new multichannel FES gait system based on a purely reflexive mechanism that is aimed at assisting gait locomotion. | Muscles: RF, BF, LG, and TA. Wave type: not specified. Pulse width: 350 μs. Frequency: 40 Hz. Intensity: superior to the motor threshold and below the pain threshold. Stimulation timing: during swing (TA, BF), at the terminal swing (TA, BF, RF), the pre-swing (LG), and the loading response (RF, LG). Trigger device: force-sensitive resistors and inertial measurement units. | The participants performed walking trials on a treadmill in two conditions: (1) 3 min at preferential speed and (2) 1 min with stimulation applied on eight muscles, at the same speed as in the first condition. | Hip, knee, and ankle angles in the sagittal plane. | Five participants obtained a higher peak of ankle plantar flexion angle in the pre-swing phase and a higher peak of ankle dorsiflexion angle in the swing phase. Knee and hip extension were reduced in the stance phase, whereas flexion angles were increased during swing phase. |
| Azmi et al. (2018) [26] | 12 young adults (5M and 7F, mean age: 26 years). | To investigate the effect of stimulating the biceps femoris in stance phase on the internal rotation torque and the anterior tibial shear force during gait. | Stimulated muscle: BF long head. Wave type: not specified. Pulse width: not specified. Frequency: 40 Hz. Intensity: below the pain threshold; had to generate a knee flexion angle. Stimulation pattern: start with 1 s ramp up, 4 s with maximum current, and 1 s ramp down. Stimulation timing: heel-strike to toe-off. Trigger device: hand switch. | Subjects performed 6 walking trials without stimulation and 6 with stimulation at their self-preferred speed. Stimulation current was at its maximum value from when the heel of the right foot strikes the force plate until toe-off. | Knee joint torque, anterior shear force, knee contact force, patella tendon force, and gait speed. | Stimulation of BF in stance phase reduced the gait speed, the peak value of the tibial internal rotation torque, and the anterior shear force at the knee. In contrast, it increased the peak of lateral knee compressive force and the peak of patella tendon force. |
| Chen et al. (2018) [27] | 9 young adults (5M and 4F, mean age 23) and 10 post-stoke adults. | To compare two methods of triggering FES for drop foot correction during walking. | Stimulated muscle: TA. Wave type: rectangular pulse. Pulse width: 400 μs. Frequency: 40 Hz. Intensity: intensity when the subjects achieved a neutral ankle angle (0°) in a seated position with the foot hanging freely in a plantar-flexed position. Stimulation timing: heel-off to heel strike. Trigger device: foot switch. | Healthy controls walked on a treadmill at 4 speeds (0.3, 0.6, 0.9, and 1.2 m/s) under 3 stimulation conditions: (1) FES triggered by the heel-off event (HOS), (2) FES triggered by a speed-adaptive algorithm (SAS), and (3) without FES (NS). | Peak of the knee flexion angle, maximum dorsiflexion angle during the swing phase, and ankle angle at the toe-off event. | Higher peak of dorsiflexion angle during swing phase and a decrease in plantar flexion angle in SAS condition compared with NS condition. Peak knee flexion angle in the NS condition was similar to that in the SAS condition at most speeds. |
| Okamura et al. (2018) [28] | 20M young adults (21 years). | To examine the effect of reinforcing the plantar intrinsic foot muscles (PIFMs) via electrical stimulation on foot dynamics during gait. | Muscle: abductor hallucis of the right leg. Wave type: not specified. Pulse width: 250 μs. Frequency: 20 Hz. Intensity: below the pain threshold. Stimulation timing: PIFMs were stimulated from mid-stance to pre-swing. Trigger device: hand switch. | Two groups performed 5 walking trials at their self-selected preferred speed on an 8 m walkway. Afterward, 5 trials were conducted again with FES only in the experimental group. | Stance duration, foot kinematics, ankle moments, and GRF. | FES slowed the deformation of the medial longitudinal arch, decreased forefoot abduction, and reduced the second peak of the vertical ground reaction force. No effect on gait speed, stance duration, forefoot eversion, ankle dorsiflexion, or anteroposterior and mediolateral GRF. |
| Ding et al. (2019) [15] | 13 young adults (5M and 8F, mean age: 26 years). | To quantify the effect of stimulating biceps femoris during the stance phase of gait and validating in a musculoskeletal model. | Stimulated muscle: BF long head. Wave type: not specified. Pulse width: 120 μs. Frequency: 40 Hz. Intensity: 40 mA, 60 mA, and 80 mA (each intensity had to generate a knee flexion angle). Stimulation timing: delivered at the early stance on the muscle activation duration. Trigger device: manual (hand switch). | Participants performed 6 walking trials without stimulation and 6 walking trials per intensity of stimulation (3 intensities) at their self-selected preferred speed on a 6 m walkway. | Gait speed. | Stimulation of BF during stance phase did not affect the gait speed. GMAX EMG peak and impulse during stance phase increased with stimulation intensity of BFLH. |
| Thorp et Adamczyk (2020) [29] | 8F young (College-aged). | To examine the effects of electrical stimulation of gastrocnemius at various phases of the gait cycle on treadmill and overground walking. | Muscle: right medial gastrocnemius (MG). Wave type: biphasic wave. Pulse width: 350 μs. Frequency: 40 Hz. Intensity: minimum motor threshold (Min), maximum tolerable intensity (Max), and 2/3 between Min and Max. Delay: 0–1 s. Stimulation timing: 8 distinct subphases within stance phase (0–49% of the gait cycle in 7% increments and 49–60%) and 4 subphases within swing phase (60–100% in 10% increments) for 100 ms. Trigger device: inertial measurement unit. | Participants preformed four trials of treadmill gait for two minutes at their preferred speed. A 1 min break was established between trials. In parallel, four overground walking trials were performed. Each stimulation pulse train was separated by a random integer (from 4 to 6) of normal strides. | Stride duration. | Depending on the stimulation timing, stride duration was influenced by the stimulation of MG. The stride period was shorter when stimulation was applied around the push-off phase and was longer when stimulation was applied around foot contact. |
| Dong et al. (2022) [30] | 10 young adults (mean age: 25 years). | To validate a FES walking assistance system with an adaptive control method of the stimulation based on temporal gait parameters and sagittal shank angle. | Stimulated muscles: RF, BF, LG, and TA of both legs. Wave type: not specified. Pulse width: 250 to 500 μs. Frequency: not specified. Intensity: adaptative. Stimulation timing: during swing (TA, BF), at the terminal swing (TA, BF, RF), at the pre-swing (LG), and at the loading response (RF, LG). Trigger device: force-sensitive resistors and inertial measurement unit. | A total of 3 conditions of walking on treadmill: without FES (NFC), with reflexive FES controller (RFC), and with adaptative and reflexive FES controller (ARFC). The walking speed increased from 1.0 to 2.0 km/h and then decreased to 1.0 km/h, with 0.2-km/h steps. | Joint kinematics of the hip, knee, and ankle in the sagittal plane. | Combined stimulation of various muscles increased the ROM angle of the ankle, knee, and hip. ARFC had a greater effect than RFC on all kinematics parameters at different walking speeds. |
| Gottlieb et al. (2022) [31] | 24 adults (13M and 11F, mean age 30 years) and 24 adults (17M and 7F, mean age 30 years) with chronic ankle instability (CAI). | To study the effects of a single gait training session with peroneal FES on ankle kinematics and peroneal activity in individuals with and without CAI. | Stimulated muscle: below the head of the fibula and over the peroneus longus belly. Wave type: biphasic symmetrical pulse. Pulse width: 200 μs. Frequency: 35 Hz. Intensity: above the motor threshold and below the discomfort threshold (between 33 mA and 40 Hz). Stimulation timing: was delivered between 0% and 80% of the stance phase. Trigger device: foot switch. | Participants walked for 10 min with FES on a treadmill at a pace 20% faster than their preferred walking speed. | Ankle kinematics and peroneal activity (EMG). | After a single gait training session with FES, healthy controls had significantly more ankle eversion angle at early and late stance than before the intervention in this phase, without a change in the peroneal muscle activity. |
| Park et al. (2022) [16] | 10M and 19F old adults (75 years). | To examine the immediate effects of wearable EMG-controlled FES on the lower limb muscle morphology, balance, and gait in older adults. | Muscles: RF, BF, TA, and MG. Wave type: rectangular biphasic wave. Pulse width: 250 μs. Frequency: 40 Hz. Intensity: below the pain threshold (range: 10–40 mA). Pulse duration: not specified. Stimulation timing: delivered at different moments of stance and swing phase, without specifying the events. Trigger device: EMGs. | After a familiarization phase with the EMG-controlled FES (5 to 10 min), walking trials were carried out with and without FES (six trials in total) in a randomized order. Participants walked at a self-selected speed on a walkway approximately 9 m long and on a 5 m GAITRite mat with 2 m acceleration and deceleration periods. | Spatiotemporal gait parameters | FES led to an increase in gait speed and cadence. No effect on stride length, step length, and step width. |
| Authors | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score | Reliability (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stewart et al., 2007 [14] | N | Y | Y | Y | Y | N | N | Y | Y | N | N | 6/11 | 54% |
| Hernandez et al., 2010 [21] | N | N | N | Y | N | N | N | Y | Y | Y | Y | 5/11 | 45% |
| Francis et al., 2013 [22] | N | Y | Y | Y | Y | N | N | Y | Y | Y | N | 7/11 | 63% |
| Lenhart et al., 2014 [13] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9/11 | 81% |
| Talis et al., 2015 [23] | N | N | N | Y | N | N | N | Y | Y | Y | Y | 5/11 | 45% |
| Rane and Bull, 2016 [24] | N | N | N | Y | N | N | N | Y | Y | Y | Y | 5/11 | 45% |
| Meng et al., 2017 [25] | N | N | N | Y | Y | N | N | Y | Y | Y | Y | 6/11 | 54% |
| Azmi et al., 2018 [26] | N | N | N | Y | N | Y | N | Y | Y | Y | Y | 6/11 | 54% |
| Chen et al., 2018 [27] | N | Y | Y | N | Y | N | N | Y | Y | Y | Y | 7/11 | 63% |
| Okamura et al., 2018 [28] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9/11 | 81% |
| Ding et al., 2019 [15] | N | N | N | Y | N | N | N | Y | Y | Y | Y | 5/11 | 45% |
| Thorp and Adamczyk, 2020 [29] | N | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8/11 | 72% |
| Dong et al., 2022 [30] | N | N | N | Y | N | N | N | Y | Y | Y | Y | 5/11 | 45% |
| Gottlieb et al., 2022 [31] | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | 8/11 | 72% |
| Park et al., 2022 [16] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9/11 | 81% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aout, T.; Begon, M.; Jegou, B.; Peyrot, N.; Caderby, T. Effects of Functional Electrical Stimulation on Gait Characteristics in Healthy Individuals: A Systematic Review. Sensors 2023, 23, 8684. https://doi.org/10.3390/s23218684
Aout T, Begon M, Jegou B, Peyrot N, Caderby T. Effects of Functional Electrical Stimulation on Gait Characteristics in Healthy Individuals: A Systematic Review. Sensors. 2023; 23(21):8684. https://doi.org/10.3390/s23218684
Chicago/Turabian StyleAout, Thomas, Mickael Begon, Baptiste Jegou, Nicolas Peyrot, and Teddy Caderby. 2023. "Effects of Functional Electrical Stimulation on Gait Characteristics in Healthy Individuals: A Systematic Review" Sensors 23, no. 21: 8684. https://doi.org/10.3390/s23218684
APA StyleAout, T., Begon, M., Jegou, B., Peyrot, N., & Caderby, T. (2023). Effects of Functional Electrical Stimulation on Gait Characteristics in Healthy Individuals: A Systematic Review. Sensors, 23(21), 8684. https://doi.org/10.3390/s23218684








